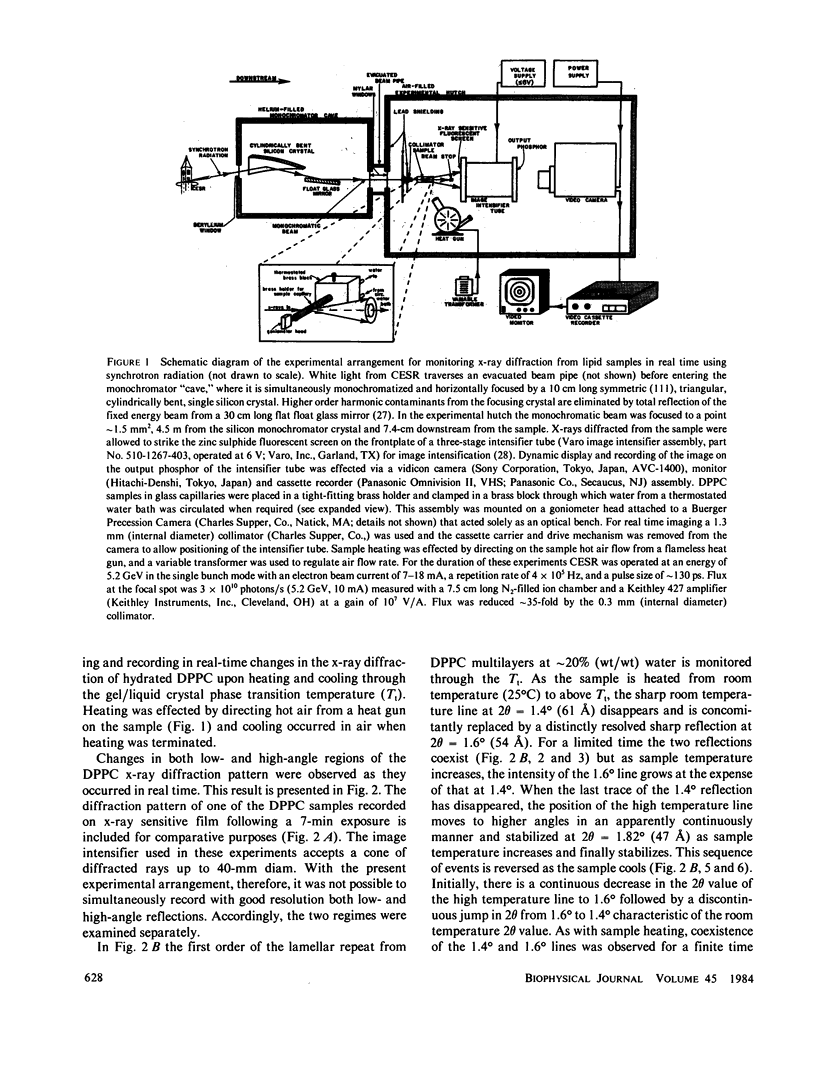
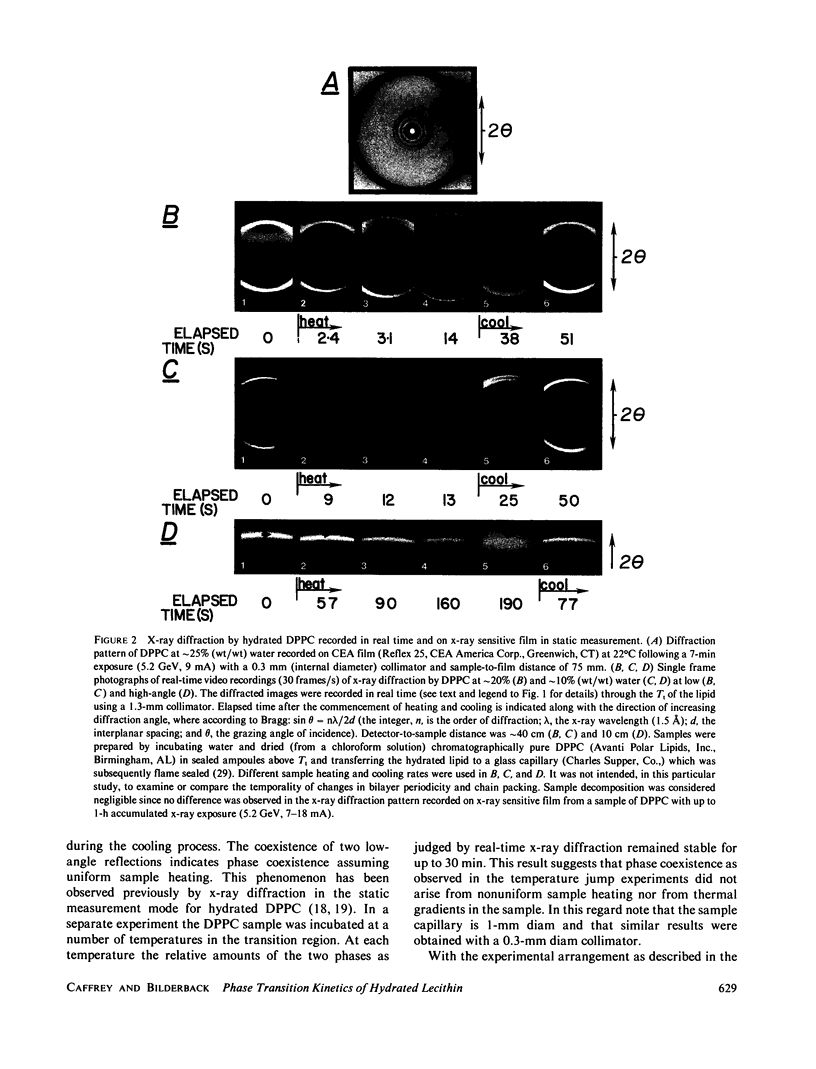
Abstract
A method is described for observing and recording in real-time x-ray diffraction from an unoriented hydrated membrane lipid, dipalmitoylphosphatidylcholine (DPPC), through its thermotropic gel/liquid crystal phase transition. Synchrotron radiation from the Cornell High Energy Synchrotron Source (Ithaca, New York) was used as an x-ray source of extremely high brilliance and the dynamic display of the diffraction image was effected using a three-stage image intensifier tube coupled to an external fluorescent screen. The image on the output phosphor was sufficiently intense to be recorded cinematographically and to be displayed on a television monitor using a vidicon camera at 30 frames X s1. These measurements set an upper limit of 2 s on the DPPC gel----liquid crystal phase transition and indicate that the transition is a two-state process. The real-time method couples the power of x-ray diffraction as a structural probe with the ability to follow kinetics of structural changes. The method does not require an exogenous probe, is relatively nonperturbing, and can be used with membranes in a variety of physical states and with unstable samples. The method has the additional advantage over its static measurement counterpart in that it is more likely to detect transiently stable intermediates if present.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batterman B. W., Ashcroft N. W. CHESS: The New Synchrotron Radiation Facility at Cornell. Science. 1979 Oct 12;206(4415):157–161. doi: 10.1126/science.206.4415.157. [DOI] [PubMed] [Google Scholar]
- Brady G. W., Fein D. B. An analysis of the X-ray interchain peak profile in dipalmitoylglycerophosphocholine. Biochim Biophys Acta. 1977 Jan 21;464(2):249–259. doi: 10.1016/0005-2736(77)90001-3. [DOI] [PubMed] [Google Scholar]
- Caffrey M., Feigenson G. W. Fluorescence quenching in model membranes. 3. Relationship between calcium adenosinetriphosphatase enzyme activity and the affinity of the protein for phosphatidylcholines with different acyl chain characteristics. Biochemistry. 1981 Mar 31;20(7):1949–1961. doi: 10.1021/bi00510a034. [DOI] [PubMed] [Google Scholar]
- Dupont Y., Gabriel A., Chabre M., Gulik-Krzywicki T., Schechter E. Use of a new detector for x-ray diffraction and kinetics of the ordering of the lipids in E. coli membranes and model systems. Nature. 1972 Aug 11;238(5363):331–333. doi: 10.1038/238331a0. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Levine Y. K. Low-angle x-ray diffraction. Mol Biol Biochem Biophys. 1981;31:437–487. doi: 10.1007/978-3-642-81537-9_9. [DOI] [PubMed] [Google Scholar]
- Gottlieb M. H., Eanes E. D. Coexistence of rigid crystalline and liquid crystalline phases in lecithin-water mixtures. Biophys J. 1974 May;14(5):335–342. doi: 10.1016/S0006-3495(74)85920-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiak M. J., Small D. M., Shipley G. G. Nature of the Thermal pretransition of synthetic phospholipids: dimyristolyl- and dipalmitoyllecithin. Biochemistry. 1976 Oct 19;15(21):4575–4580. doi: 10.1021/bi00666a005. [DOI] [PubMed] [Google Scholar]
- Johnson M. L., Winter T. C., Biltonen R. L. The measurement of the kinetics of lipid phase transitions: a volume-perturbation kinetic calorimeter. Anal Biochem. 1983 Jan;128(1):1–6. doi: 10.1016/0003-2697(83)90335-4. [DOI] [PubMed] [Google Scholar]
- Leigh J. B. Synchrotron x-ray sources: a new tool in biological structural and kinetic analysis. Annu Rev Biophys Bioeng. 1976;5:239–270. doi: 10.1146/annurev.bb.05.060176.001323. [DOI] [PubMed] [Google Scholar]
- Ranck J. L., Mateu L., Sadler D. M., Tardieu A., Gulik-Krzywicki T., Luzzati V. Order-disorder conformational transitions of the hydrocarbon chains of lipids. J Mol Biol. 1974 May 15;85(2):249–277. doi: 10.1016/0022-2836(74)90363-5. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Chapman D., Larsson K. Tilted hydrocarbon chains of dipalmitoyl lecithin become perpendicular to the bilayer before melting. Biophys J. 1975 Nov;15(11):1117–1124. doi: 10.1016/S0006-3495(75)85888-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D. M. Phase equilibria and structure of dry and hydrated egg lecithin. J Lipid Res. 1967 Nov;8(6):551–557. [PubMed] [Google Scholar]
- Tardieu A., Luzzati V., Reman F. C. Structure and polymorphism of the hydrocarbon chains of lipids: a study of lecithin-water phases. J Mol Biol. 1973 Apr 25;75(4):711–733. doi: 10.1016/0022-2836(73)90303-3. [DOI] [PubMed] [Google Scholar]
- Wilkins M. H., Blaurock A. E., Engelman D. M. Bilayer structure in membranes. Nat New Biol. 1971 Mar 17;230(11):72–76. doi: 10.1038/newbio230072a0. [DOI] [PubMed] [Google Scholar]
- Yager P., Peticolas W. L. The kinetics of the main phase transition of aqueous dispersions of phospholipids induced by pressure jump and monitored by Raman spectroscopy. Biochim Biophys Acta. 1982 Jun 28;688(3):775–785. doi: 10.1016/0005-2736(82)90291-7. [DOI] [PubMed] [Google Scholar]