Abstract
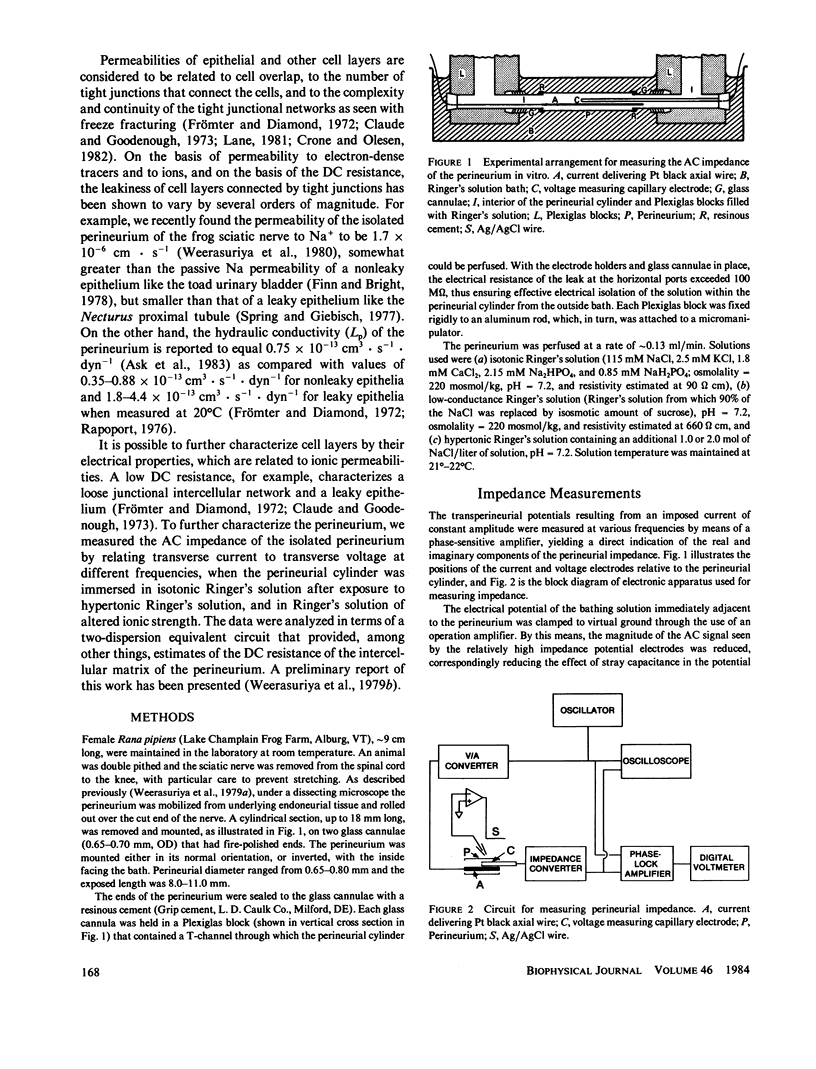
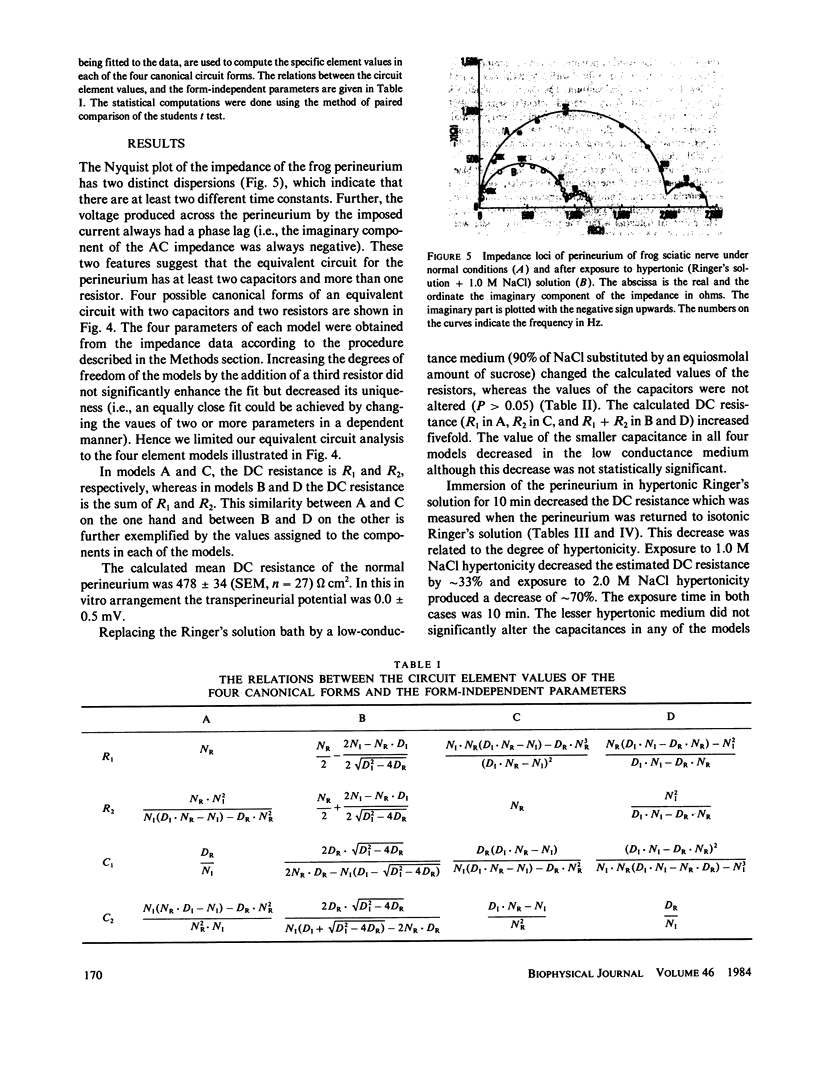
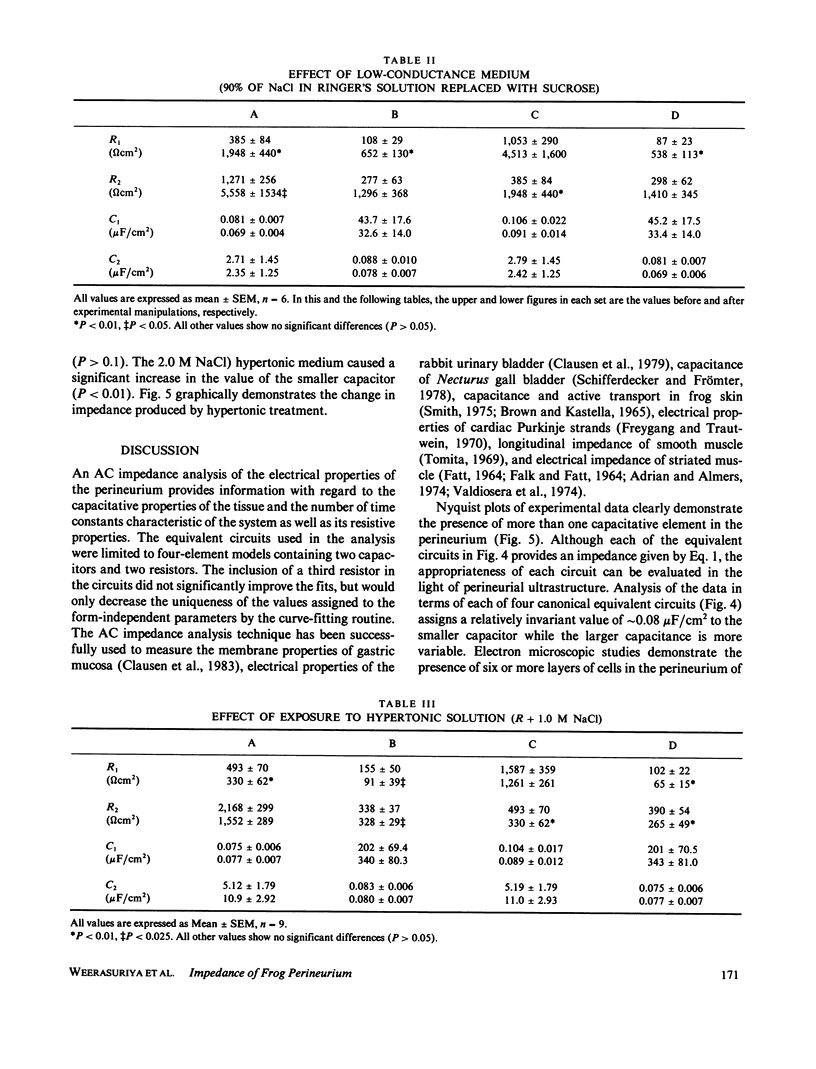
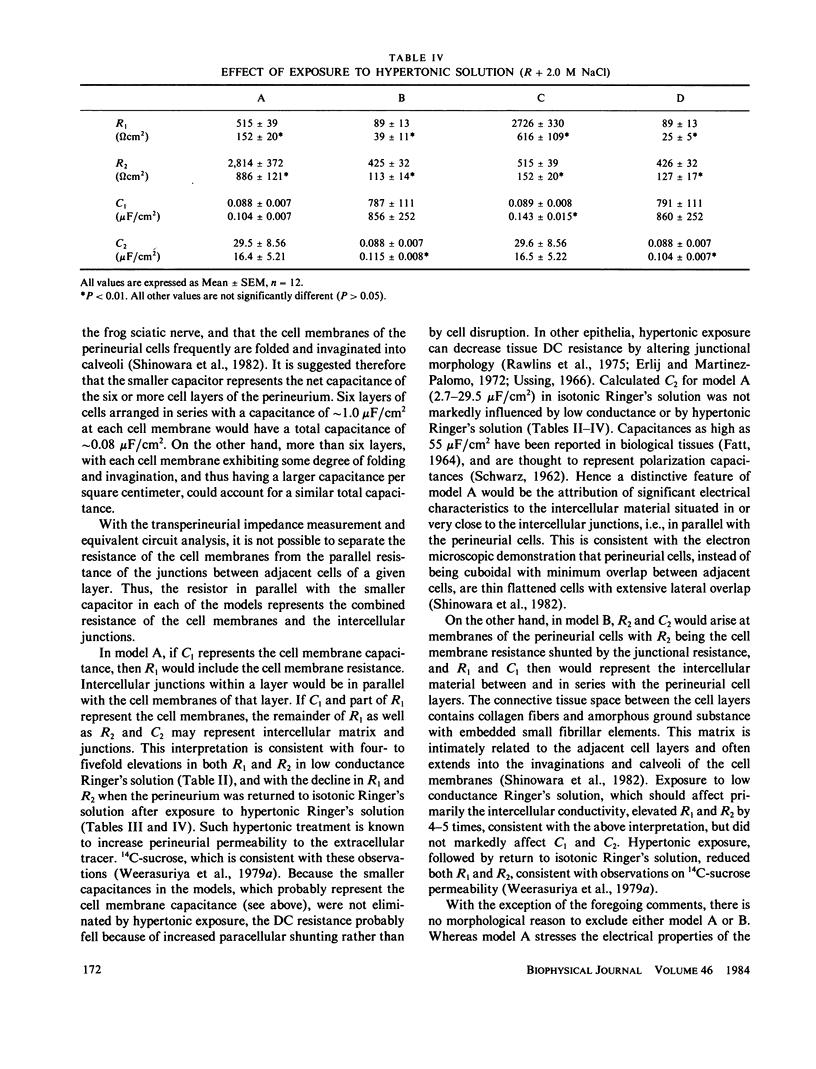
The AC impedance of the isolated perineurium of the frog sciatic nerve was examined at frequencies from 2 Hz to 100 kHz. A Nyquist plot of the imaginary and real components of the impedance demonstrated more than 1 capacitative element, and a DC resistance of 478 +/- 34 (SEM, n = 27) omega cm2. Transperineurial potential in the absence of externally applied current was 0.0 +/- 0.5 mV. The impedance data were fitted by nonlinear least squares to an equation representing the generalized impedance of four equivalent circuits each with two resistive and two capacitative elements. Only two of these circuits were consistent with perineurial morphology, however. In both, the perineurial cells were represented by a resistive and capacitative element in parallel, where capacitance was less than 0.1 microF/cm2. The extracellular matrix and intercellular junctions of the perineurium were represented as single resistive and capacitative elements in parallel or in series, where capacitance exceeded 2 microF/cm2. Immersion of the perineurium in low conductance Ringer's solution increased DC resistive elements as compared with their values in isotonic Ringer's solution, whereas treatment for 10 min with a hypertonic Ringer's solution (containing an additional 1.0 or 2.0 mol NaCl/liter of solution) reduced DC resistive elements, consistent with changes in perineurial permeability. The results indicate that (a) perineurial impedance contains two time constants and can be analyzed in terms of contributions from cellular and extracellular elements, and (b) transperineurial DC resistance, which is intermediate between DC resistance for leaky and nonleaky epithelia, represents intercellular resistance and can be experimentally modified by hypertonicity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Almers W. Membrane capacity measurements on frog skeletal muscle in media of low ion content. J Physiol. 1974 Mar;237(3):573–605. doi: 10.1113/jphysiol.1974.sp010499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akert K., Sandri C., Weibel E. R., Peper K., Moor H. The fine structure of the perineural endothelium. Cell Tissue Res. 1976 Jan 27;165(3):281–295. doi: 10.1007/BF00222433. [DOI] [PubMed] [Google Scholar]
- Ask P., Levitan H., Robinson P. J., Rapoport S. I. Peripheral nerve as an osmometer: role of the perineurium in frog sciatic nerve. Am J Physiol. 1983 Jan;244(1):C75–C81. doi: 10.1152/ajpcell.1983.244.1.C75. [DOI] [PubMed] [Google Scholar]
- Brown A. C., Kastella K. G. The AC impedance of frog skin and its relation to active transport. Biophys J. 1965 Jul;5(4):591–606. doi: 10.1016/S0006-3495(65)86736-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkel W. E. The histological fine structure of perineurium. Anat Rec. 1967 Jun;158(2):177–189. doi: 10.1002/ar.1091580207. [DOI] [PubMed] [Google Scholar]
- CRESCITELLI F. Nerve sheath as a barrier to the action of certain substances. Am J Physiol. 1951 Aug;166(2):229–240. doi: 10.1152/ajplegacy.1951.166.2.229. [DOI] [PubMed] [Google Scholar]
- Claude P., Goodenough D. A. Fracture faces of zonulae occludentes from "tight" and "leaky" epithelia. J Cell Biol. 1973 Aug;58(2):390–400. doi: 10.1083/jcb.58.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen C., Lewis S. A., Diamond J. M. Impedance analysis of a tight epithelium using a distributed resistance model. Biophys J. 1979 May;26(2):291–317. doi: 10.1016/S0006-3495(79)85250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen C., Machen T. E., Diamond J. M. Use of AC impedance analysis to study membrane changes related to acid secretion in amphibian gastric mucosa. Biophys J. 1983 Feb;41(2):167–178. doi: 10.1016/S0006-3495(83)84417-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C., Olesen S. P. Electrical resistance of brain microvascular endothelium. Brain Res. 1982 Jun 3;241(1):49–55. doi: 10.1016/0006-8993(82)91227-6. [DOI] [PubMed] [Google Scholar]
- Erlij D., Martínez-Palomo A. Opening of tight junctions in frog skin by hypertonic urea solutions. J Membr Biol. 1972;9(3):229–240. [PubMed] [Google Scholar]
- FALK G., FATT P. LINEAR ELECTRICAL PROPERTIES OF STRIATED MUSCLE FIBRES OBSERVED WITH INTRACELLULAR ELECTRODES. Proc R Soc Lond B Biol Sci. 1964 Apr 14;160:69–123. doi: 10.1098/rspb.1964.0030. [DOI] [PubMed] [Google Scholar]
- FATT P. AN ANALYSIS OF THE TRANSVERSE ELECTRICAL IMPEDANCE OF STRIATED MUSCLE. Proc R Soc Lond B Biol Sci. 1964 Mar 17;159:606–651. doi: 10.1098/rspb.1964.0023. [DOI] [PubMed] [Google Scholar]
- FENG T. P., LIU Y. M. The connective tissue sheath of the nerve as effective diffusion barrier. J Cell Physiol. 1949 Aug;34(1):1–16. doi: 10.1002/jcp.1030340102. [DOI] [PubMed] [Google Scholar]
- Finn A. L., Bright J. The paracellular pathway in toad urinary bladder: permselectivity and kinetics of opening. J Membr Biol. 1978 Dec 8;44(1):67–83. doi: 10.1007/BF01940574. [DOI] [PubMed] [Google Scholar]
- Freygang W. H., Trautwein W. The structural implications of the linear electrical properties of cardiac Purkinje strands. J Gen Physiol. 1970 Apr;55(4):524–547. doi: 10.1085/jgp.55.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frömter E., Diamond J. Route of passive ion permeation in epithelia. Nat New Biol. 1972 Jan 5;235(53):9–13. doi: 10.1038/newbio235009a0. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K. Some observations on perfused frog sciatic nerves. J Physiol. 1954 Feb 26;123(2):338–356. doi: 10.1113/jphysiol.1954.sp005055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott G. D. Mlab--a mathematical modeling tool. Comput Programs Biomed. 1979 Dec;10(3):271–280. doi: 10.1016/0010-468x(79)90075-8. [DOI] [PubMed] [Google Scholar]
- NICELY M. Measurement of the potential difference across the connective tissue sheath of frog sciatic nerve. Experientia. 1955 May 15;11(5):199–200. doi: 10.1007/BF02161318. [DOI] [PubMed] [Google Scholar]
- Olsson Y., Reese T. S. Permeability of vasa nervorum and perineurium in mouse sciatic nerve studied by fluorescence and electron microscopy. J Neuropathol Exp Neurol. 1971 Jan;30(1):105–119. doi: 10.1097/00005072-197101000-00011. [DOI] [PubMed] [Google Scholar]
- RASHBASS C., RUSHTON W. A. H. The relation of structure to the spread of excitation in the frog's sciatic trunk. J Physiol. 1949 Dec 15;110(1-2):110–135. doi: 10.1113/jphysiol.1949.sp004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reale E., Luciano L., Spitznas M. Freeze-fracture faces of the perineurial sheath of the rabbit sciatic nerve. J Neurocytol. 1975 Jun;4(3):261–270. doi: 10.1007/BF01102112. [DOI] [PubMed] [Google Scholar]
- Schifferdecker E., Frömter E. The AC impedance of Necturus gallbladder epithelium. Pflugers Arch. 1978 Nov 14;377(2):125–133. doi: 10.1007/BF00582842. [DOI] [PubMed] [Google Scholar]
- Shinowara N. L., Michel M. E., Rapoport S. I. Morphological correlates of permeability in the frog perineurium: vesicles and "transcellular channels". Cell Tissue Res. 1982;227(1):11–22. doi: 10.1007/BF00206328. [DOI] [PubMed] [Google Scholar]
- Smith P. G. Frequency dependence of the frog skin impedance. Biochim Biophys Acta. 1975 Jan 14;375(1):124–129. doi: 10.1016/0005-2736(75)90077-2. [DOI] [PubMed] [Google Scholar]
- Spring K. R., Giebisch G. Tracer Na fluxes in Necturus proximal tubule. Am J Physiol. 1977 May;232(5):F461–F470. doi: 10.1152/ajprenal.1977.232.5.F461. [DOI] [PubMed] [Google Scholar]
- Tomita T. The longitudinal tissue impedance of the smooth muscle of guinea-pig taenia coli. J Physiol. 1969 Mar;201(1):145–159. doi: 10.1113/jphysiol.1969.sp008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ussing H. H. Anomalous transport of electrolytes and sucrose through the isolated frog skin induced by hypertonicity of the outside bathing solution. Ann N Y Acad Sci. 1966 Jul 14;137(2):543–555. doi: 10.1111/j.1749-6632.1966.tb50180.x. [DOI] [PubMed] [Google Scholar]
- Valdiosera R., Clausen C., Eisenberg R. S. Impedance of frog skeletal muscle fibers in various solutions. J Gen Physiol. 1974 Apr;63(4):460–491. doi: 10.1085/jgp.63.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerasuriya A., Rapoport S. I., Taylor R. E. Ionic permeabilities of the frog perineurium. Brain Res. 1980 Jun 9;191(2):405–415. doi: 10.1016/0006-8993(80)91290-1. [DOI] [PubMed] [Google Scholar]
- Weerasuriya A., Rapoport S. I., Taylor R. E. Modification of permeability of frog perineurium to [14C]-sucrose by stretch and hypertonicity. Brain Res. 1979 Sep 21;173(3):503–512. doi: 10.1016/0006-8993(79)90244-0. [DOI] [PubMed] [Google Scholar]


