Abstract
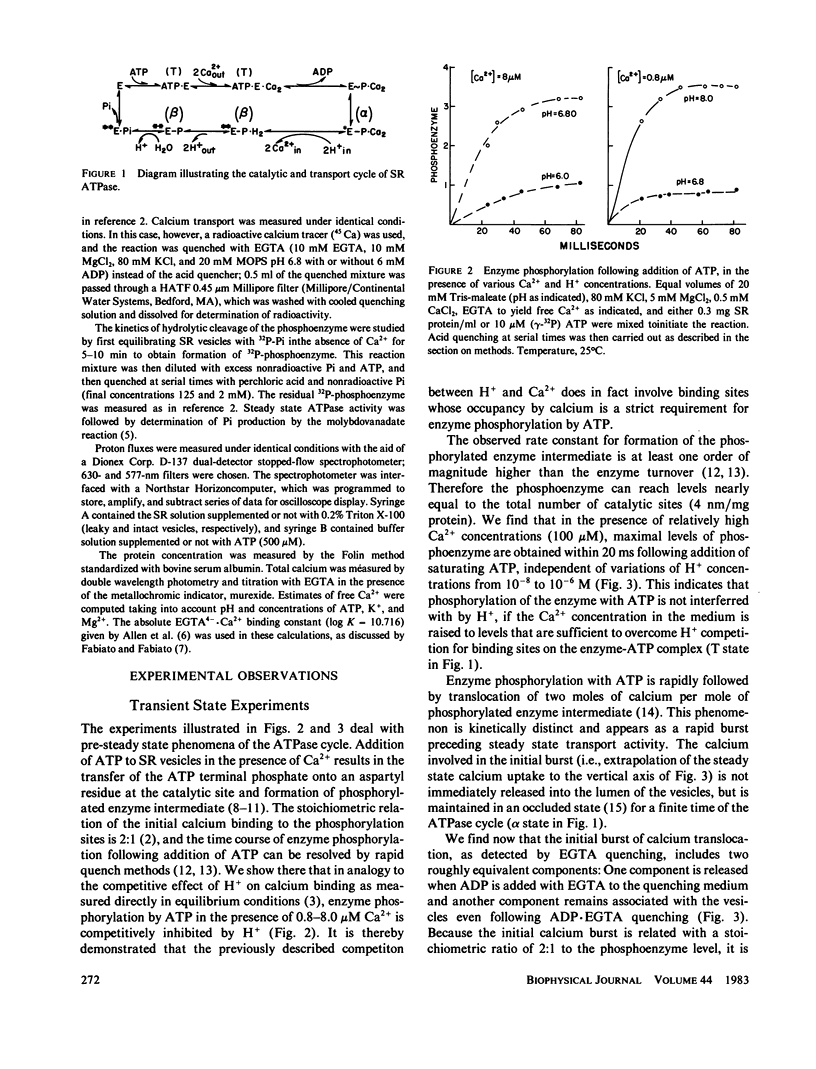
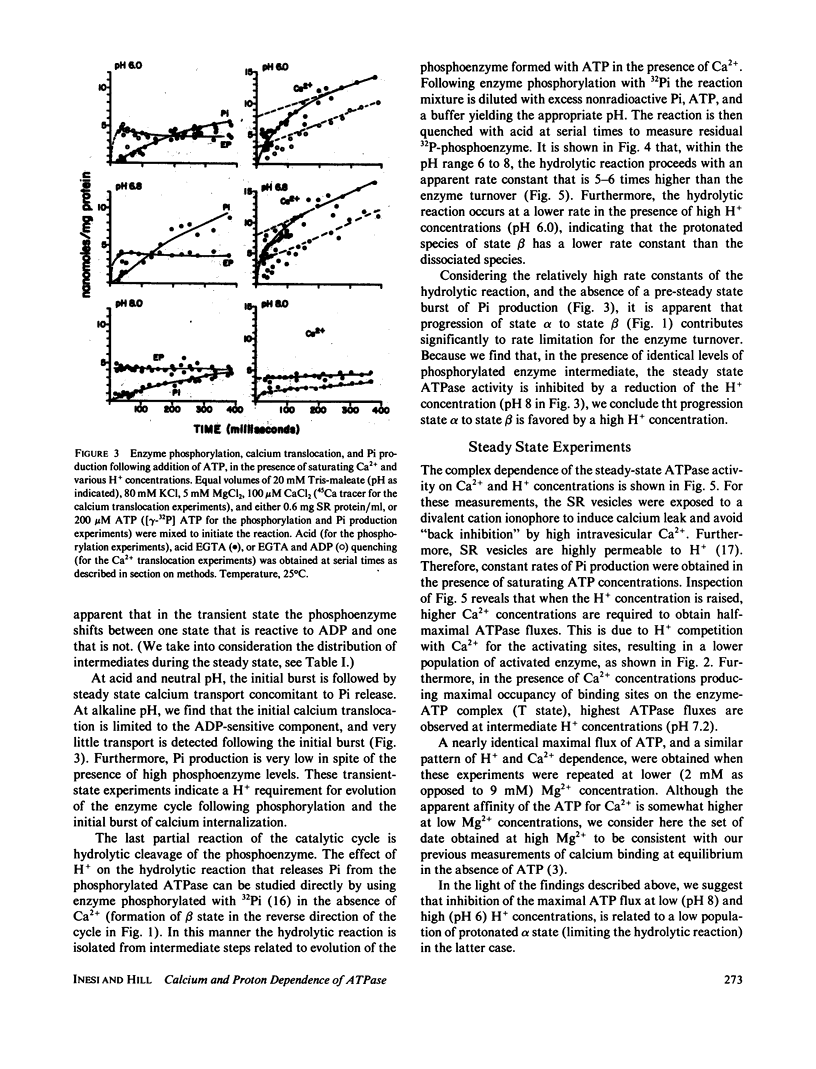
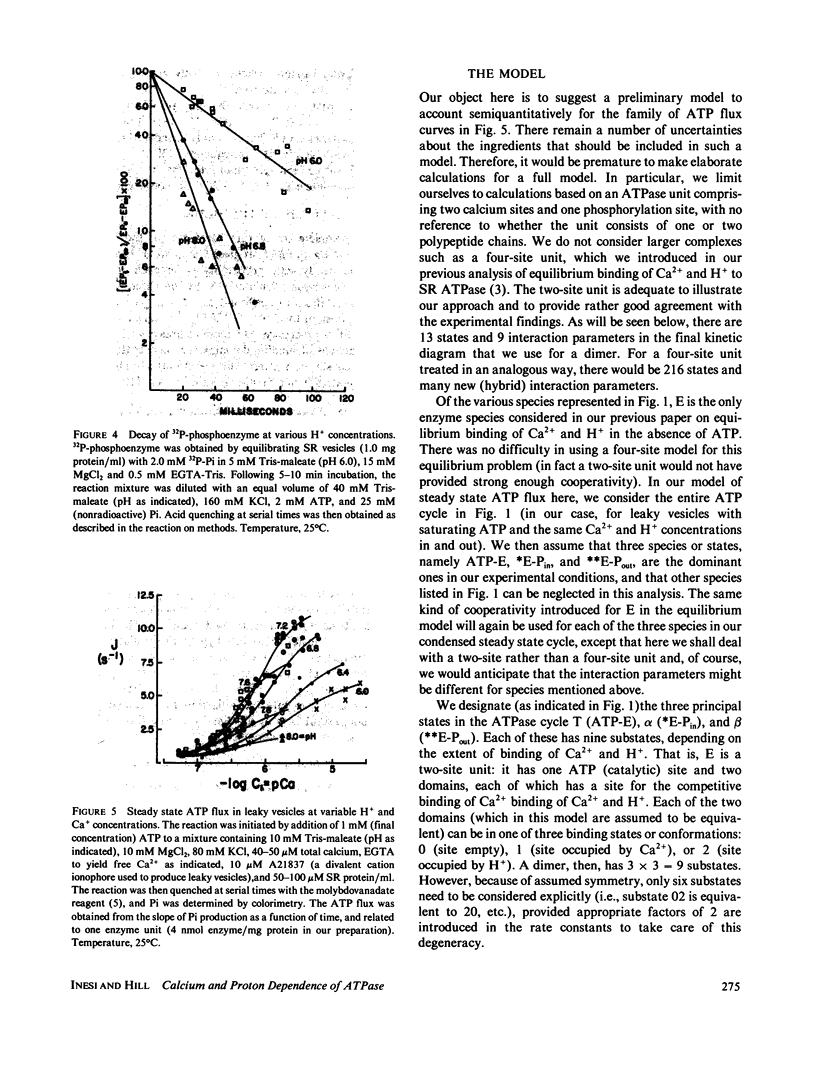
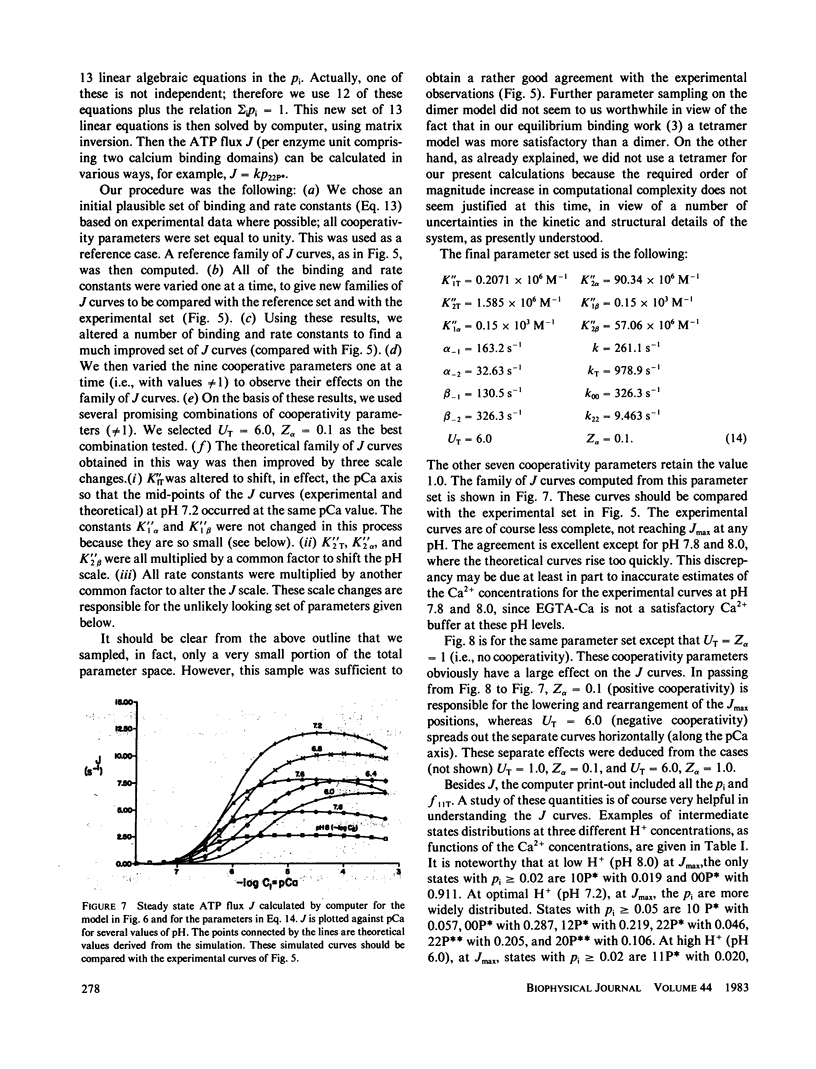
The influence of Ca2+ and H+ concentrations on the sequential reactions of the ATPase cycle was studied by a series of pre-steady state and steady state experiments with sarcoplasmic reticulum vesicles. It is shown that H+ competition with calcium binding results in a reduced population of activated enzyme, which is manifested by a lower level of phosphorylated enzyme intermediate following addition of ATP. Further effects of Ca2+ and H+ are demonstrated on the progression of the phosphoenzyme through the reaction cycle and on the final hydrolytic cleavage of Pi. The overall dependence of steady state ATP flux on Ca2+ and H+ concentrations in leaky vesicles is expressed by a series of curves showing that as the H+ concentration is raised higher Ca2+ concentrations are required to obtain half-maximal ATP fluxes. At saturating Ca2+, maximal ATP fluxes are observed at an intermediate H+ concentration (pH 7.2), while lower levels are obtained as the H+ concentration is reduced (to pH 8) or increased (to pH 6). A preliminary model is then proposed based on the presence of two interacting domains permitting competitive binding of Ca2+ or H+, per each catalytic site undergoing phosphorylation by ATP. The model considers three main states and thirteen substates (depending on the occupancy of the binding sites in each state by Ca2+, H+, or neither) in the progression of the ATP cycle, coupled to transport of Ca2+ and counter transport of H+ in leaky vesicles. Considering the preliminary nature of the model and the experimental scatter, a rather satisfactory agreement is noted between a family of curves generated by theoretical analysis and the ATP flux curves obtained experimentally.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Blinks J. R., Prendergast F. G. Aequorin luminescence: relation of light emission to calcium concentration--a calcium-independent component. Science. 1977 Mar 11;195(4282):996–998. doi: 10.1126/science.841325. [DOI] [PubMed] [Google Scholar]
- Bastide F., Meissner G., Fleischer S., Post R. L. Similarity of the active site of phosphorylation of the adenosine triphosphatase from transport of sodium and potassium ions in kidney to that for transport of calcium ions in the sarcoplasmic reticulum of muscle. J Biol Chem. 1973 Dec 25;248(24):8385–8391. [PubMed] [Google Scholar]
- Carvalho A. P. Binding and release of cations by sarcoplasmic reticulum before and after removal of lipid. Eur J Biochem. 1972 Jun 9;27(3):491–502. doi: 10.1111/j.1432-1033.1972.tb01865.x. [DOI] [PubMed] [Google Scholar]
- Chiesi M., Inesi G. Adenosine 5'-triphosphate dependent fluxes of manganese and and hydrogen ions in sarcoplasmic reticulum vesicles. Biochemistry. 1980 Jun 24;19(13):2912–2918. doi: 10.1021/bi00554a015. [DOI] [PubMed] [Google Scholar]
- Degani C., Boyer P. D. A borohydride reduction method for characterization of the acyl phosphate linkage in proteins and its application to sarcoplasmic reticulum adenosine triphosphatase. J Biol Chem. 1973 Dec 10;248(23):8222–8226. [PubMed] [Google Scholar]
- Dupont Y. Occlusion of divalent cations in the phosphorylated calcium pump of sarcoplasmic reticulum. Eur J Biochem. 1980 Aug;109(1):231–238. doi: 10.1111/j.1432-1033.1980.tb04788.x. [DOI] [PubMed] [Google Scholar]
- Eletr S., Inesi G. Phospholipid orientation in sarcoplasmic membranes: spin-label ESR and proton MNR studies. Biochim Biophys Acta. 1972 Sep 1;282(1):174–179. doi: 10.1016/0005-2736(72)90321-5. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Froehlich J. P., Taylor E. W. Transient state kinetic studies of sarcoplasmic reticulum adenosine triphosphatase. J Biol Chem. 1975 Mar 25;250(6):2013–2021. [PubMed] [Google Scholar]
- Hill T. L. Further study of the effect of enzyme-enzyme interactions on steady-state enzyme kinetics. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4111–4115. doi: 10.1073/pnas.74.10.4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L., Inesi G. Equilibrium cooperative binding of calcium and protons by sarcoplasmic reticulum ATPase. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3978–3982. doi: 10.1073/pnas.79.13.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L., Levitzki A. Subunit neighbor interactions in enzyme kinetics: half-of-the-sites reactivity in a dimer. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5741–5745. doi: 10.1073/pnas.77.10.5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L. Theoretical study of the effect of enzyme-enzyme interactions on steady-state enzyme kinetics. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3632–3636. doi: 10.1073/pnas.74.9.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inesi G., Kurzmack M., Coan C., Lewis D. E. Cooperative calcium binding and ATPase activation in sarcoplasmic reticulum vesicles. J Biol Chem. 1980 Apr 10;255(7):3025–3031. [PubMed] [Google Scholar]
- Kurzmack M., Verjovski-Almeida S., Inesi G. Detection of an initial burst of Ca2+ translocation in sarcoplasmic reticulum. Biochem Biophys Res Commun. 1977 Sep 23;78(2):772–776. doi: 10.1016/0006-291x(77)90246-7. [DOI] [PubMed] [Google Scholar]
- Lin T. I., Morales M. F. Application of a one-step procedure for measuring inorganic phosphate in the presence of proteins: the actomyosin ATPase system. Anal Biochem. 1977 Jan;77(1):10–17. doi: 10.1016/0003-2697(77)90284-6. [DOI] [PubMed] [Google Scholar]
- Madeira V. M. Proton gradient formation during transport of Ca2+ by sarcoplasmic reticulum. Arch Biochem Biophys. 1978 Jan 30;185(2):316–325. doi: 10.1016/0003-9861(78)90173-x. [DOI] [PubMed] [Google Scholar]
- Makinose M. The phosphorylation of the membranal protein of the sarcoplasmic vesicles during active calcium transport. Eur J Biochem. 1969 Aug;10(1):74–82. [PubMed] [Google Scholar]
- Masuda H., de Meis L. Phosphorylation of the sarcoplasmic reticulum membrane by orthophosphate. Inhibition by calcium ions. Biochemistry. 1973 Nov 6;12(23):4581–4585. doi: 10.1021/bi00747a006. [DOI] [PubMed] [Google Scholar]
- McKinley D., Meissner G. Evidence for a K+, Na+ permeable channel in sarcoplasmic reticulum. J Membr Biol. 1978 Dec 15;44(2):159–186. doi: 10.1007/BF01976037. [DOI] [PubMed] [Google Scholar]
- Ueno T., Sekine T. A role of H+ flux in active Ca2+ transport into sarcoplasmic reticulum vesicles. I. Effect of an artificially imposed H+ gradient on Ca2+ uptake. J Biochem. 1981 Apr;89(4):1239–1246. [PubMed] [Google Scholar]
- Verjovski-Almeida S., Kurzmack M., Inesi G. Partial reactions in the catalytic and transport cycle of sarcoplasmic reticulum ATPase. Biochemistry. 1978 Nov 14;17(23):5006–5013. doi: 10.1021/bi00616a023. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Tonomura Y. Reaction mechanism of the Ca++ -dependent ATPase of sarcoplasmic reticulum from skeletal muscle. I. Kinetic studies. J Biochem. 1967 Nov;62(5):558–575. doi: 10.1093/oxfordjournals.jbchem.a128706. [DOI] [PubMed] [Google Scholar]
- de Meis L., Vianna A. L. Energy interconversion by the Ca2+-dependent ATPase of the sarcoplasmic reticulum. Annu Rev Biochem. 1979;48:275–292. doi: 10.1146/annurev.bi.48.070179.001423. [DOI] [PubMed] [Google Scholar]


