Abstract
The movement of guard cells in stomatal complexes controls water loss and CO2 uptake in plants. Examination of the dual-affinity nitrate transporter gene AtNRT1.1 (CHL1) revealed that it is expressed and functions in Arabidopsis guard cells. CHL1 promoter–β-glucuronidase and CHL1 promoter–green fluorescent protein constructs showed strong expression in guard cells, and immunolocalization experiments with anti-CHL1 antibody confirmed these results. To assess CHL1 function, chl1 mutant plants grown in the presence of nitrate were examined. Compared with wild-type plants, chl1 mutants had reduced stomatal opening and reduced transpiration rates in the light or when deprived of CO2 in the dark. These effects result in enhanced drought tolerance in chl1 mutants. At the cellular level, chl1 mutants showed reduced nitrate accumulation in guard cells during stomatal opening and failed to show nitrate-induced depolarization of guard cells. In wild-type guard cells, nitrate induced depolarization, and nitrate concentrations increased threefold during stomatal opening. These results identify an anion transporter that functions in stomatal opening and demonstrate that CHL1 supports stomatal function in the presence of nitrate.
INTRODUCTION
Stomata act as ports that regulate the uptake of CO2 for photosynthesis and the evaporation of water for transpiration in plants. Gas exchange through stomatal pores in the leaves of plants is regulated by the turgor-driven expansion and contraction of guard cells in response to environmental and internal signals, including light, humidity, CO2, phytohormones, calcium, and reactive oxygen species (reviewed by Blatt, 2000; Assmann and Wang, 2001; Dietrich et al., 2001; Schroeder et al., 2001; Roelfsema and Hedrich, 2002). Changes in guard cell turgor are driven by fluxes of K+ and Cl− and, depending on the growth conditions and the time of day, the accumulation or loss of malate and Suc (reviewed by Talbott and Zeiger, 1998; Blatt, 2000; Assmann and Wang, 2001; Dietrich et al., 2001; Schroeder et al., 2001). For example, light-induced stomatal opening involves activation of the plasma membrane H+-ATPase, which results in the hyperpolarization of the plasma membrane and the opening of inward-rectifying K+ channels. The influx of K+ is accompanied by an influx of Cl− and an accumulation of malate.
The search for ion channel/transporter genes involved in stomatal movement has led to the identification of multiple K channel genes that are expressed and function in guard cells (Dietrich et al., 2001; Kwak et al., 2001; Pilot et al., 2001; Schroeder et al., 2001; Szyroki et al., 2001). It is thought that multiple K channels, and not just one, are essential for stomatal opening (Dietrich et al., 2001; Szyroki et al., 2001). No anion channel/transporter gene responsible for stomatal opening has been identified to date. Cl− influx during stomatal opening has been proposed to occur via H+/Cl− symport, but no electrophysiological data or molecular analyses to support this model have been reported (Assmann and Wang, 2001; Dietrich et al., 2001). Stomatal closing involves the efflux of potassium via outward K channels and of anions via two types of anion channels: slow or S-type and rapid or R-type (guard cell anion channel [GCAC]) channels (Schroeder and Keller, 1992; Dietrich and Hedrich, 1994). The S-type channels may be composed of or be regulated by ATP binding cassette proteins (Leonhardt et al., 1997, 1999, 2001). One ATP binding cassette transporter, AtMRP5, has been shown to be required for gliben-clamide (a modulator of K-ATP and cystic fibrosis transmem-brane conductance regulator chloride channels)–induced opening of stomates and thus may serve as a channel or channel regulator (Gaedeke et al., 2001).
The Arabidopsis NRT1.1 (CHL1) gene encodes a dual-affinity nitrate transporter that contributes to both low- and high-affinity uptake in roots of Arabidopsis seedlings (Tsay et al., 1993; Touraine and Glass, 1997; Wang et al., 1998; Liu et al., 1999). Recent studies have shown that CHL1 is expressed preferentially in proliferating regions of roots and shoots (e.g., lateral root primordia and young leaves) and contributes to the growth of nascent organs (Guo et al., 2001). Further analysis of CHL1 has revealed a surprising result: CHL1 is expressed in guard cells (see below). This observation led us to examine possible functions for CHL1 in stomatal movements and function. Because CHL1 is a nitrate transporter, we examined stomatal opening and gas exchange in the presence or absence of nitrate. The results from these experiments are described below.
RESULTS
CHL1 Functions in Light-Induced Stomatal Opening and Nitrate Accumulation in Guard Cells
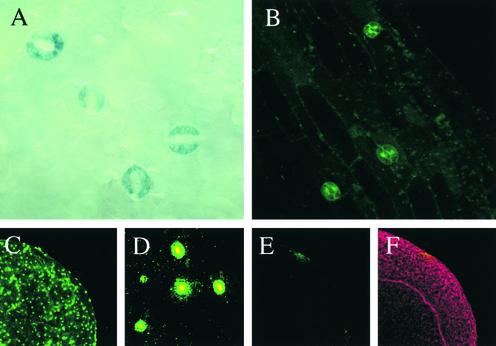
Recent studies of CHL1 showed only low levels of CHL1 expression in mature shoot organs and root tissues using transgenic plants containing CHL1–β-glucuronidase/green fluorescent protein (GUS/GFP) fusion constructs (Guo et al., 2001). Close examination of shoots from these lines, however, revealed strong CHL1 expression in guard cells of mature leaves (Figure 1A) and hypocotyls (Figure 1B). Strong GUS staining also was found in guard cells of floral organs (data not shown). Immunolocalization experiments with affinity-purified anti-CHL1 antibody confirmed these findings, showing high levels of CHL1 protein in guard cells (Figures 1C and 1D; staining with preimmune serum is shown in Figures 1E and 1F).
Figure 1.
Localization of CHL1 Expression and Protein in Guard Cells.
(A) Portion of a leaf from a plant carrying the HaeII CHL1-GUS fusion construct (Guo et al., 2001). Strong GUS staining indicative of CHL1 expression is found in guard cells.
(B) Section of a hypocotyl from a 7-day-old transgenic plant carrying the HaeII CHL1-GFP construct (Guo et al., 2001). Strong GFP signals are found only in guard cells using confocal laser scanning microscopy.
(C) Immunofluorescence from a portion of a leaf stained with anti-CHL1 antibody. Whole-mount assays were performed with affinity-purified anti-CHL1 antibody and Alexa Flour 488–conjugated secondary antibody (Molecular Probes) using confocal laser scanning microscopy (Guo et al., 2001).
(D) Higher magnification of a section of the image in (C) showing immunofluorescence from individual stomata.
(E) Control experiment using preimmune serum showing little immunofluorescence from a leaf.
(F) Outline of the leaf shown in (E) by propidium iodide staining.
The finding of strong CHL1 expression in guard cells suggests that CHL1 may play a role in stomatal function. Because CHL1 is a nitrate transporter, stomatal function was examined in the presence or absence of nitrate in wild-type and chl1 mutant plants. Light-induced stomatal opening was examined first. chl1 mutants (two alleles tested) were impaired significantly (reduced to one-third the wild-type level) in stomatal opening in white light when detached leaves were incubated with 30 mM KNO3 (Figure 2A). This deficiency was dependent on nitrate, because no significant difference (P < 0.05 by t test) in stomatal opening was observed between mutant and wild-type cells if NO3− was replaced with Cl− (Figure 2A). Thus, CHL1 contributes substantially to light-induced opening of stomates when nitrate is the anion but is dispensable for light-induced opening when chloride is the anion.
Figure 2.
CHL1 Functions in Light-Induced Stomatal Opening.
(A) Stomatal apertures were determined for wild-type and chl1 mutant plants (chl1-4 and chl1-5) undergoing light-induced stomatal opening. Data were averaged from three separate experiments; n = 40 aperture measurements per experiment. Error bars represent standard deviations.
(B) Nitrate accumulation was measured in guard cell protoplasts isolated from detached leaves of wild-type and chl1 mutant plants during light-induced stomatal opening in the presence of nitrate. Error bars represent standard deviations (n = 4).
One explanation for these results is that CHL1 mediates nitrate uptake into guard cells. Because nitrate is an anion, it could serve as a counter-ion for K+ influx during stomatal opening. Nitrate accumulation in guard cells was measured during light-induced opening in wild-type and mutant plants. In wild-type guard cells, nitrate concentrations increased nearly threefold after 3 h of light treatment (Figure 2B). chl1 mutant guard cells, however, showed no significant increase in nitrate accumulation during the same treatment (Figure 2B). These results show that net nitrate influx occurs during stomatal opening and that this influx is dependent on CHL1.
CHL1 Supports Stomatal Function in Intact Plants
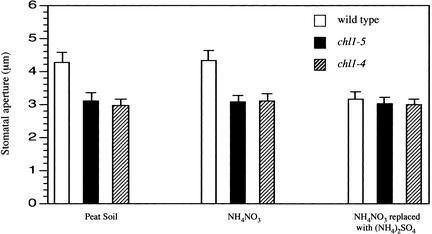
The experiments described above were performed with detached leaves floating on liquid medium. To examine the role of CHL1 in stomatal function further, stomatal apertures and gas exchange were examined for leaves on intact plants. Plants were grown in a 16-h-light/8-h-dark cycle with a continuous supply of nitrate (i.e., in organic peat soil or with vermiculite irrigated with NH4NO3). During the middle of the light period, stomatal apertures were found to be 35 to 45% greater in wild-type plants than in chl1 mutants (Figure 3). For plants deprived of nitrate for 10 days, there was little difference between wild-type and mutant plants as a result of the decrease in stomatal aperture in wild-type plants (Figure 3).
Figure 3.
Stomatal Apertures in Intact Plants.
Stomatal apertures were determined for leaves from intact plants during the middle of the light period (16 h of light and 8 h of dark) as described in Methods. Data were averaged from three separate experiments; n = 40 aperture measurements per experiment.
One explanation for these results is that the reductions in stomatal apertures observed in the mutants are attributable to indirect and detrimental effects of chl1 mutations on photosynthetic activity, which could increase intercellular CO2 levels and cause stomates to close. To test this possibility, stomatal conductance was measured in mature leaves of wild-type and chl1 mutant plants in the dark, when no photosynthesis occurs. When stomates were induced to open by decreasing CO2 levels (treating plants with CO2-free air), chl1 mutants (two alleles tested) showed much reduced increases in stomatal conductance (Figure 4A) and stomatal aperture (Figure 4B, measured at 40 min after CO2 depletion) compared with the wild type. Thus, CHL1 functions to increase stomatal aperture in plants treated continuously with nitrate, and this function is not mediated by an indirect effect on intercellular CO2 levels.
Figure 4.
Stomatal Conductance and Apertures in Plants Depleted of CO2 in the Dark.
(A) Stomatal conductance was measured in wild-type and chl1 mutant plants in the dark when CO2 levels (400 mL/L) were decreased to 0 mL/L (CO2-free air). Squares, wild type (n = 6); circles, chl1-4 (n = 5); triangles, chl1-5 (n = 5). Error bars represent standard deviations.
(B) Stomatal apertures in leaves of the wild type and chl1 mutants after 40 min of treatment with CO2-free air. Error bars represent standard deviations (n = 5).
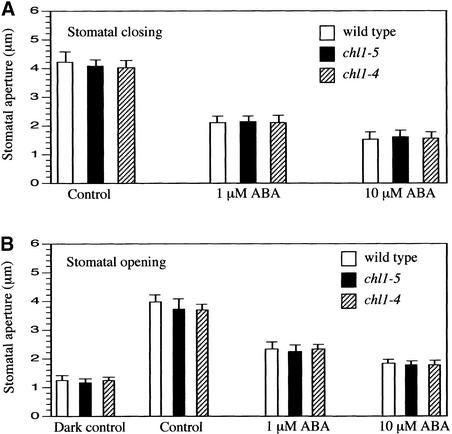
chl1 Mutations Do Not Affect Abscisic Acid–Induced Stomatal Closing or Abscisic Acid Inhibition of Stomatal Opening
One explanation for the effect of chl1 mutations on stomatal aperture is that they affect stomatal responses to abscisic acid. Abscisic acid induces stomates to close. To determine if chl1 mutants are altered in abscisic acid–induced stomatal closing, leaves from wild-type and mutant plants were placed in the light with a solution of 30 mM KCl to open the stomates and then treated with 1 and 10 μM abscisic acid to induce closing. After 2 h, wild-type and mutant stomates closed to the same extent (Figure 5A). As another test of abscisic acid response, light-induced stomatal opening was measured in the presence of abscisic acid, which inhibits opening. Both mutant and wild-type plants showed similar inhibition of stomatal opening by abscisic acid (Figure 5B). We conclude that the reduced stomatal aperture in chl1 mutants is not the result of effects on abscisic acid responses.
Figure 5.
Abscisic Acid Effects on Stomatal Opening and Closing of Wild-Type and chl1 Mutant Plants
(A) Stomatal apertures were determined for wild-type and chl1 mutant plants (chl1-4 and chl1-5) undergoing abscisic acid (ABA)–induced stomatal closing as described in Methods. Data were averaged from three separate experiments; n = 40 aperture measurements per experiment. Error bars represent standard deviations.
(B) Light-induced stomatal opening was measured in the wild type and chl1 mutants with or without abscisic acid treatment. Dark control data represent stomatal apertures from leaves taken from seedlings kept in the dark for 5 h. Data were averaged from three separate experiments; n = 40 aperture measurements per experiment. Error bars represent standard deviations.
Nitrate Induces Depolarization in Wild-Type but Not chl1 Mutant Guard Cells
Further confirmation that CHL1 functions in guard cells was obtained by examining changes in membrane potential in guard cells in response to nitrate. In roots, CHL1 functions as an electrogenic nitrate transporter that depolarizes the plasma membrane during nitrate uptake (Tsay et al., 1993; Wang et al., 1998). Thus, changes in membrane potential are indicative of nitrate uptake into the cell. To test for such activity in guard cells, the voltage-sensitive dye bis-oxonol was used to examine changes in membrane potential induced by nitrate. This dye has been used to examine membrane potential changes in potato leaves (Hedrich et al., 2001) and animal cells (Dall'Asta et al., 1997). When epidermal strips from wild-type plants were exposed to 100 mM NO3−, fluorescence signals from guard cells increased dramatically (indicating depolarization) and reached a peak after 30 s (Figures 6A and 6E). Fluorescence increases also were observed with lower concentrations of NO3−: 30-s peak values were 80 for 10 mM NO3− and 120 for 50 mM NO3−. No significant increase in fluorescence was found in mutant guard cells (two chl1 alleles tested) exposed to 100 mM NO3− (Figures 6B and 6E) or 10 and 50 mM NO3− (data not shown). Cs+ was used as the counter-ion in these experiments to minimize the effect of cation-induced depolarization (Tsay et al., 1993; Ichida et al., 1997). To show that fluorescence signals obtained with CsNO3 were in the range observed with KCl, wild-type guard cells were treated with 100 mM KCl. The resulting fluorescence signals showed a similar time course of induction, but peak levels were a bit lower compared with those recorded at 100 mM CsNO3 (Figures 6C and 6E). chl1 mutant guard cells showed an almost identical response to wild-type cells with 100 mM KCl (Figures 6D and 6E). To demonstrate that these fluorescence increases were caused by depolarization of the cellular membrane, wild-type and chl1 mutant guard cells were depolarized with 50 μM dinitrophenol (a H+ ionophore) in the presence of the bis-oxonol dye and found to display increased fluorescence (data not shown). These data show that nitrate induces CHL1-dependent membrane depolarization in guard cells, indicative of nitrate uptake into these cells.
Figure 6.
Nitrate Induces Depolarization of Membrane Potential in Guard Cells of Wild-Type but Not chl1 Plants.
Depolarization of membrane potentials is represented as an increase in fluorescence.
(A) Fluorescence from a representative wild-type guard cell at 0, 15, 30, and 60 s after adding CsNO3 to a final concentration of 100 mM.
(B) Fluorescence from a representative mutant (chl1-5) guard cell at 0, 15, 30, and 60 s after adding CsNO3 to a final concentration of 100 mM.
(C) Fluorescence from a representative wild-type guard cell at 0, 15, 30, and 60 s after adding KCl to a final concentration of 100 mM.
(D) Fluorescence from a representative mutant (chl1-5) guard cell at 0, 15, 30, and 60 s after adding KCl to a final concentration of 100 mM.
(E) Quantified fluorescence signals from the stomates for each genotype or treatment. Error bars represent standard deviations (n = 5).
chl1 Mutant Plants Have Reduced Water Loss and Are Drought Tolerant
Given that chl1 mutations affect stomatal aperture, one would expect these mutations to also affect transpiration and water loss in whole plants. To assess CHL1 function in these processes, water loss was measured by two methods. First, the fresh weight of detached leaves was determined for wild-type and chl1 plants during desiccation. For plants treated continuously with nitrate, water loss was faster in wild-type compared with mutant leaves (Figure 7A, left and middle), whereas no significant difference was found between leaves from wild-type and mutant plants deprived of nitrate for 10 days (Figure 7A, right). Second, water loss from mature plants undergoing drought stress for 20 days was measured. After 5 days of drought treatment, the rosette leaves in wild-type plants showed severe wilting and chlorosis, whereas the leaves of chl1 mutants were turgid and remained green (Figure 7B). Measurements of soil water content showed that water loss was faster from pots containing wild-type plants compared with pots containing mutants during the first 12 days of desiccation (Figure 7C).
Figure 7.
Chl1 Mutants Are More Drought Tolerant than Wild-Type Plants under Drought Stress.
(A) Water loss from the leaves of wild-type and chl1 plants grown with the indicated nitrogen sources. Data show percentage of initial fresh weight (F.W.) lost from leaves from three individual plants per genotype. Results from one of three experiments are shown. Squares, wild type; circles, chl1-4; triangles, chl1-5. Error bars represent standard deviations.
(B) Wild-type and chl1 mutant plants after 5 days of drought stress. Plants were irrigated for 22 days with nitrate-containing medium and then drought stressed by terminating irrigation. Ten pots per genotype were examined in one of three separate experiments.
(C) Soil water contents were determined by weighing pots and plotted as a percentage of initial fresh weight in chl1 and wild-type pots during desiccation. Pots were covered to minimize soil evaporation. Squares, wild type; circles, chl1-4; triangles, chl1-5. Error bars represent standard deviations.
DISCUSSION
Work on nitrate transporters in plants has focused on nitrate uptake into roots (reviewed by Crawford and Glass, 1998; Forde, 2000; Glass et al., 2001; Crawford and Forde, 2002). Some nitrate transporters are expressed in shoots, but there have been no indications that they function in guard cells. The results described here show that CHL1 is expressed in guard cells and functions as a nitrate transporter and promotes stomatal opening when nitrate is the sole anion. Because chl1 mutants grown with nitrate medium have reduced stomatal apertures and transpiration, CHL1 is involved in these processes as well. Our results suggest that it is the impaired uptake of nitrate into guard cells of the mutant that results in reduced opening of stomates and in reduced stomatal aperture and transpiration. However, the relative importance of nitrate uptake compared with chloride uptake or malate accumulation in guard cells of Arabidopsis plants grown in soil is not known. At present, there is no evidence for an obligate role for nitrate/CHL1 in stomatal function. However, it is possible that nitrate, chloride, and ma-late all play roles of different importance depending on the environmental conditions (such as the external nitrate and chloride concentrations and the time of day). In addition, reduced stomatal aperture and transpiration in the mutants could involve effects mediated by reduced nitrate uptake in the roots or nascent organs in the mutants. Further experiments are needed to address these issues.
We performed some experiments to exclude any indirect effects of chl1 mutations on transpiration. We examined stomatal distribution and density in leaves of chl1 mutants and found no difference between wild-type and mutant plants (data not shown). These findings are consistent with results reported for lettuce, which when grown in N-limited medium has equivalent numbers of adaxial and abaxial stomata compared with plants supplied with NO3− (Broadley et al., 2001). It also appears that CHL1 does not serve as a general anion transporter because chl1 mutations have little effect on stomatal opening when chloride is the only anion in the bath solution. We measured conductance in stomates induced to open in the dark by the depletion of CO2 and found that chl1 mutations impaired stomatal opening, showing that the effect from the chl1 mutations is not dependent on an indirect effect on photosynthesis and intercellular CO2 levels. Finally, we examined abscisic acid–induced responses on stomates and found no difference between the wild type and the mutants.
The findings reported here identify an anion transporter gene that functions in stomatal opening and indicate that nitrate can play a role in this process. These results were surprising because there has been little mention of nitrate in stomatal movements and no reports of a nitrate transporter being expressed or functioning in guard cells. Previous work had focused on Cl− and malate, which are well known as primary anions that counterbalance the influx of K+ during stomatal opening, leading to models proposing that Cl− transporters provide for anion transport during stomatal opening (Raschke and Fellows, 1971; Allaway, 1973; Raschke and Schnabl, 1978; Blatt, 2000; Assmann and Wang, 2001; Dietrich et al., 2001). However, there are data in the literature that have implicated nitrate as being important in stomatal movements. KNO3 supports the opening of stomates as well as KCl in Vicia epidermal strips (Humble and Hsiao, 1969). In addition, the anion channels that mediate stomatal closure show their highest selectivity for nitrate. The R-type GCAC1 channel is fourfold more selective (Hedrich and Marten, 1993), and the S-type anion channels are 21 times more permeable to nitrate than to chloride (Schmidt and Schroeder, 1994). It would make sense that anion channels mediating stomatal closure would have a preference for nitrate if nitrate were important in mediating stomatal opening, as indicated by our results.
The results from our work also suggest that the presence or absence of nitrate in the growth environment can influence stomatal conductance and that this effect is mediated in part by the CHL1 transporter. We found that water loss was greater for wild-type plants in nitrate-containing soil than for wild-type plants in soil depleted of nitrate or for chl1 mutant plants in nitrate-replete soil (Figures 7A and 7C). In addition, wild-type plants were more drought sensitive than chl1 mutants, which are defective in nitrate uptake into guard cells (Figure 7B). Consistent with these findings are reports indicating that higher nitrate levels correlate with greater stomatal conductance in plants. Nitrate-fed soybeans have a higher transpiration rate per unit leaf area than nitrogen-fixing seedlings when both are under water stress (Ines Minguez and Sau, 1989). A dramatic reduction in stomatal conductance is observed in lettuce plants when NH4NO3 is replaced in hydroponic medium with an equivalent concentration of CaSO4 (Broadley et al., 2001). In cotton, stomates show increased sensitivity to water stress (close at higher water potentials) with decreasing levels of supplied nitrate (Radin and Ackerson, 1981). In addition, NH4NO3-sufficient common beans were reported to suffer more drought-induced senescence than control plants grown under conditions of N2 fixation after 10 days of desiccation stress (Lodeiro et al., 2000). These studies could not distinguish between general nitrogen effects and specific effects of nitrate. The finding that CHL1 functions as a nitrate transporter and supports stomatal opening provides evidence for a role for nitrate and provides insights into the relationship between stomatal conductance and nitrate.
METHODS
Plant Material and Growth
Wild-type and chl1 mutant plants of Arabidopsis thaliana ecotype Columbia were grown in peat soil (Sun Gro Horticulture, Bellevue, WA) with fertilizer (Pete's 20-10-20; McConkey, Sumner, WA) or on vermiculite irrigated with nutrient solution (pH 5.5, 6 mM NH4NO3, 0.5 mM K2HPO4-KH2PO4, 0.2 mM MgSO4, 0.1 mM CaCl2, 0.05 mM FeSO4-EDTA, 50 μM H3BO3, 12 μM MnSO4, 1 μM ZnCl2, 1 μM CuSO4, and 0.2 μM Na2MoO4) for 18 days under a light cycle of 16 h of light and 8 h of dark at 24°C. The vermiculite-grown plants then were flushed five times with 400 mL of double-distilled water with a repeat treatment the next day. Half of the pots (16 total) then were irrigated with the same NH4NO3 nutrient solution described above; the other half were irrigated with the same solution except that the NH4NO3 was replaced with 6 mM (NH4)2SO4. Both sets of plants grew and appeared the same after 10 days of treatment.
The chl1-4 and chl1-5 mutants were generated by γ-irradiation (Tsay et al., 1993). chl1-4 has reduced levels of apparently full-length mRNA, but chl1-5 has a deletion that results in no detectable mRNA (Tsay et al., 1993).
Constructs and Plant Transformation
CHL1 promoter–β-glucuronidase/green fluorescent protein fusion constructs and plant transformation were generated as described (Guo et al., 2001).
Light and Confocal Microscopy
Histochemical localization of β-glucuronidase was performed and observed using light microscopy as described (Guo et al., 2001). Green fluorescent protein and immunolocalization (using antibodies raised against the N-terminal 15 amino acids of CHL1) were analyzed using a confocal laser scanning microscope as described (Guo et al., 2001).
Stomatal Aperture Measurements
Plants were grown for 4 weeks in peat soil and then placed in the dark overnight to close the stomata. Detached leaves then were floated in 30 mM KNO3 or KCl solutions (with 0.1 mM CaCl2 and 5 mM Mes-KOH, pH 6.15) under light (80 μmol·m−2·s−1) for 3 h to induce stomatal opening, which was measured as aperture width (Pei et al., 1997; Szyroki et al., 2001). For plants grown in different nitrogen sources or treated with CO2-free air, detached leaves were blended and the resulting epidermal fragments were taken to examine stomatal aperture immediately (Pei et al., 1997; Szyroki et al., 2001). To measure stomatal closing, detached leaves were floated in 30 mM KCl solution as described above under light for 2 h. After 2 h, abscisic acid (Sigma) was added to the buffer solution. After an additional 2 h, leaves were blended and apertures were measured. To measure abscisic acid effect on stomatal opening, 4-week-old seedlings were kept in the dark for 5 h to close the stomata. Detached leaves were floated in 30 mM KCl solution with or without abscisic acid for 2 h under light. Detached leaves then were blended and stomatal apertures were measured.
Stomatal Conductance Measurements
Stomatal conductance was measured using the infrared gas analyzer technique (Hedrich et al., 2001) with the LI-6400 Portable Photosynthesis System (Li-Cor, Lincoln, NE). Plants were grown on vermiculite and irrigated with NH4NO3 nutrient solution as described above. Four-week-old plants were transferred from pots to 10-mL flasks with 8 mL of NH4NO3 nutrient solution, grown for 12 h under continuous light at 24°C, and then kept in the dark for 5 h to close the stomates. The fully expanded leaves of wild-type and chl1 mutant plants were used to examine stomatal conductance in the dark at 24°C. Gas flow through the cuvette was 500 mL/min. The CO2 level was switched from 400 to 0 mL/L (CO2-free air) when the leaf showed a stable gas-exchange rate and stomatal conductance was measured. Detached leaves were taken to examine stomatal aperture after 40 min of CO2-free air treatment, as described (Pei et al., 1997; Szyroki et al., 2001).
Water Loss and Drought Tolerance Measurements
Water loss from the leaves of wild-type and chl1 plants grown with the indicated nitrogen sources was determined as described (Figure 7A; Leung et al., 1997; Wang et al., 2001). Plants were irrigated for 22 days and then drought stressed by terminating irrigation, as described previously (Vartanian et al., 1994; Pei et al., 1998).
Guard Cell Protoplast Isolation and Nitrate Measurements
Guard cell protoplasts were isolated as described (Miedema and Assmann, 1996) with some modifications. After 3 h of incubation in 30 mM KNO3 solution, as described above, detached leaves were blended for 1 min and filtered through a 100-μm nylon mesh. Harvested epidermal peels were transferred to a conical flask containing 50 mL of digestion solution 1 (0.7% [w/v] Cellulysin [Calbiochem, La Jolla, CA], 0.1% [w/v] PVP40 [Sigma], and 0.25% [w/v] BSA in basic medium [5 mM MES, pH 5.5, 0.5 mM CaCl2, 0.5 mM MgCl2, 10 mM KH2PO4, 0.5 mM ascorbic acid, 450 mM sorbitol]) and digested for up to 2 h in a vigorously shaking water bath. When all mesophyll cells were digested, the peels were filtered and transferred to digestion solution 2 (1.3% [w/v] Cellulase RS [Yakult Honsha, Tokyo, Japan], 0.0075% [w/v] Pectolyase Y-23 [Seishin, Tokyo, Japan], and 0.25% [w/v] BSA in basic medium). Peels were examined for lack of intact guard cells. When digested, protoplasts were filtered through a 10-μm nylon mesh folded into four layers and washed with the basal medium. Guard cell protoplasts were harvested by filtering with a 10-μm nylon mesh. Cells were collected by centrifugation at 200g for 4 min. Cell numbers were counted in the samples using a hemacytometer (Hausser Scientific, Horsham, PA). The purity of guard cell protoplasts was ∼92% (1000 cells counted). An average diameter of 6 μm was used to calculate the volume of a protoplast. The cells (∼1 × 108) were ground frozen into a powder. Distilled water (0.5 mL) was added to the ground pellet; this was boiled for 5 min and then spun. The supernatant was used to measure nitrate concentration by HPLC (Thayer and Huffaker, 1980).
Imaging Membrane Potential Changes in Intact Guard Cells
Epidermal strips from detached leaves were made as described (Pei et al., 1997) and then incubated in loading buffer (5 mM Mes-KOH, pH 5.7, 0.25 mM KCl, and 1 mM CaCl2). Peels were mounted onto a copper mesh as described (Allen et al., 1999) and then incubated with 200 μL of loading buffer containing 1 μM dye [bis-(1,3-dibutylbarbituric acid)trimethine oxonol (B-438; Molecular Probes, Eugene, OR)] for 10 min. B-438 fluorescent signals were detected using a confocal laser scanning microscope (MRC-600; Bio-Rad). The dye was excited using a fluorescein isothiocyanate filter set (488 nm), and images were collected using an emission filter (530 ± 15 nm). The images were collected at 0, 15, 30, and 60 s after adding CsNO3 or KCl solutions. Signal intensities were quantified using Photoshop (Adobe Systems, San Jose, CA).
Upon request, all novel materials described in this article will be made available in a timely manner for noncommercial research purposes.
Acknowledgments
We thank June Kwak, Nathalie Leonhardt, Gethyn Allen, and Julian Schroeder for their invaluable technical advice and assistance with guard cell measurements, and Rongchen Wang and Rudi Tischner for their advice in measuring nitrate by HPLC. This work was supported by National Institutes of Health Grant GM40672.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.006312.
References
- Allaway, W.G. (1973). Accumulation of malate in guard cells of Vicia faba during stomatal opening. Planta 110, 63–70. [DOI] [PubMed] [Google Scholar]
- Allen, G.J., Kuchitsu, K., Chu, S.P., Murata, Y., and Schroeder, J.I. (1999). Arabidopsis abi1-1 and abi2-1 mutations impair abscisic acid induced cytosolic calcium rises in guard cells. Plant Cell 11, 1785–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann, S.M., and Wang, X.-Q. (2001). From milliseconds to millions of years: Guard cells and environmental responses. Curr. Opin. Plant Biol. 4, 421–428. [DOI] [PubMed] [Google Scholar]
- Blatt, M.R. (2000). Cellular signaling and volume control in stomatal movements in plants. Annu. Rev. Cell Dev. Biol. 16, 221–241. [DOI] [PubMed] [Google Scholar]
- Broadley, M.R., Escobar-Gutierrez, A.J., Burns, A., and Burns, I.G. (2001). Nitrogen-limited growth of lettuce is associated with lower stomatal conductance. New Phytol. 152, 97–106. [DOI] [PubMed] [Google Scholar]
- Crawford, N.M., and Forde, B.G. (2002). Molecular and developmental biology of inorganic nitrogen nutrition. In The Arabidopsis Book, E. Meyerowitz and C. Somerville, eds (Rockville, MD: American Society of Plant Biologists). [DOI] [PMC free article] [PubMed]
- Crawford, N.M., and Glass, A.D.M. (1998). Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci. 3, 389–395. [Google Scholar]
- Dall'Asta, V., Gatti, R., Orlandini, G., Rossi, P.A., Rotoli, B.M., Sala, R., Bussolati, O., and Gazzola, G.C. (1997). Membrane potential changes visualized in complete growth media through confocal laser scanning microscopy of bis-oxonol-loaded cells. Exp. Cell Res. 231, 260–268. [DOI] [PubMed] [Google Scholar]
- Dietrich, P., and Hedrich, R. (1994). Interconversion of fast and slow gating modes of GCAC1, a guard cell anion channel. Planta 195, 301–304. [Google Scholar]
- Dietrich, P., Sanders, D., and Hedrich, R. (2001). The role of ion channels in light-dependent stomatal opening. J. Exp. Bot. 52, 1959–1967. [DOI] [PubMed] [Google Scholar]
- Forde, B.G. (2000). Nitrate transporters in plants: Structure, function and regulation. Biochim. Biophys. Acta 1465, 219–235. [DOI] [PubMed] [Google Scholar]
- Gaedeke, N., Klein, M., Kolukisaoglu, U., Forestier, C., Muller, A., Ansorge, M., Becker, D., Mamnun, Y., Kuchler, K., Schulz, B., Mueller-Roeber, B., and Martinoia, E. (2001). The Arabidopsis thaliana ABC transporter AtMRP5 controls root development and stomata movement. EMBO J. 20, 1875–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass, A.D.M., Brito, D.T., Kaiser, B.N., Kronzucker, H.J., Kumar, A., Okamoto, M., Rawat, S.R., Siddiqi, M.Y., Silim, S.M., Vidmar, J.J., and Zhuo, D. (2001). Nitrogen transport in plants, with an emphasis on the regulation of fluxes to match plant demand. J. Plant Nutr. Soil Sci. 164, 199–207. [Google Scholar]
- Guo, F.-Q., Wang, R., Chen, M., and Crawford, N.M. (2001). The Arabidopsis dual-affinity nitrate transporter gene AtNRT1.1 (CHL1) is activated and functions in nascent organ development during vegetative and reproductive growth. Plant Cell 13, 1761–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich, R., and Marten, I. (1993). Malate-induced feedback regulation of plasma membrane anion channels could provide a CO2 sensor to guard cells. EMBO J. 12, 897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich, R., Spidola, N., Savchenko, G., Felle, H.H., Kaiser, W.M., and Heber, U. (2001). Changes in apoplastic pH and membrane potential in leaves in relation to stomatal responses to CO2, malate, abscisic acid or interruption of water supply. Planta 213, 594–601. [DOI] [PubMed] [Google Scholar]
- Humble, G.D., and Hsiao, T.C. (1969). Specific requirement of potassium for light-activated opening of stomata in epidermal strips. Plant Physiol. 44, 230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichida, A.M., Pei, Z.M., Baizabal-Aguirre, V.M., Turner, K.J., and Schroeder, J.I. (1997). Expression of a Cs+-resistant guard cell K+ channel confers Cs+-resistant, light-induced stomatal opening in transgenic Arabidopsis. Plant Cell 9, 1843–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ines Minguez, M., and Sau, F. (1989). Response of nitrate-fed and nitrogen-fixing soybeans to progressive water stress. J. Exp. Bot. 213, 497–502. [Google Scholar]
- Kwak, J., Murata, Y., Baizabal-Aguirre, V.M., Merrill, J., Wang, M., Kemper, A., Hawke, S.D., Tallman, G., and Schroeder, J.I. (2001). Dominant negative guard cell K+ channel mutants reduce inward-rectifying K+ currents and light-induced stomatal opening in Arabidopsis. Plant Physiol. 127, 473–485. [PMC free article] [PubMed] [Google Scholar]
- Leonhardt, N., Bazin, I., Richaud, P., Marin, E., Vavasseur, A., and Forestier, C. (2001). Antibodies to the CFTR modulate the turgor pressure of guard cell protoplasts via slow anion channels. FEBS Lett. 494, 15–18. [DOI] [PubMed] [Google Scholar]
- Leonhardt, N., Marin, E., Vavasseur, A., and Forestier, C. (1997). Evidence for the existence of a sulfonylurea-receptor-like protein in plants: Modulation of stomatal movements and guard cell potassium channels by sulfonylureas and potassium channel openers. Proc. Natl. Acad. Sci. USA 94, 14156–14161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt, N., Vavasseur, A., and Forestier, C. (1999). ATP binding cassette modulators control abscisic acid-regulated slow anion channels in guard cells. Plant Cell 11, 1141–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, J., Merlot, S., and Giraudat, J. (1997). The Arabidopsis ABSCISIC ACID-INSENSITIVE2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell 9, 759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, K.-H., Huang, C.-Y., and Tsay, Y.-F. (1999). CHL1 is a dual-affinity nitrate transporter of Arabidopsis involving multiple phases of nitrate uptake. Plant Cell 11, 865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodeiro, A.R., Gonzalez, P., Hernandez, A., Balague, L.J., and Favelukes, G. (2000). Comparison of drought tolerance in nitrogen-fixing and inorganic nitrogen-grown common beans. Plant Sci. 154, 31–41. [DOI] [PubMed] [Google Scholar]
- Miedema, H., and Assmann, S.M. (1996). A membrane-delimited effect of internal pH on the K+ outward rectifier of Vicia faba guard cells. J. Membr. Biol. 154, 227–237. [DOI] [PubMed] [Google Scholar]
- Pei, Z.M., Ghassemian, M., Kwak, C.M., McCourt, P., and Schroeder, J.I. (1998). Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science 282, 287–290. [DOI] [PubMed] [Google Scholar]
- Pei, Z.M., Kuchitsu, K., Ward, J.M., Schwarz, M., and Schroeder, J.I. (1997). Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild type and abi1 and abi2 mutants. Plant Cell 9, 409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilot, G., Lacombe, B., Gaymard, F., Cherel, I., Boucherez, J., Thibaud, J.-B., and Sentenac, H. (2001). Guard cell inward K+ channel activity in Arabidopsis involves expression of the twin channel subunits KAT1 and KAT2. J. Biol. Chem. 276, 3215–3221. [DOI] [PubMed] [Google Scholar]
- Radin, J.W., and Ackerson, R.C. (1981). Water relations of cotton plants under nitrogen deficiency. III. Stomatal conductance, photosynthesis, and abscisic acid accumulation during drought. Plant Physiol. 67, 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschke, K., and Fellows, M.P. (1971). Stomatal movement in Zea mays: Shuttle of potassium and chloride between guard cells and subsidiary cells. Planta 101, 296–316. [DOI] [PubMed] [Google Scholar]
- Raschke, K., and Schnabl, H. (1978). Availability of chloride affects the balance between potassium chloride and potassium malate in guard cells of Vicia faba L. Plant Physiol. 62, 84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema, M.R.G., and Hedrich, R. (2002). Studying guard cells in the intact plant: Modulation of stomatal movement by apoplastic factors. New Phytol. 153, 425–431. [DOI] [PubMed] [Google Scholar]
- Schmidt, C., and Schroeder, J.I. (1994). Anion selectivity of slow anion channels in the plasma membrane of guard cells: Large nitrate permeability. Plant Physiol. 106, 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, J.I., Allen, G.J., Hugouvieux, V., Kwak, J.M., and Waner, D. (2001). Guard cell signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 627–658. [DOI] [PubMed] [Google Scholar]
- Schroeder, J.I., and Keller, B.U. (1992). Two types of anion channel currents in guard cells with distinct voltage regulation. Proc. Natl. Acad. Sci. USA 89, 5025–5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyroki, A., Ivashikina, N., Dietrich, P., Roelfsema, M.R., Ache, P., Reintanz, B., Deeken, R., Godde, M., Felle, H., Steinmeyer, R., Palme, K., and Hedrich, R. (2001). KAT1 is not essential for stomatal opening. Proc. Natl. Acad. Sci. USA 98, 2917–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott, L.-D., and Zeiger, E. (1998). The role of sucrose in guard cell osmoregulation. J. Exp. Bot. 49, 329–337. [Google Scholar]
- Thayer, J.R., and Huffaker, R.C. (1980). Determination of nitrate and nitrite by HPLC: Comparison with other methods for nitrate determination. Anal. Biochem. 102, 110–119. [DOI] [PubMed] [Google Scholar]
- Touraine, B., and Glass, A.D.M. (1997). NO3− and ClO3− fluxes in the chl1-5 mutant of Arabidopsis thaliana: Does the CHL1-5 gene encode a low-affinity NO3− transporter? Plant Physiol. 114, 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay, Y.-F., Schroeder, J.I., Feldmann, K.A., and Crawford, N.M. (1993). A herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell 72, 705–713. [DOI] [PubMed] [Google Scholar]
- Vartanian, N., Marcotte, L., and Giraudat, J. (1994). Drought rhizogenesis in Arabidopsis thaliana: Differential responses of hormonal mutants. Plant Physiol. 104, 761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R., Liu, D., and Crawford, N.M. (1998). The Arabidopsis CHL1 protein plays a major role in high affinity nitrate uptake. Proc. Natl. Acad. Sci. USA 95, 15134–15139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X.Q., Hemayet, U., Jones, A.M., and Assmann, S.M. (2001). G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science 292, 2070–2072. [DOI] [PubMed] [Google Scholar]