Abstract
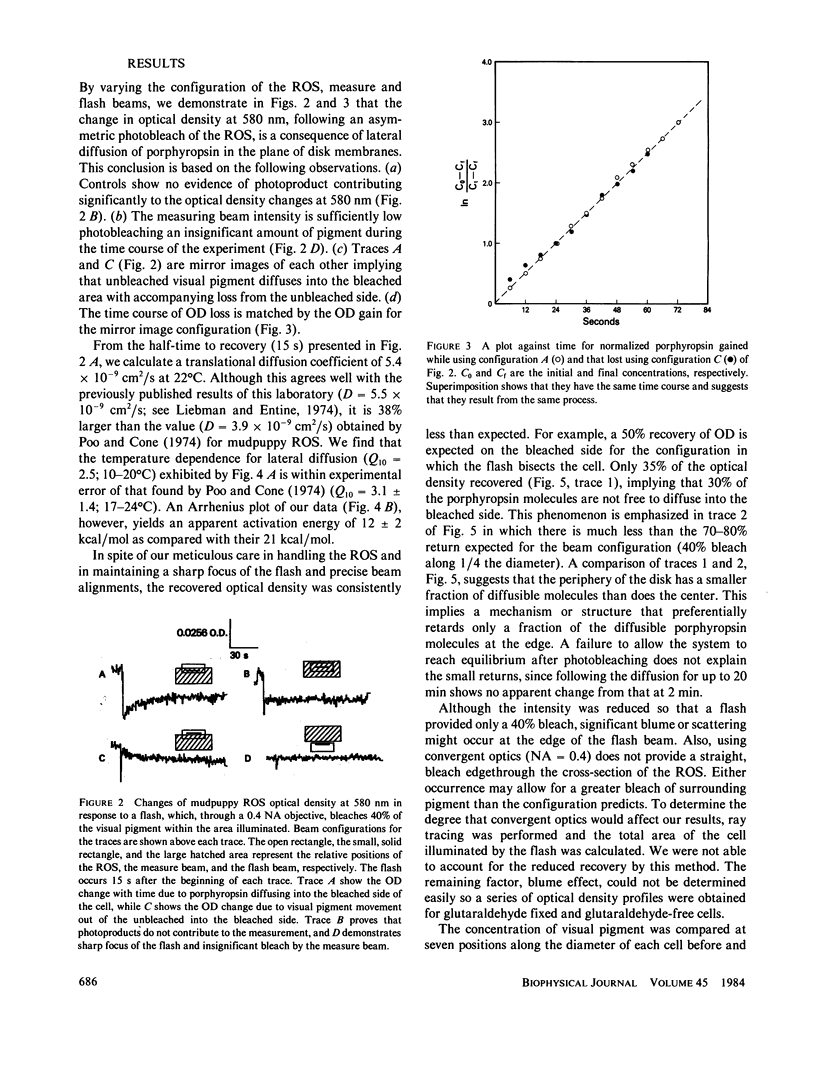
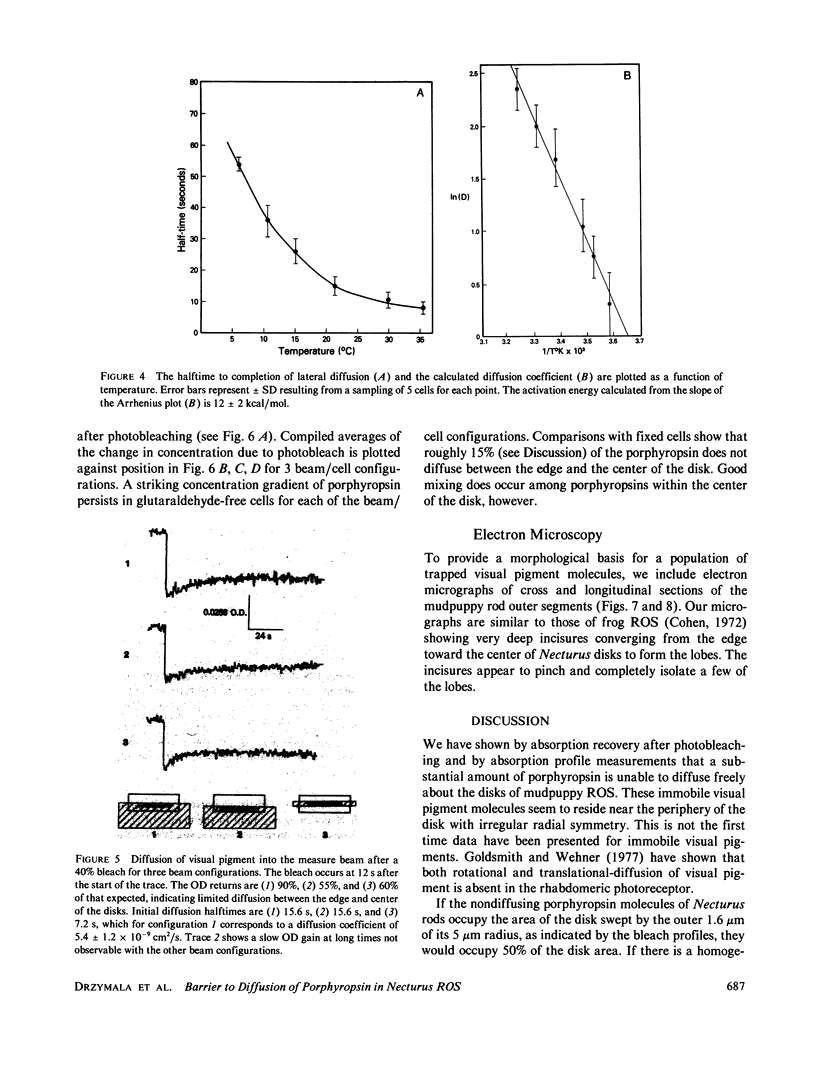
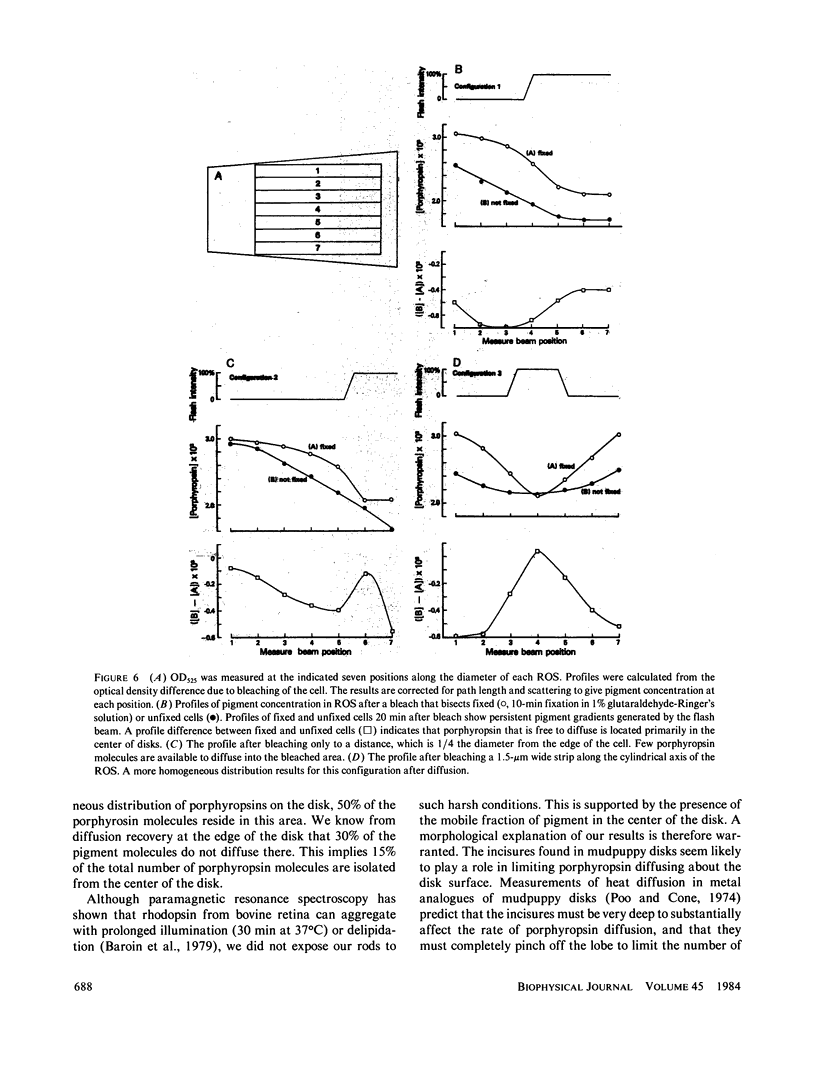
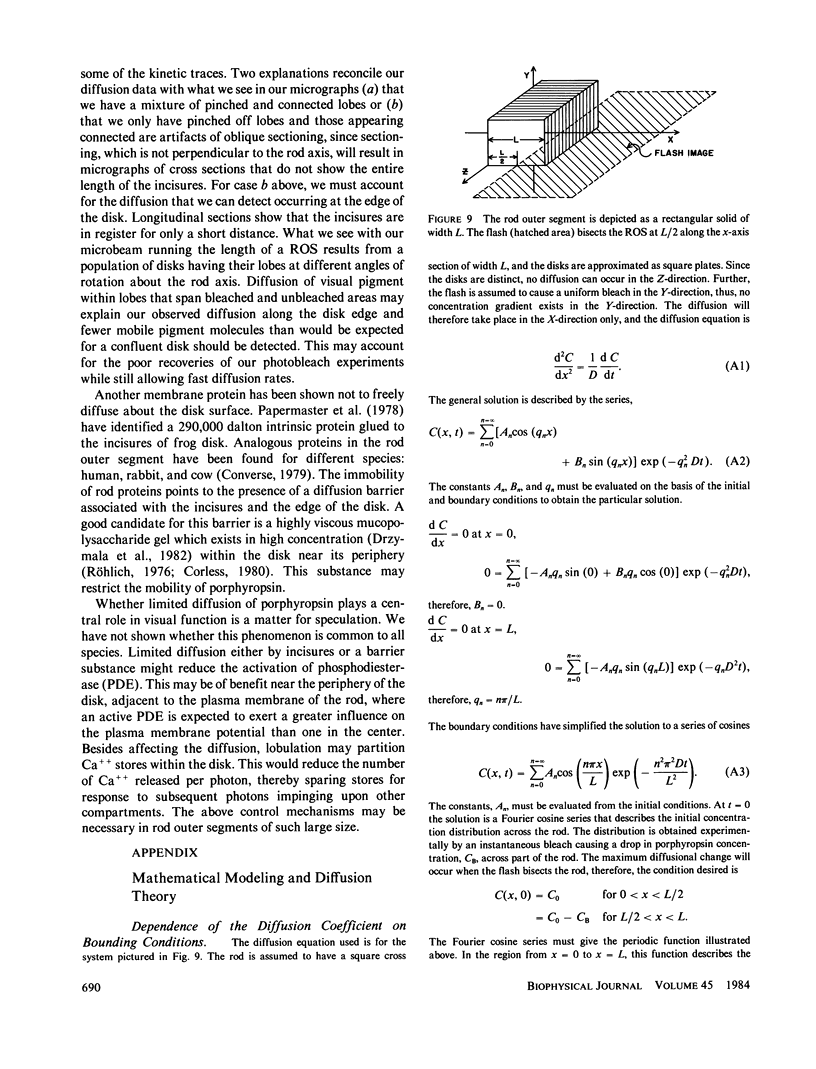
Microspectrophotometry was used to study lateral diffusion of the visual pigment, porphyropsin , in the disk membrane in intact mudpuppy (Necturus maculosus) rod outer segments (ROS), isolated in frog Ringer's solution. A concentration gradient of unbleached visual pigment was produced on the disks by rapidly photobleaching 40% of the pigment in an area spanning 1/4 or 1/2 of the cell's width. The change in optical density of the cells at 580 nm was then followed with time on either the bleached or unbleached side. The temperature dependence of porphyropsin diffusion yielded a Q10 of 2.5 between 10 and 20 degrees C with an activation energy of 12 +/- 2 kcal. At completion of pigment diffusion, the center and edge of the disk had, respectively, attained only 90 and 55% of the concentration expected. Computed diffusion coefficients (5.4 X 10(-9) cm2/s) were similar at the center and periphery of the disk immediately after the flash, however, an additional slow component for diffusion was detected at the periphery. A comparison of optical density at 525 nm along the diameter of ROS before and after the flash showed a persistent (20 min) postbleach concentration gradient of unbleached porphyropsin . This suggests that 15% of the prophyropsins may be sequestered into distinct areas on a mudpuppy disk and are not free to diffuse over the whole surface. This argument is supported by the observation that mudpuppy disks are separated into petal -shaped regions by incisures, some of which penetrate nearly to the disk center.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. J. Purification and quantitation of glutaraldehyde and its effect on several enzyme activities in skeletal muscle. J Histochem Cytochem. 1967 Aug;15(11):652–661. doi: 10.1177/15.11.652. [DOI] [PubMed] [Google Scholar]
- Baroin A., Bienvenue A., Devaux P. F. Spin-label studies of protein-protein interactions in retinal rod outer segment membranes. Saturation transfer electron paramagnetic resonance spectroscopy. Biochemistry. 1979 Apr 3;18(7):1151–1155. doi: 10.1021/bi00574a005. [DOI] [PubMed] [Google Scholar]
- Borggreven J. M., Daemen F. J., Bonting S. L. Biochemical aspects of the visual process. VI. The lipid composition of native and hexane-extracted cattle rod outer segments. Biochim Biophys Acta. 1970 Mar 10;202(2):374–381. [PubMed] [Google Scholar]
- Brown P. K. Rhodopsin rotates in the visual receptor membrane. Nat New Biol. 1972 Mar 15;236(63):35–38. doi: 10.1038/newbio236035a0. [DOI] [PubMed] [Google Scholar]
- Chambers R. W., Bowling M. C., Grimley P. M. Glutaraldehyde fixation in routine histopathology. Arch Pathol. 1968 Jan;85(1):18–30. [PubMed] [Google Scholar]
- Cherry R. J., Bürkli A., Busslinger M., Schneider G., Parish G. R. Rotational diffusion of band 3 proteins in the human erythrocyte membrane. Nature. 1976 Sep 30;263(5576):389–393. doi: 10.1038/263389a0. [DOI] [PubMed] [Google Scholar]
- Cone R. A. Rotational diffusion of rhodopsin in the visual receptor membrane. Nat New Biol. 1972 Mar 15;236(63):39–43. doi: 10.1038/newbio236039a0. [DOI] [PubMed] [Google Scholar]
- Converse C. A. The large intrinsic membrane protein in rod outer segments: in vitro synthesis in cattle, and comparison in humans and rabbits. Exp Eye Res. 1979 Oct;29(4):409–416. doi: 10.1016/0014-4835(79)90057-5. [DOI] [PubMed] [Google Scholar]
- Corless J. M. The carbohydrates in frog retinal rod outer segments. Prog Histochem Cytochem. 1980;12(2):1–57. doi: 10.1016/s0079-6336(80)80010-6. [DOI] [PubMed] [Google Scholar]
- Drzymala R. E., Liebman P. A., Romhányi G. Acid polysaccharide content of frog rod outer segments determined by metachromatic toluidine blue staining. Histochemistry. 1982;76(3):363–379. doi: 10.1007/BF00543958. [DOI] [PubMed] [Google Scholar]
- FRASCA J. M., PARKS V. R. A ROUTINE TECHNIQUE FOR DOUBLE-STAINING ULTRATHIN SECTIONS USING URANYL AND LEAD SALTS. J Cell Biol. 1965 Apr;25:157–161. doi: 10.1083/jcb.25.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith T. H., Wehner R. Restrictions on rotational and translational diffusion of pigment in the membranes of a rhabdomeric photoreceptor. J Gen Physiol. 1977 Oct;70(4):453–490. doi: 10.1085/jgp.70.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller J., Ostwald T. J., Bok D. The osmotic behavior of rod photoreceptor outer segment discs. J Cell Biol. 1971 Mar;48(3):633–649. doi: 10.1083/jcb.48.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenstein R., Hess B. Immobilization of bacteriorhodopsin and orientation of its transition moment in purple membrane. FEBS Lett. 1978 May 1;89(1):15–20. doi: 10.1016/0014-5793(78)80512-2. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Entine G. Lateral diffusion of visual pigment in photorecptor disk membranes. Science. 1974 Aug 2;185(4149):457–459. doi: 10.1126/science.185.4149.457. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Pugh E. N., Jr The control of phosphodiesterase in rod disk membranes: kinetics, possible mechanisms and significance for vision. Vision Res. 1979;19(4):375–380. doi: 10.1016/0042-6989(79)90097-x. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Weiner H. L., Drzymala R. E. Lateral diffusion of visual pigment in rod disk membranes. Methods Enzymol. 1982;81:660–668. doi: 10.1016/s0076-6879(82)81091-4. [DOI] [PubMed] [Google Scholar]
- Lux S. E. Dissecting the red cell membrane skeleton. Nature. 1979 Oct 11;281(5731):426–429. doi: 10.1038/281426a0. [DOI] [PubMed] [Google Scholar]
- Nigg E. A., Cherry R. J. Anchorage of a band 3 population at the erythrocyte cytoplasmic membrane surface: protein rotational diffusion measurements. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4702–4706. doi: 10.1073/pnas.77.8.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir I., Pease D. C. Ultrastructural aspects of discs in rod outer segments. Exp Eye Res. 1973 Jul;16(3):173–182. doi: 10.1016/0014-4835(73)90211-x. [DOI] [PubMed] [Google Scholar]
- Papermaster D. S., Schneider B. G., Zorn M. A., Kraehenbuhl J. P. Immunocytochemical localization of a large intrinsic membrane protein to the incisures and margins of frog rod outer segment disks. J Cell Biol. 1978 Aug;78(2):415–425. doi: 10.1083/jcb.78.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M., Cone R. A. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974 Feb 15;247(5441):438–441. doi: 10.1038/247438a0. [DOI] [PubMed] [Google Scholar]
- Röhlich P. Photoreceptor membrane carbohydrate on the intradiscal surface of retinal rod disks. Nature. 1976 Oct 28;263(5580):789–791. doi: 10.1038/263789a0. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takezoe H., Yu H. Lateral diffusion of photopigments in photoreceptor disk membrane vesicles by the dynamic Kerr effect. Biochemistry. 1981 Sep 1;20(18):5275–5281. doi: 10.1021/bi00521a028. [DOI] [PubMed] [Google Scholar]
- Trauble H., Sackmann E. Lipid motion and phodopsin rotation. Nature. 1973 Sep 28;245(5422):210–211. doi: 10.1038/245210a0. [DOI] [PubMed] [Google Scholar]
- Wey C. L., Cone R. A., Edidin M. A. Lateral diffusion of rhodopsin in photoreceptor cells measured by fluorescence photobleaching and recovery. Biophys J. 1981 Feb;33(2):225–232. doi: 10.1016/S0006-3495(81)84883-7. [DOI] [PMC free article] [PubMed] [Google Scholar]




