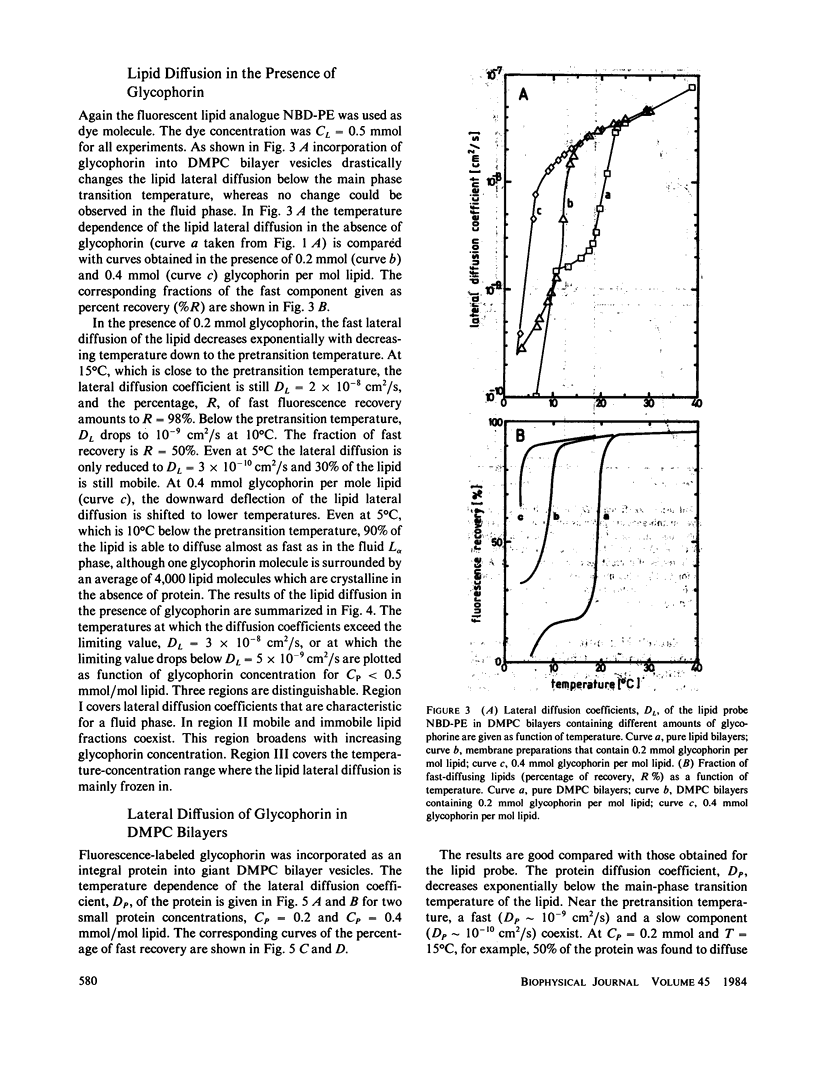
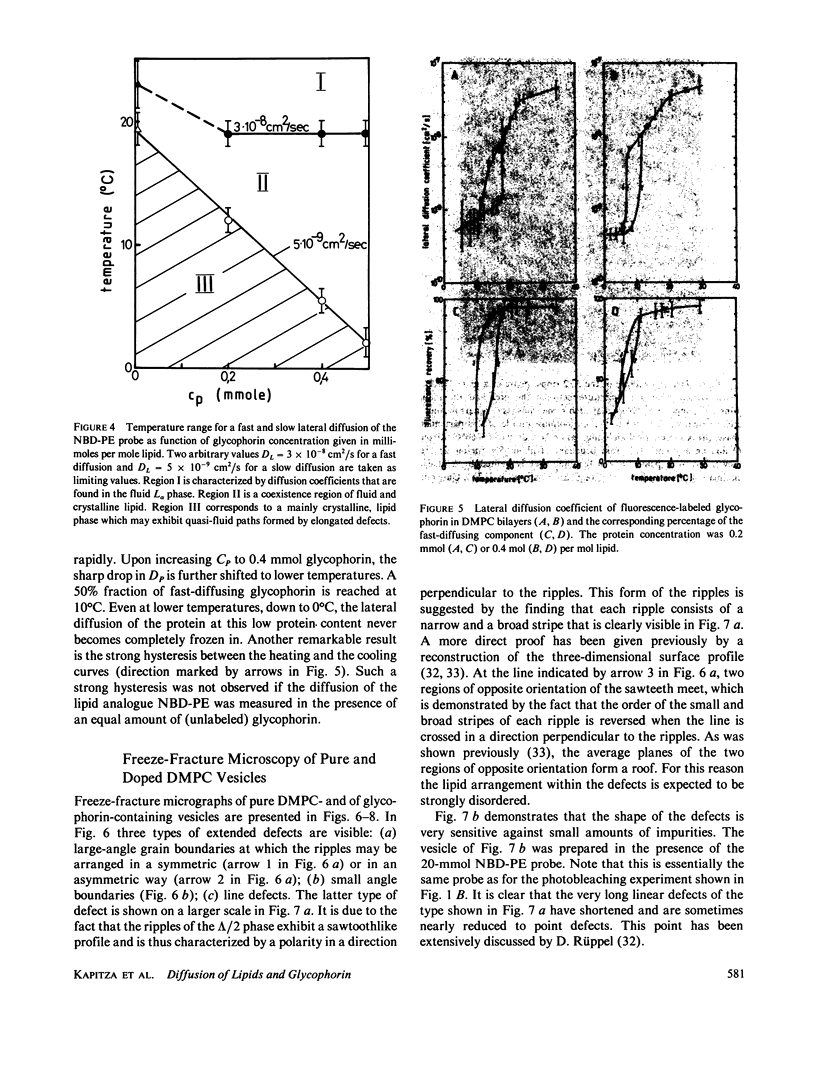
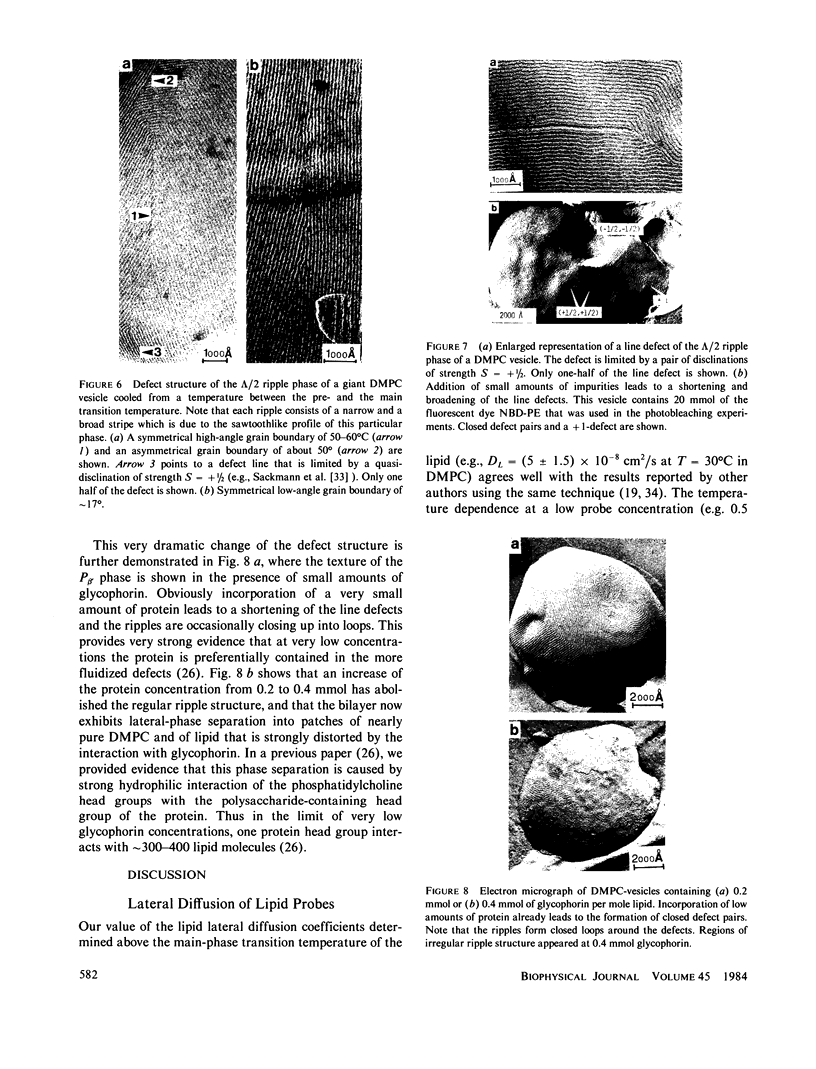
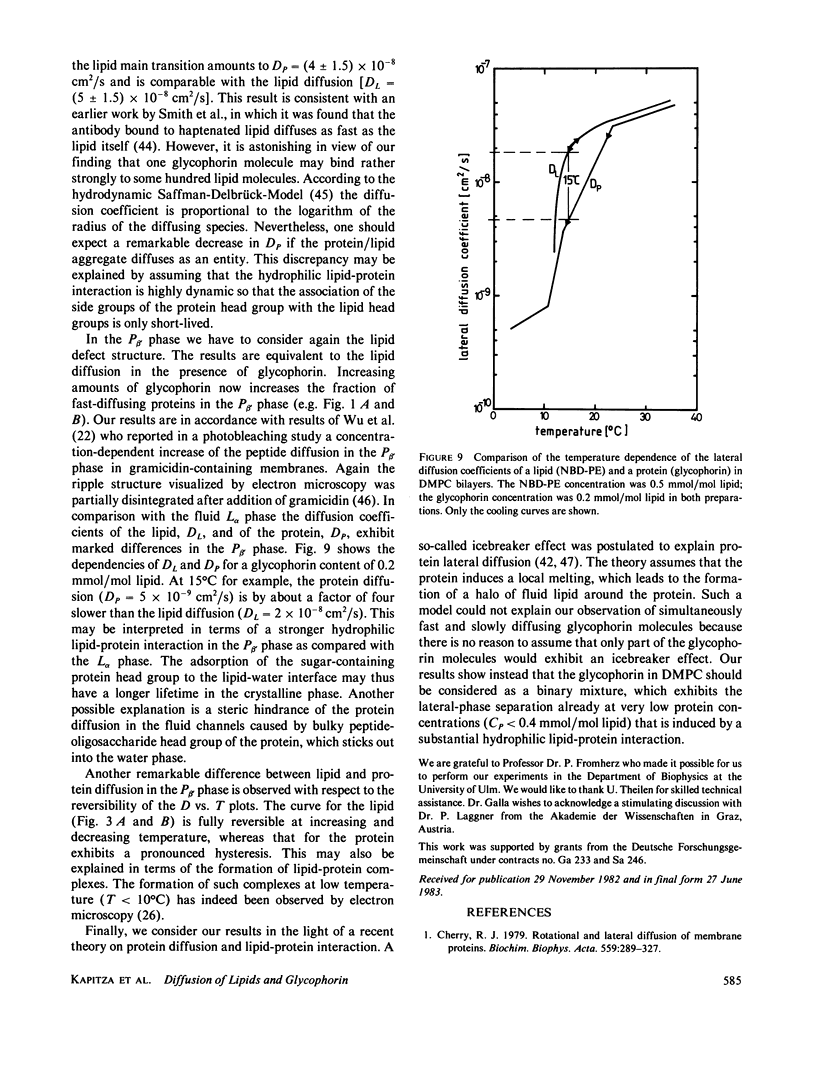
Abstract
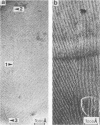
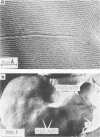
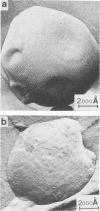
The lateral mobility of the lipid analog N-4-nitrobenzo-2-oxa-1,3 diazole phosphatidylethanolamine and of the integral protein glycophorin in giant dimyristoylphosphatidylcholine vesicles was studied by the photobleaching technique. Above the temperature of the chain-melting transition (Tm = 23 degrees C), the diffusion coefficient, Dp, of the protein [Dp = (4 +/- 2) X 10(-8) cm2/s at 30 degrees C] was within the experimental errors equal to the corresponding values DL of the lipid analog. In the P beta 1 phase the diffusion of lipid and glycophorin was studied as a function of the probe and the protein concentration. (a) At low lipid-probe content (cL less than 5 mmol/mol of total lipid), approximately 20% of the probe diffuses fast (D approximately equal to 10(-8) - 10(-9) cm2/s), while the mobility of the rest is strongly reduced (D less than 10(-10) cm2/s). At a higher concentration (cp approximately 20 mmol), all probe is immobilized (D less than 10(-10) cm2/s). (b) Incorporation of glycophorin up to cp = 0.4 mmol/mol of total lipid leads to a gradual increase of the fraction of mobile lipid probe due to the lateral-phase separation into a pure P beta 1 phase and a fraction of lipid that is fluidized by strong hydrophilic lipid-protein interaction. (c) The diffusion of the glycophorin molecules is characterized by a slow and a fast fraction. The latter increases with increasing protein content, which is again due to the lateral-phase separation caused by the hydrophilic lipid-protein interaction. The results are interpreted in terms of a fast transport along linear defects in the P beta 1 phase, which form quasi-fluid paths for a nearly one dimensional and thus very effective transport. Evidence for this interpretation of the diffusion measurements is provided by freeze-fracture electron microscopy.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brûlet P., McConnell H. M. Protein-lipid interactions glycophorin and dipalmitolyphosphatidylcholine. Biochem Biophys Res Commun. 1976 Jan 26;68(2):363–368. doi: 10.1016/0006-291x(76)91153-0. [DOI] [PubMed] [Google Scholar]
- Chapman D., Cornell B. A., Ellasz A. W., Perry A. Interactions of helical polypepetide segments which span the hydrocarbon region of lipid bilayers. Studies of the gramicidin A lipid-water system. J Mol Biol. 1977 Jul 5;113(3):517–538. doi: 10.1016/0022-2836(77)90236-4. [DOI] [PubMed] [Google Scholar]
- Cherry R. J. Rotational and lateral diffusion of membrane proteins. Biochim Biophys Acta. 1979 Dec 20;559(4):289–327. doi: 10.1016/0304-4157(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Derzko Z., Jacobson K. Comparative lateral diffusion of fluorescent lipid analogues in phospholipid multibilayers. Biochemistry. 1980 Dec 23;19(26):6050–6057. doi: 10.1021/bi00567a016. [DOI] [PubMed] [Google Scholar]
- Devaux P., McConnell H. M. Lateral diffusion in spin-labeled phosphatidylcholine multilayers. J Am Chem Soc. 1972 Jun 28;94(13):4475–4481. doi: 10.1021/ja00768a600. [DOI] [PubMed] [Google Scholar]
- Fahey P. F., Koppel D. E., Barak L. S., Wolf D. E., Elson E. L., Webb W. W. Lateral diffusion in planar lipid bilayers. Science. 1977 Jan 21;195(4275):305–306. doi: 10.1126/science.831279. [DOI] [PubMed] [Google Scholar]
- Falkovitz M. S., Seul M., Frisch H. L., McConnell H. M. Theory of periodic structures in lipid bilayer membranes. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3918–3921. doi: 10.1073/pnas.79.12.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. W., James T. L. Lateral diffusion of the phospholipid molecule in dipalmitoylphosphatidylcholine bilayers. An investigation using nuclear spin--lattice relaxation in the rotating frame. Biochemistry. 1978 Apr 4;17(7):1177–1183. doi: 10.1021/bi00600a007. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Hartmann W. Excimer-forming lipids in membrane research. Chem Phys Lipids. 1980 Oct;27(3):199–219. doi: 10.1016/0009-3084(80)90036-5. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Hartmann W., Theilen U., Sackmann E. On two-dimensional passive random walk in lipid bilayers and fluid pathways in biomembranes. J Membr Biol. 1979 Jul 31;48(3):215–236. doi: 10.1007/BF01872892. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Sackmann E. Chemically induced lipid phase separation in model membranes containing charged lipids: a spin label study. Biochim Biophys Acta. 1975 Sep 2;401(3):509–529. doi: 10.1016/0005-2736(75)90249-7. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Sackmann E. Lateral diffusion in the hydrophobic region of membranes: use of pyrene excimers as optical probes. Biochim Biophys Acta. 1974 Feb 26;339(1):103–115. doi: 10.1016/0005-2736(74)90336-8. [DOI] [PubMed] [Google Scholar]
- Grant C. W., McConnell H. M. Glycophorin in lipid bilayers. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4653–4657. doi: 10.1073/pnas.71.12.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitza H. G., Sackmann E. Local measurement of lateral motion in erythrocyte membranes by photobleaching technique. Biochim Biophys Acta. 1980;595(1):56–64. doi: 10.1016/0005-2736(80)90247-3. [DOI] [PubMed] [Google Scholar]
- Lee A. G. Analysis of the defect structure of gel-phase lipid. Biochemistry. 1977 Mar 8;16(5):835–841. doi: 10.1021/bi00624a004. [DOI] [PubMed] [Google Scholar]
- Lee A. G. Fluorescence studies of chlorophyll a incorporated into lipid mixtures, and the interpretation of "phase" diagrams. Biochim Biophys Acta. 1975 Nov 17;413(1):11–23. doi: 10.1016/0005-2736(75)90054-1. [DOI] [PubMed] [Google Scholar]
- Lindblom G., Wennerström H., Arvidson G., Lindman B. Lecithin translational diffusion studied by pulsed nuclear magnetic resonance. Biophys J. 1976 Nov;16(11):1287–1295. doi: 10.1016/S0006-3495(76)85774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R., Peters J., Tews K. H., Bähr W. A microfluorimetric study of translational diffusion in erythrocyte membranes. Biochim Biophys Acta. 1974 Nov 15;367(3):282–294. doi: 10.1016/0005-2736(74)90085-6. [DOI] [PubMed] [Google Scholar]
- Pink D. A., Lookman T., MacDonald A. L., Zuckermann M. J., Jan N. Lateral diffusion of gramicidin S, M-13 coat protein and glycophorin in bilayers of saturated phospholipids. Mean field and Monte Carlo studies. Biochim Biophys Acta. 1982 Apr 23;687(1):42–56. doi: 10.1016/0005-2736(82)90168-7. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L., Smith B. A., McConnell H. M. Lateral diffusion in binary mixtures of cholesterol and phosphatidylcholines. Proc Natl Acad Sci U S A. 1979 Jan;76(1):15–18. doi: 10.1073/pnas.76.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackmann E., Träuble H. Studies of the crystalline-liquid crystalline phase transition of lipid model membranes. I. Use of spin labels and optical probes as indicators of the phase transition. J Am Chem Soc. 1972 Jun 28;94(13):4482–4491. doi: 10.1021/ja00768a013. [DOI] [PubMed] [Google Scholar]
- Saffman P. G., Delbrück M. Brownian motion in biological membranes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3111–3113. doi: 10.1073/pnas.72.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. A., McConnell H. M. Determination of molecular motion in membranes using periodic pattern photobleaching. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2759–2763. doi: 10.1073/pnas.75.6.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. M., Parce J. W., Smith B. A., McConnell H. M. Antibodies bound to lipid haptens in model membranes diffuse as rapidly as the lipids themselves. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4177–4179. doi: 10.1073/pnas.76.9.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. M., Rubenstein J. L., Parce J. W., McConnell H. M. Lateral diffusion of M-13 coat protein in mixtures of phosphatidylcholine and cholesterol. Biochemistry. 1980 Dec 9;19(25):5907–5911. doi: 10.1021/bi00566a037. [DOI] [PubMed] [Google Scholar]
- Strittmatter P., Rogers M. J. Apparent dependence of interactions between cytochrome b5 and cytochrome b5 reductase upon translational diffusion in dimyristoyl lecithin liposomes. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2658–2661. doi: 10.1073/pnas.72.7.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz W. L., Jacobson K., Wu E. S., Derzko Z. Lateral mobility of an amphipathic apolipoprotein, ApoC-III, bound to phosphatidylcholine bilayers with and without cholesterol. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5645–5649. doi: 10.1073/pnas.76.11.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz W. L., Kapitza H. G., Stümpel J., Sackmann E., Jovin T. M. Translational mobility of glycophorin in bilayer membranes of dimyristoylphosphatidylcholine. Biochemistry. 1981 Mar 3;20(5):1392–1396. doi: 10.1021/bi00508a055. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J., Ververgaert P. H., van Deenen L. L., Elbers P. F. Phase transitions of phospholipid bilayers and membranes of Acholeplasma laidlawii B visualized by freeze fracturing electron microscopy. Biochim Biophys Acta. 1972 Nov 2;288(2):326–332. doi: 10.1016/0005-2736(72)90253-2. [DOI] [PubMed] [Google Scholar]
- Webb W. W. Applications of fluorescence correlation spectroscopy. Q Rev Biophys. 1976 Feb;9(1):49–68. doi: 10.1017/s0033583500002158. [DOI] [PubMed] [Google Scholar]
- Wey C. L., Cone R. A., Edidin M. A. Lateral diffusion of rhodopsin in photoreceptor cells measured by fluorescence photobleaching and recovery. Biophys J. 1981 Feb;33(2):225–232. doi: 10.1016/S0006-3495(81)84883-7. [DOI] [PMC free article] [PubMed] [Google Scholar]