Abstract
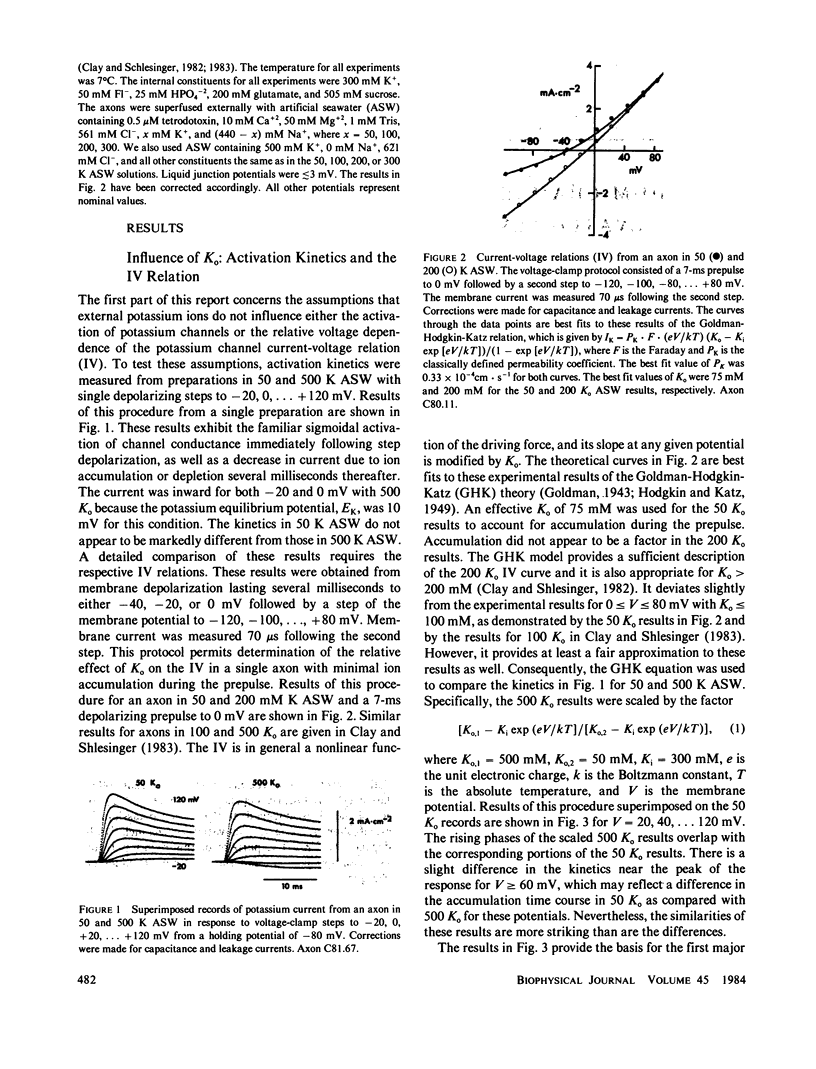
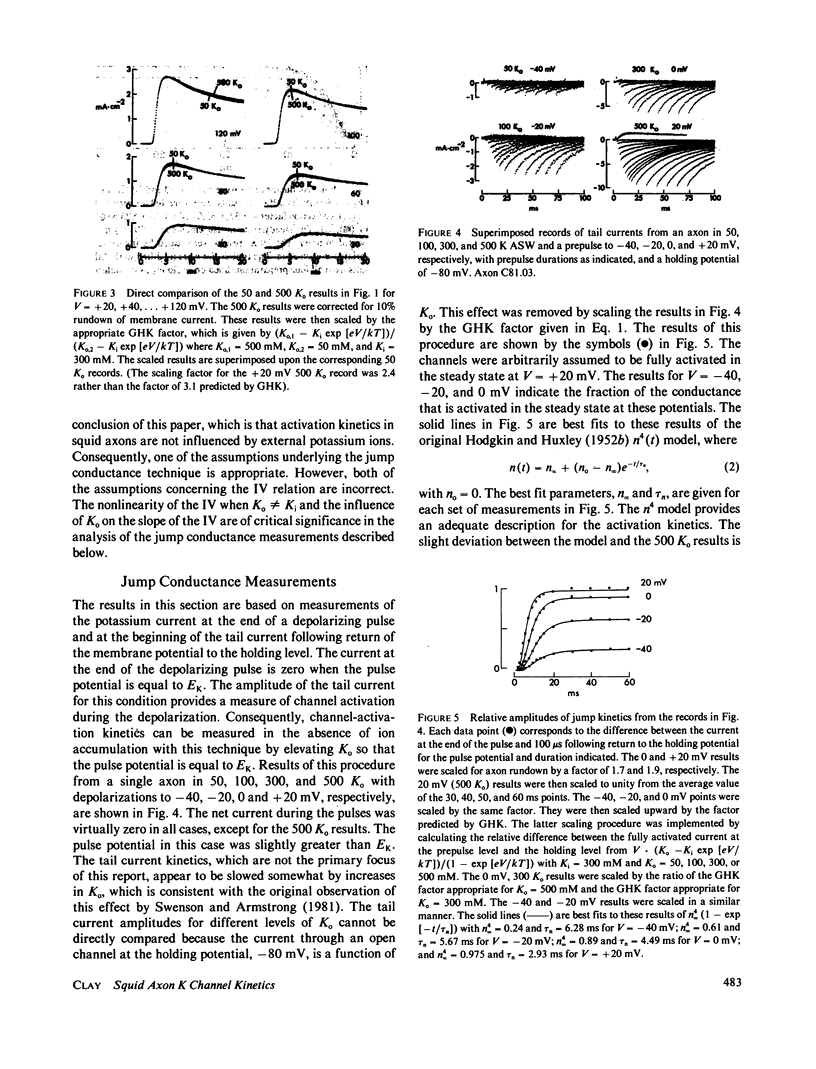
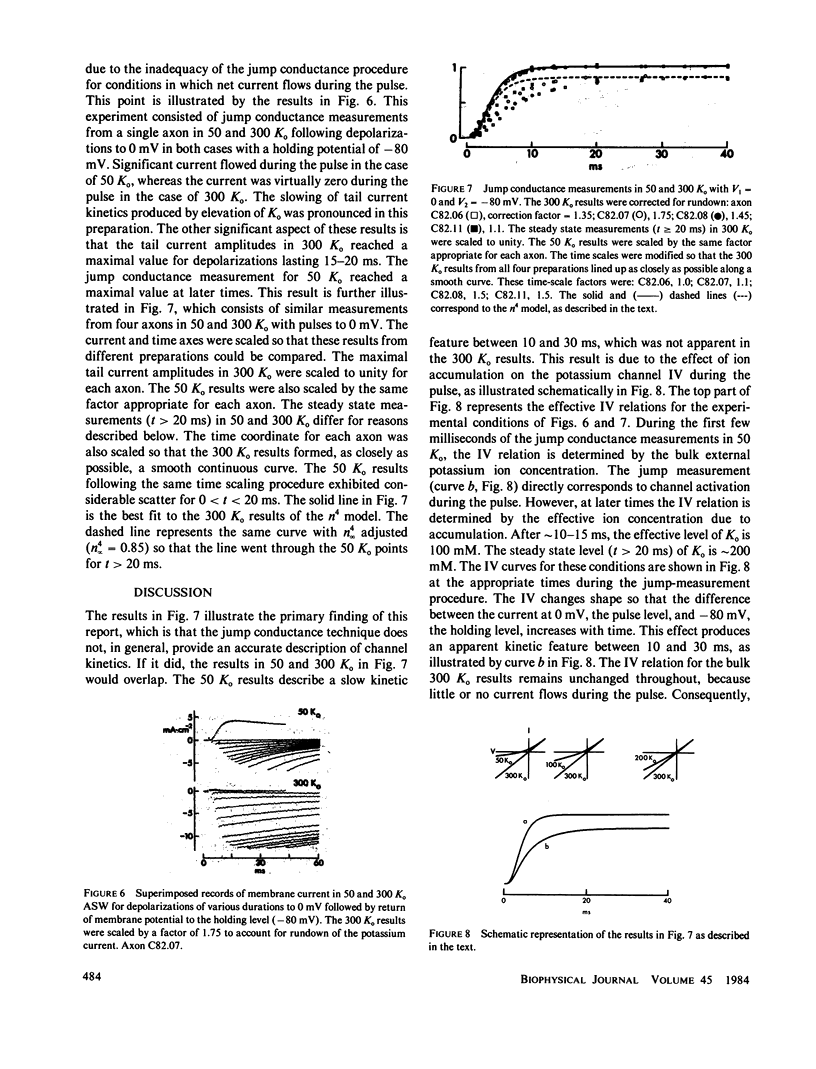
Potassium ion current in squid axons is usually modified by the effects of ion accumulation in the periaxonal space during voltage-clamp depolarization. The time course of potassium channel activation and ion accumulation usually overlap. A widely accepted procedure for circumventing the effects of accumulation in measurements of activation kinetics consists of measuring the difference in the current at the end of a depolarizing pulse and immediately following return of the membrane potential to the holding level. This instantaneous jump procedure is based upon the assumptions that the potassium channel current-voltage relation (IV) is a linear function of the driving force, and that the IV and the potassium channel-gating kinetics are both independent of ion accumulation. The latter assumption appears to be appropriate for activation kinetics. However, both assumptions concerning the IV are incorrect, in general. Consequently, the jump procedure provides a misleading view of gating kinetics for membrane depolarizations that produce net current flow. Jump conductance measurements for depolarizations that produce little or no net current indicate that the Hodgkin-Huxley n4 model of potassium channel kinetics is appropriate for the physiological range of membrane potentials.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clay J. R., Shlesinger M. F. Delayed kinetics of squid axon potassium channels do not always superpose after time translation. Biophys J. 1982 Mar;37(3):677–680. [PMC free article] [PubMed] [Google Scholar]
- Clay J. R., Shlesinger M. F. Effects of external cesium and rubidium on outward potassium currents in squid axons. Biophys J. 1983 Apr;42(1):43–53. doi: 10.1016/S0006-3495(83)84367-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., Wanke E. Channel noise in nerve membranes and lipid bilayers. Q Rev Biophys. 1975 Nov;8(4):451–506. doi: 10.1017/s0033583500001967. [DOI] [PubMed] [Google Scholar]
- Fohlmeister J. F., Adelman W. J., Jr Anomalous potassium channel-gating rates as functions of calcium and potassium ion concentrations. Biophys J. 1982 Feb;37(2):427–431. doi: 10.1016/S0006-3495(82)84688-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilly W. F., Armstrong C. M. Divalent cations and the activation kinetics of potassium channels in squid giant axons. J Gen Physiol. 1982 Jun;79(6):965–996. doi: 10.1085/jgp.79.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson R. P., Jr, Armstrong C. M. K+ channels close more slowly in the presence of external K+ and Rb+. Nature. 1981 Jun 4;291(5814):427–429. doi: 10.1038/291427a0. [DOI] [PubMed] [Google Scholar]


