Abstract
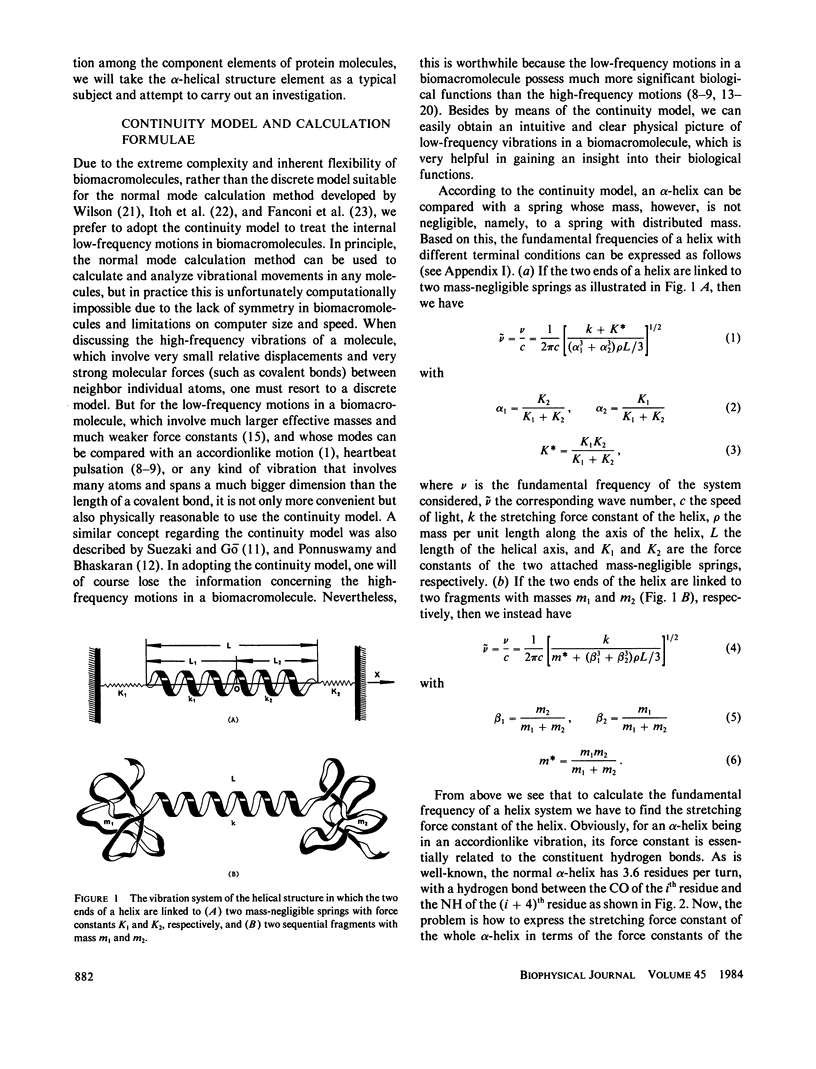
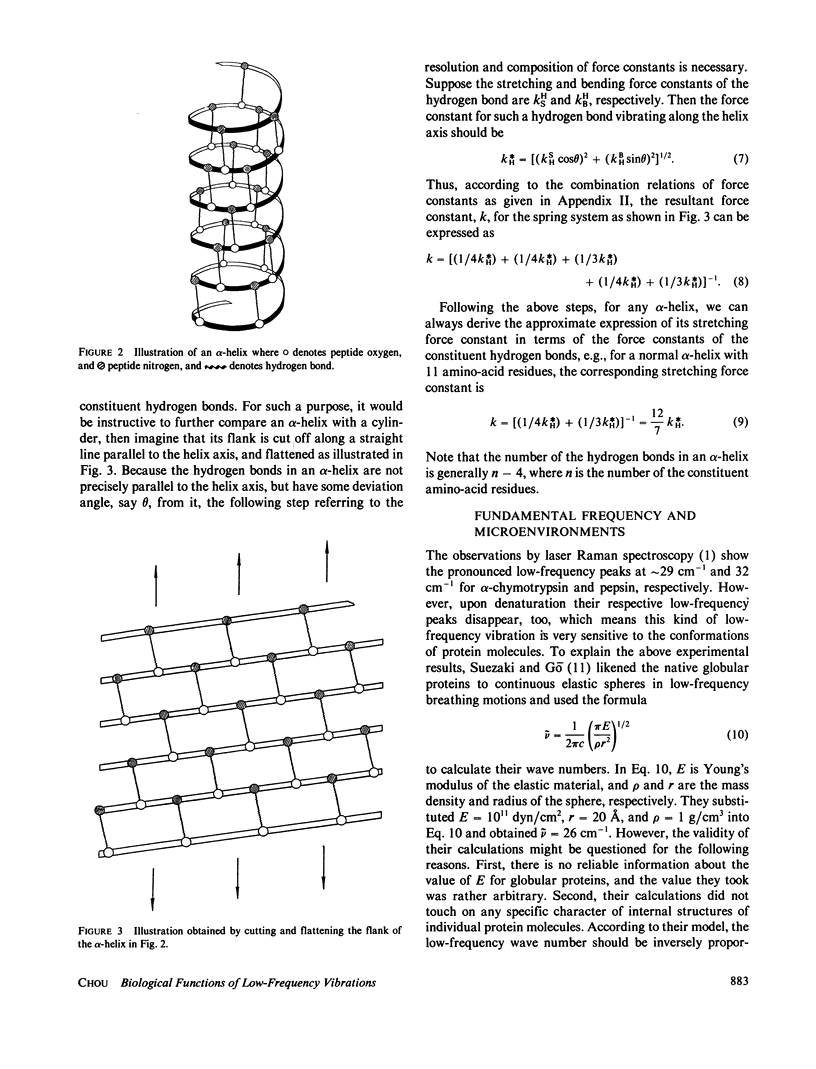
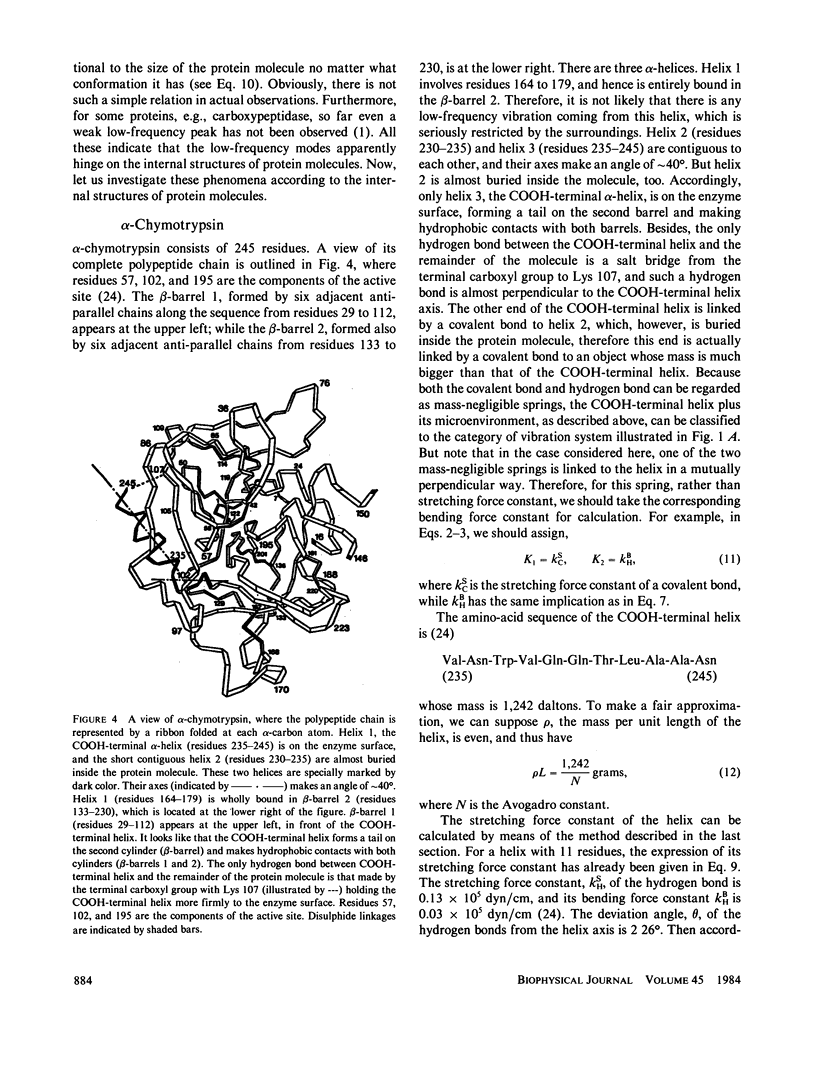
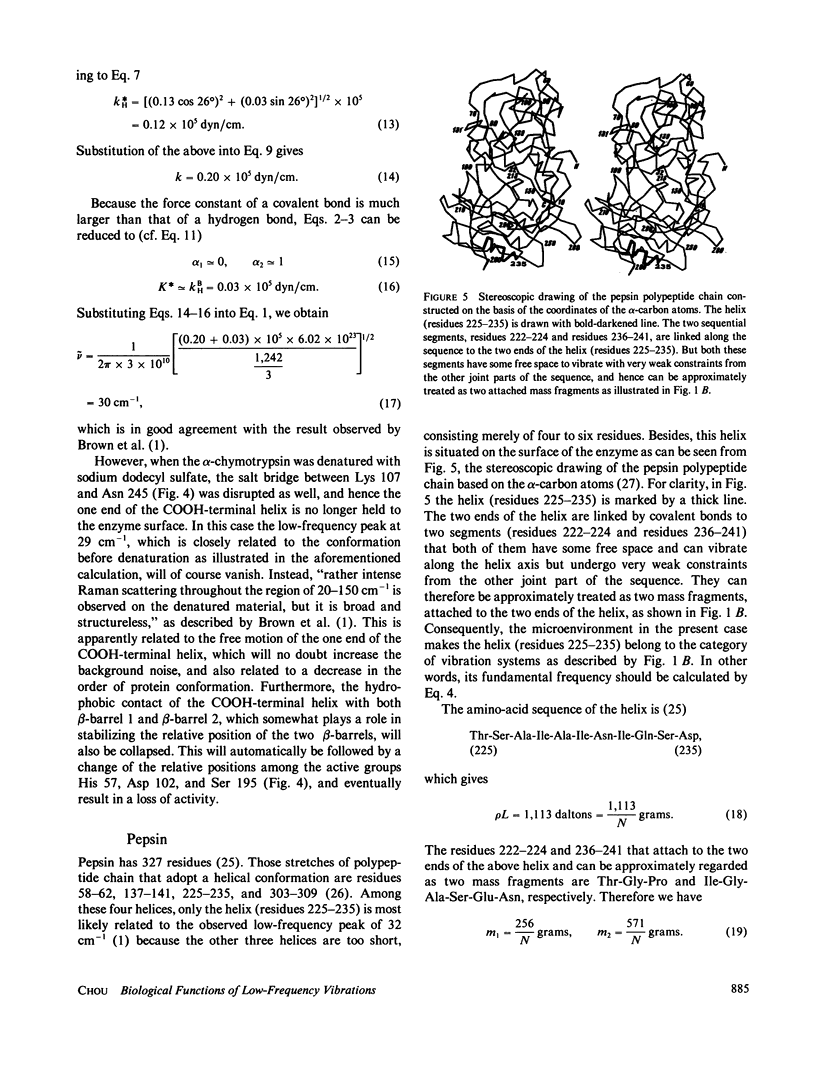
Low-frequency vibrations in biomacromolecules possess significant biological functions. In this paper, the alpha-helix element is compared with a mass-distributed spring. Based on this, a set of intuitive and easily handled equations are derived for predicting the fundamental frequencies of helical structures in protein molecules. As shown in the equations, the fundamental frequency depends not only on the constituents of a helix itself but also on its microenvironment. The calculated results agree with the observations. The calculations also demonstrate that the low-frequency vibrations with wave number of approximately 30 cm-1 do not necessarily arise from motions that involve either all or very large portions of the protein molecule as previously thought; a piece of helix containing more than 10 residues and surrounded by a proper microenvironment can also generate such low-frequency motions. Furthermore , we illustrate that the low-frequency motions are closely related to the native state of a protein molecule. Upon denaturation, which is accompanied by a radical change of the relevant microenvironment, the original fundamental frequency also disappears. Consequently, this kind of special frequency termed activating low frequency can serve as a dynamic criterion in identifying whether a biomacromolecule is in its native state. The energy of a phonon excited by this kind of low-frequency vibration is of the same order of magnitude as the average enthalpy value per residue measured during conformational change in some protein molecules. Therefore, there must be some intrinsic relation between the allosteric transitions of protein molecules and their low-frequency motions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birktoft J. J., Blow D. M. Structure of crystalline -chymotrypsin. V. The atomic structure of tosyl- -chymotrypsin at 2 A resolution. J Mol Biol. 1972 Jul 21;68(2):187–240. doi: 10.1016/0022-2836(72)90210-0. [DOI] [PubMed] [Google Scholar]
- Brown K. G., Erfurth S. C., Small E. W., Peticolas W. L. Conformationally dependent low-frequency motions of proteins by laser Raman spectroscopy. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1467–1469. doi: 10.1073/pnas.69.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careri G., Fasella P., Gratton E. Statistical time events in enzymes: a physical assessment. CRC Crit Rev Biochem. 1975 Aug;3(2):141–164. doi: 10.3109/10409237509102555. [DOI] [PubMed] [Google Scholar]
- Cooper A. Thermodynamic fluctuations in protein molecules. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2740–2741. doi: 10.1073/pnas.73.8.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanconi B., Small E. W., Peticolas W. L. Phonon dispersion curves and normal coordinate analysis of -poly-L-alanine. Biopolymers. 1971;10(8):1277–1298. doi: 10.1002/bip.360100804. [DOI] [PubMed] [Google Scholar]
- Go N., Noguti T., Nishikawa T. Dynamics of a small globular protein in terms of low-frequency vibrational modes. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3696–3700. doi: 10.1073/pnas.80.12.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu I. N., Delbaere L. T., James M. N., Hofmann T. Penicillopepsin from Penicillium janthinellum crystal structure at 2.8 A and sequence homology with porcine pepsin. Nature. 1977 Mar 10;266(5598):140–145. doi: 10.1038/266140a0. [DOI] [PubMed] [Google Scholar]
- Hull W. E., Sykes B. D. Fluorotyrosine alkaline phosphatase: internal mobility of individual tyrosines and the role of chemical shift anisotropy as a 19F nuclear spin relaxation mechanism in proteins. J Mol Biol. 1975 Oct 15;98(1):121–153. doi: 10.1016/s0022-2836(75)80105-7. [DOI] [PubMed] [Google Scholar]
- Ito K., Shimanouchi T. Vibrational frequencies and modes of alpha-helix. Biopolymers. 1970;9(4):383–399. doi: 10.1002/bip.1970.360090402. [DOI] [PubMed] [Google Scholar]
- Ji S. Energy and negentropy in enzymic catalysis. Ann N Y Acad Sci. 1974 Feb 18;227:419–437. doi: 10.1111/j.1749-6632.1974.tb14405.x. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. R., Weber G. Quenching of protein fluorescence by oxygen. Detection of structural fluctuations in proteins on the nanosecond time scale. Biochemistry. 1973 Oct 9;12(21):4171–4179. doi: 10.1021/bi00745a021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter P. C., Mosher L. E., Rhoads C. Low-frequency modes in the Raman spectra of proteins. Biopolymers. 1982 Jul;21(7):1469–1472. doi: 10.1002/bip.360210715. [DOI] [PubMed] [Google Scholar]
- Ponnuswamy P. K., Bhaskaran R. Internal fluctuations in globular proteins. Int J Pept Protein Res. 1982 May;19(5):549–555. doi: 10.1111/j.1399-3011.1982.tb02641.x. [DOI] [PubMed] [Google Scholar]
- Saviotti M. L., Galley W. C. Room temperature phosphorescence and the dynamic aspects of protein structure. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4154–4158. doi: 10.1073/pnas.71.10.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell H. M., Lozansky E. D., Lessen M. Structural and energetic considerations of wave propagation in DNA. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):11–19. doi: 10.1101/sqb.1979.043.01.004. [DOI] [PubMed] [Google Scholar]
- Suezaki Y., Go N. Breathing mode of conformational fluctuations in globular proteins. Int J Pept Protein Res. 1975;7(4):333–334. doi: 10.1111/j.1399-3011.1975.tb02448.x. [DOI] [PubMed] [Google Scholar]
- Tang J., Sepulveda P., Marciniszyn J., Jr, Chen K. C., Huang W. Y., Tao N., Liu D., Lanier J. P. Amino-acid sequence of porcine pepsin. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3437–3439. doi: 10.1073/pnas.70.12.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]


