Abstract
It has been suggested that potentials measured with conventional microelectrodes in chemically or mechanically skinned muscle fibers arise from a Donnan equilibrium due to myofilament fixed charges. This hypothesis was tested in mechanically skinned frog (Rana pipiens) semitendinosus fibers by measuring the distribution potential (Ed) between fiber and bath with 3 M KCl-filled microelectrodes and the K+ activity gradient (aik/aok) with K+ ion-selective microelectrodes (KISE). If skinned fibers are a Donnan system, Ed should become more positive as pH is decreased, altering the fixed charge on the myofilaments. Consistent with this expectation, Ed was -4.4, -0.6, and +4.8 mV in ATP-containing solutions and -6.5, -2.2, and +8.4 mV in ATP-free solutions at pH 7, 6, and 5, respectively. Donnan equilibrium also requires that all mobile ionic species be in electrochemical equilibrium. In ATP-containing solutions, this was true for K+ at pH 7. At pH 5, however, KISE indicated that K+ was not in equilibrium; average Ed was 5.9 mV positive to the K+ equilibrium potential, and aik/aok was 1.04, while the Donnan prediction was 0.83. In contrast, KISE measurements in ATP-free solutions indicated that K+ was in equilibrium at all pH studied. Skinned fibers in ATP-containing media are not equilibrium systems because ATPase reactions occur. Under our conditions, frog myofibrils hydrolyze 0.4 and 0.08 mumol ATP/min X mg myofibrillar protein at pH 7 and 5, respectively. It is suggested that in the presence of ATP, Ed is a superposition of Donnan and diffusion potentials, the latter arising from differences in the mobilities of anionic substrate and products that diffuse through the charged myofilament lattice. A coupling to diffusion of K+, the predominant counter ion, is required for macroscopic electroneutrality. This coupling may be the origin of the nonequilibrium K+ distribution.
Full text
PDF
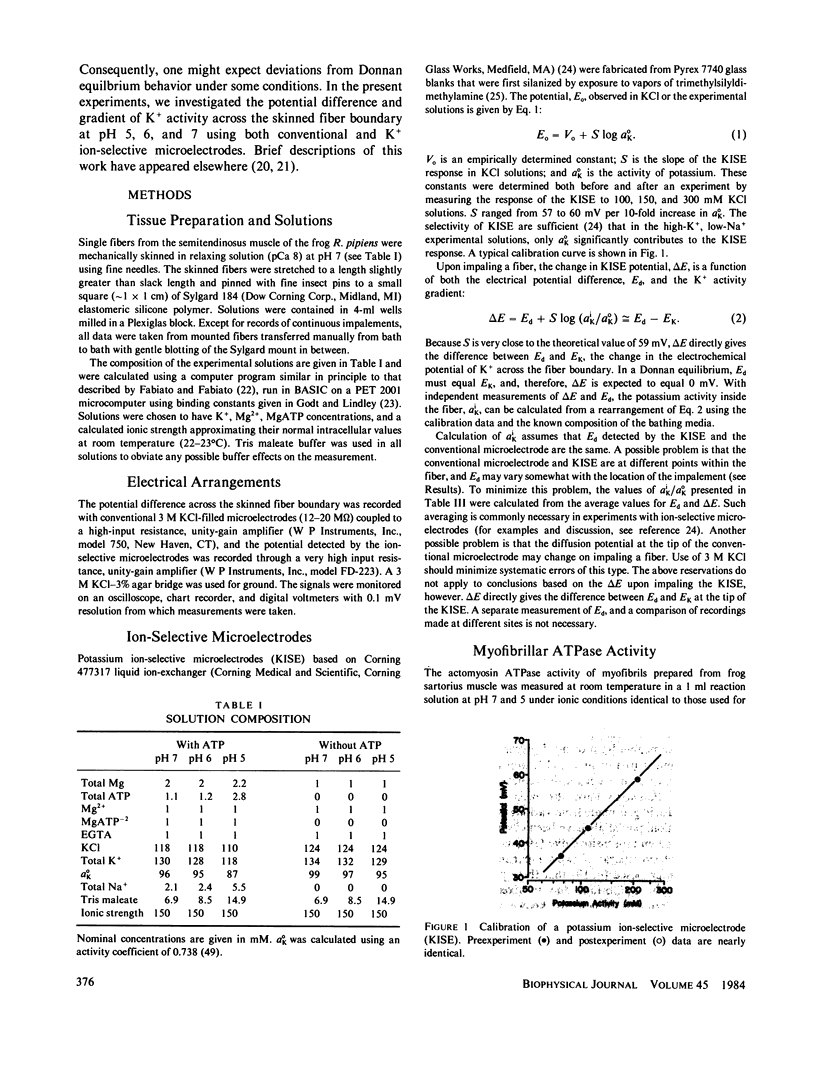

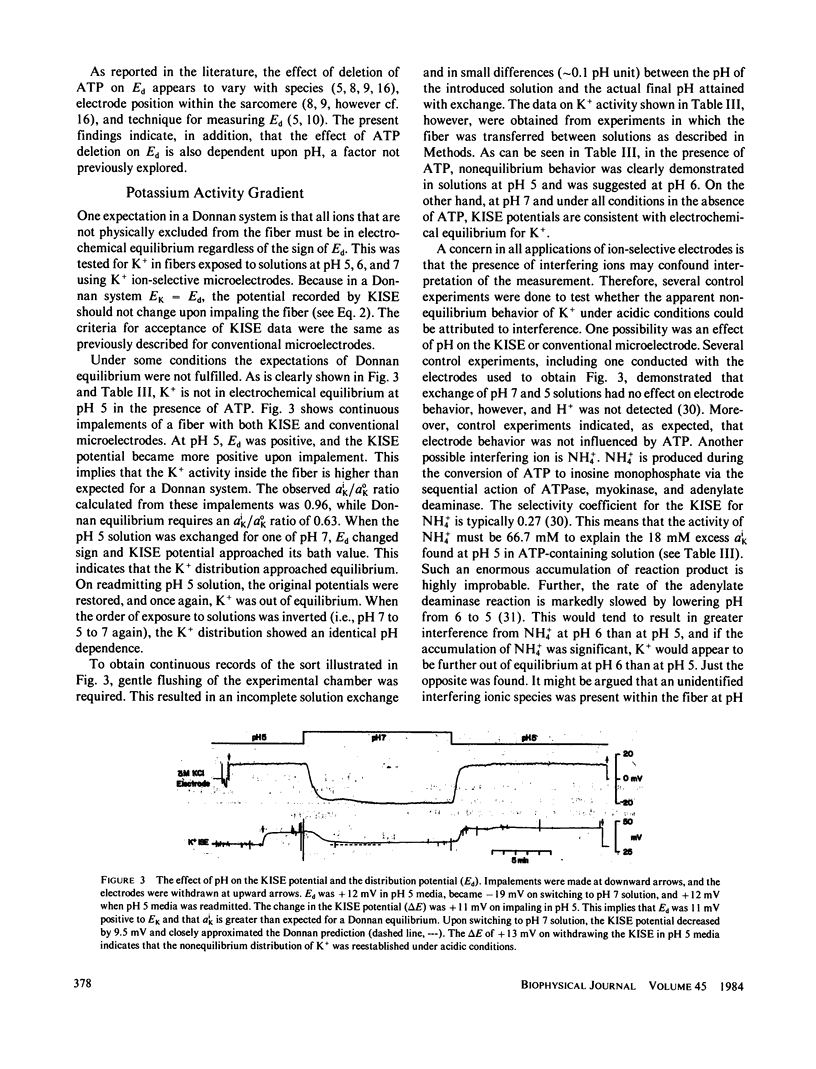




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgarten C. M. A program for calculation of activity coefficients at selected concentrations and temperatures. Comput Biol Med. 1981;11(4):189–196. doi: 10.1016/s0010-4825(81)80021-2. [DOI] [PubMed] [Google Scholar]
- Baumgarten C. M. An improved liquid ion exchanger for chloride ion-selective microelectrodes. Am J Physiol. 1981 Nov;241(5):C258–C263. doi: 10.1152/ajpcell.1981.241.5.C258. [DOI] [PubMed] [Google Scholar]
- Bunow B. Chemical reactions and membranes: a macroscopic basis for facilitated transport, chemiosmosis and active transport. Part I: Linear analysis. J Theor Biol. 1978 Nov 7;75(1):51–78. doi: 10.1016/0022-5193(78)90202-3. [DOI] [PubMed] [Google Scholar]
- Bunow B. Chemical reactions and membranes; a macroscopic basis for active transport. II. Non-linear aspects. J Theor Biol. 1978 Nov 7;75(1):79–96. doi: 10.1016/0022-5193(78)90203-5. [DOI] [PubMed] [Google Scholar]
- CHICHIBU S. Electrical properties of glycerinated crayfish muscle fiber. Tohoku J Exp Med. 1961 Jan 25;73:170–179. doi: 10.1620/tjem.73.170. [DOI] [PubMed] [Google Scholar]
- Caillé J. P. Charges fixés du protoplasme des fibres musculaires de balane. Biochim Biophys Acta. 1979 Jun 12;585(2):300–313. [PubMed] [Google Scholar]
- Collins E. W., Jr, Edwards C. Role of Donnan equilibrium in the resting potentials in glycerol-extracted muscle. Am J Physiol. 1971 Oct;221(4):1130–1133. doi: 10.1152/ajplegacy.1971.221.4.1130. [DOI] [PubMed] [Google Scholar]
- Davis T. L., Jackson J. W., Day B. E., Shoemaker R. L., Rehm W. S. Potentials in frog cornea and microelectrode artifact. Am J Physiol. 1970 Jul;219(1):178–183. doi: 10.1152/ajplegacy.1970.219.1.178. [DOI] [PubMed] [Google Scholar]
- DeSimone J. A., Caplan S. R. The determination of local reaction and diffusion parameters of enzyme membranes from global measurements. Biochemistry. 1973 Jul 31;12(16):3032–3039. doi: 10.1021/bi00740a014. [DOI] [PubMed] [Google Scholar]
- DeSimone J. A. Perturbations in the structure of the double layer at an enzymic surface. J Theor Biol. 1977 Sep 21;68(2):225–240. doi: 10.1016/0022-5193(77)90161-8. [DOI] [PubMed] [Google Scholar]
- Elliott G. F., Bartels E. M. Donnan potential measurements in extended hexagonal polyelectrolyte gels such as muscle. Biophys J. 1982 May;38(2):195–199. doi: 10.1016/S0006-3495(82)84546-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott G. F. Donnan and osmotic effects in muscle fibres without membranes. J Mechanochem Cell Motil. 1973 May;2(1):83–89. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Excitation-contraction coupling of isolated cardiac fibers with disrupted or closed sarcolemmas. Calcium-dependent cyclic and tonic contractions. Circ Res. 1972 Sep;31(3):293–307. doi: 10.1161/01.res.31.3.293. [DOI] [PubMed] [Google Scholar]
- Godt R. E., Lindley B. D. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J Gen Physiol. 1982 Aug;80(2):279–297. doi: 10.1085/jgp.80.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Godt R. E., Donaldson S. K., Harris C. E. Tension in skinned frog muscle fibers in solutions of varying ionic strength and neutral salt composition. J Gen Physiol. 1973 Nov;62(5):550–574. doi: 10.1085/jgp.62.5.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinke J. A. Water and electrolyte content of the myofilament phase in the chemically skinned barnacle fiber. J Gen Physiol. 1980 May;75(5):531–551. doi: 10.1085/jgp.75.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King E. J. The colorimetric determination of phosphorus. Biochem J. 1932;26(2):292–297. doi: 10.1042/bj0260292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- NAYLER W. G., MERRILLEES N. C. SOME OBSERVATIONS ON THE FINE STRUCTURE AND METABOLIC ACTIVITY OF NORMAL AND GLYCERINATED VENTRICULAR MUSCLE OF TOAD. J Cell Biol. 1964 Sep;22:533–550. doi: 10.1083/jcb.22.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor G. R. Average electrostatic potential between the filaments in striated muscle and its relation to a simple Donnan potential. Biophys J. 1982 May;38(2):201–204. doi: 10.1016/S0006-3495(82)84547-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. J., Ehrenfeld J., Lindemann B. Volume changes and potential artifacts of epithelial cells of frog skin following impalement with microelectrodes filled with 3 m KCl. J Membr Biol. 1978;40(Spec No):91–119. doi: 10.1007/BF02026000. [DOI] [PubMed] [Google Scholar]
- OVERBEEK J. T. The Donnan equilibrium. Prog Biophys Biophys Chem. 1956;6:57–84. [PubMed] [Google Scholar]
- Pemrick S. M., Edwards C. Differences in the charge distribution of glycerol-extracted muscle fibers in rigor, relaxation, and contraction. J Gen Physiol. 1974 Nov;64(5):551–567. doi: 10.1085/jgp.64.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scordilis S. P., Tedeschi H., Edwards C. Donnan potential of rabbit skeletal muscle myofibrils I: electrofluorochromometric detection of potential. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1325–1329. doi: 10.1073/pnas.72.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Lowenstein J. M. Adenylate deaminase. II. Purification and some regulatory properties of the enzyme from calf brain. J Biol Chem. 1967 Feb 25;242(4):607–615. [PubMed] [Google Scholar]
- Solaro R. J., Shiner J. S. Modulation of Ca2+ control of dog and rabbit cardiac myofibrils by Mg2+. Comparison with rabbit skeletal myofibrils. Circ Res. 1976 Jul;39(1):8–14. doi: 10.1161/01.res.39.1.8. [DOI] [PubMed] [Google Scholar]
- Stephenson D. G., Wendt I. R., Forrest Q. G. Non-uniform ion distributions and electrical potentials in sarcoplasmic regions of skeletal muscle fibres. Nature. 1981 Feb 19;289(5799):690–692. doi: 10.1038/289690a0. [DOI] [PubMed] [Google Scholar]
- Weiss R. M., Lazzara R., Hoffman B. F. Potentials measured from glycerinated cardiac muscle. Nature. 1967 Sep 16;215(5107):1305–1307. doi: 10.1038/2151305a0. [DOI] [PubMed] [Google Scholar]


