Abstract
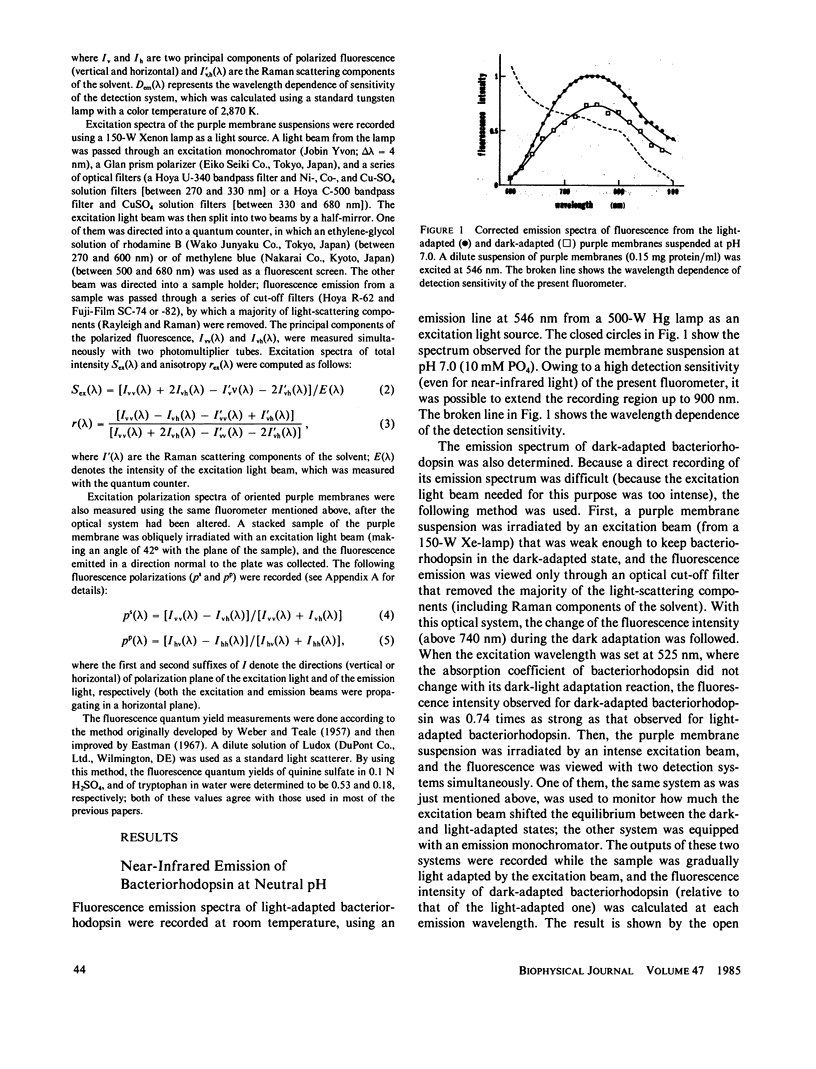
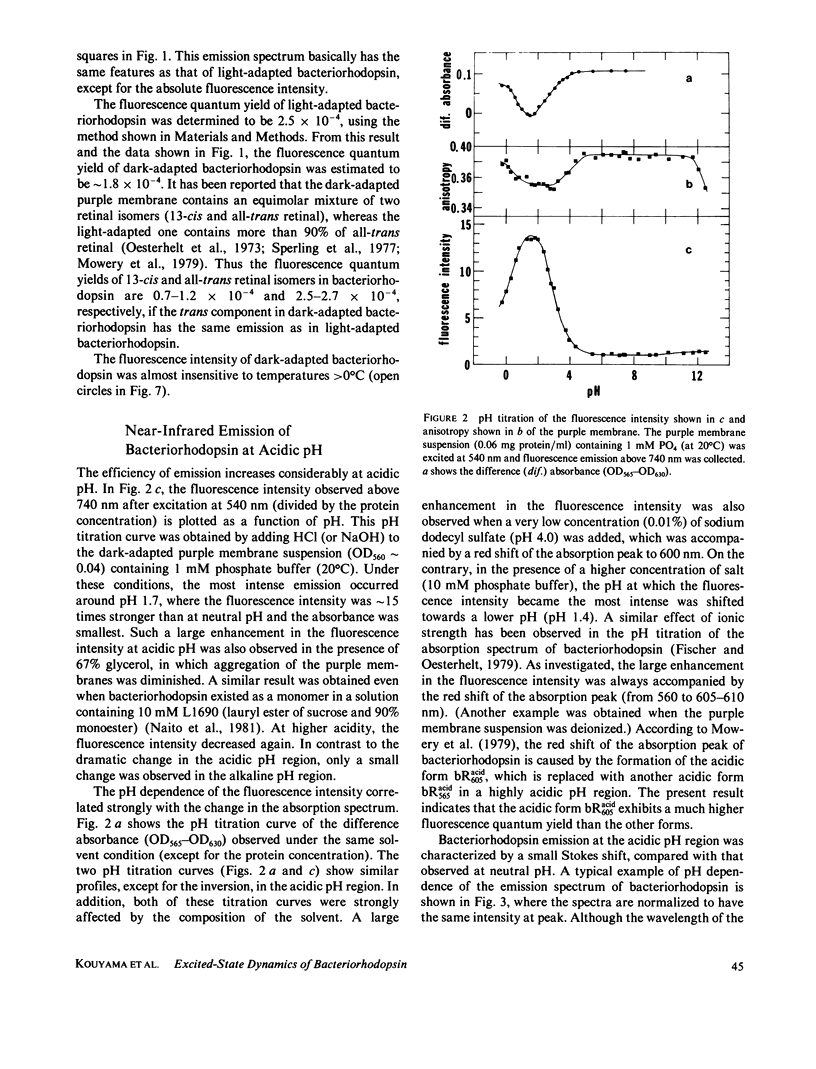
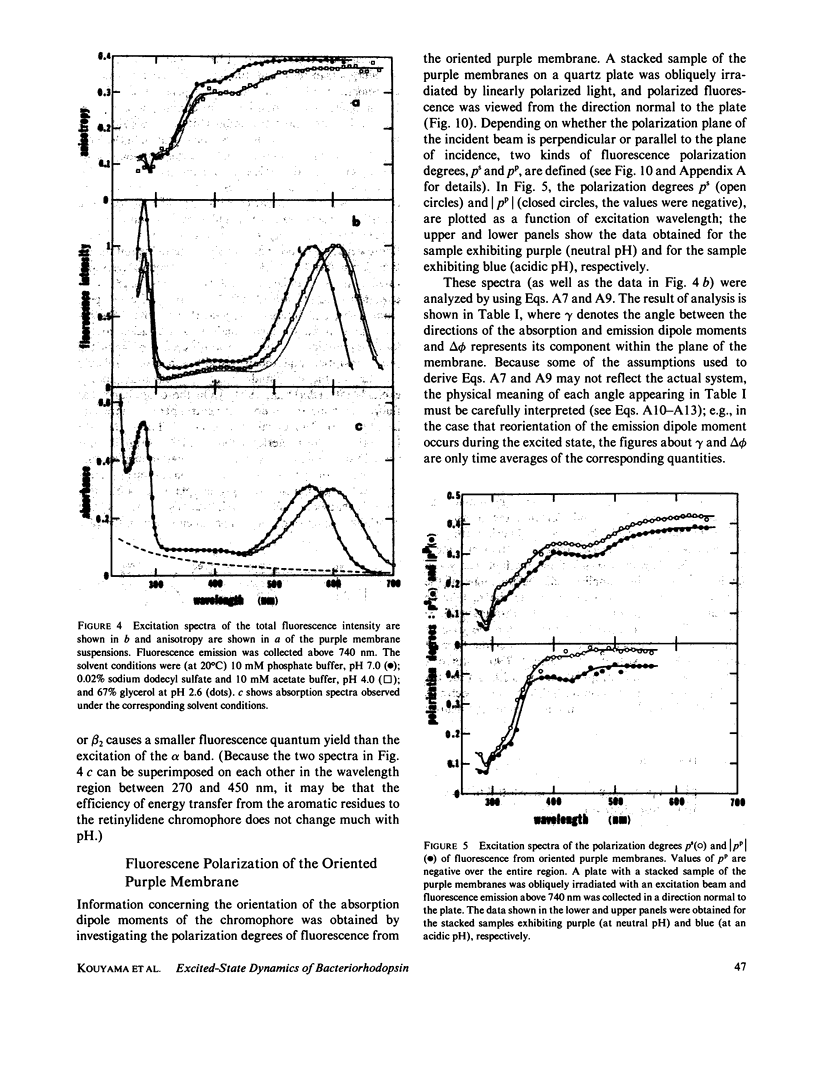
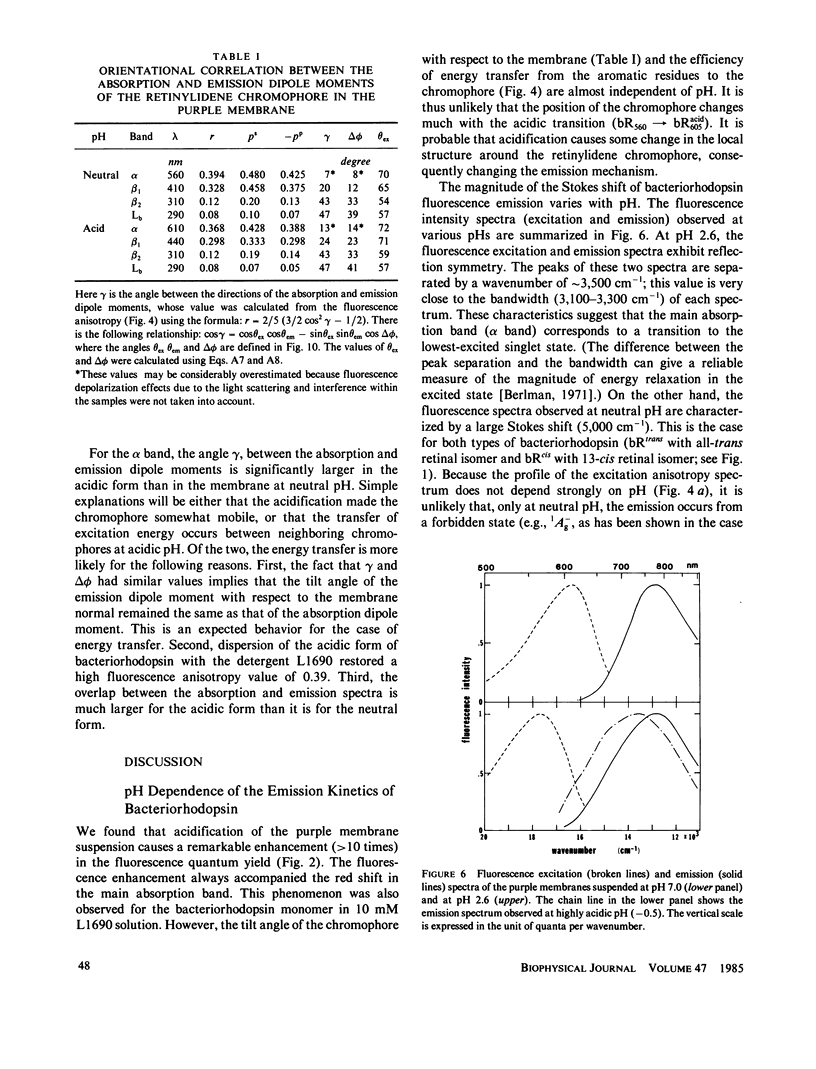
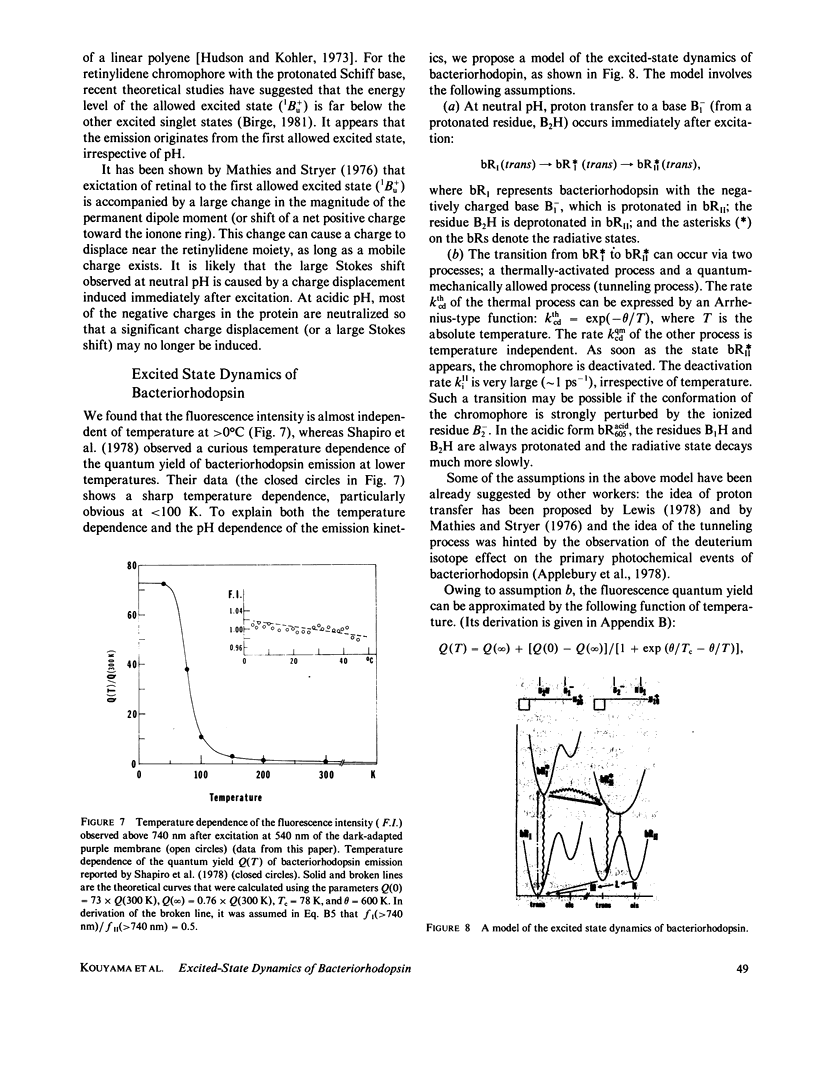
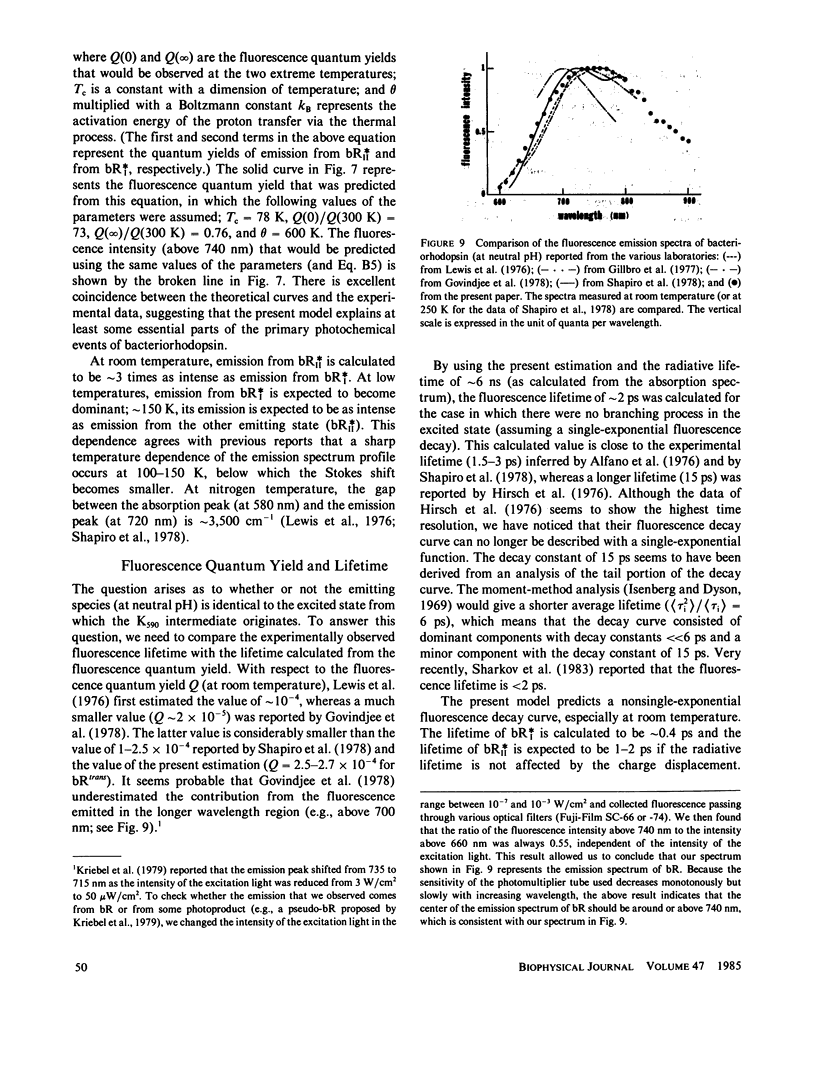
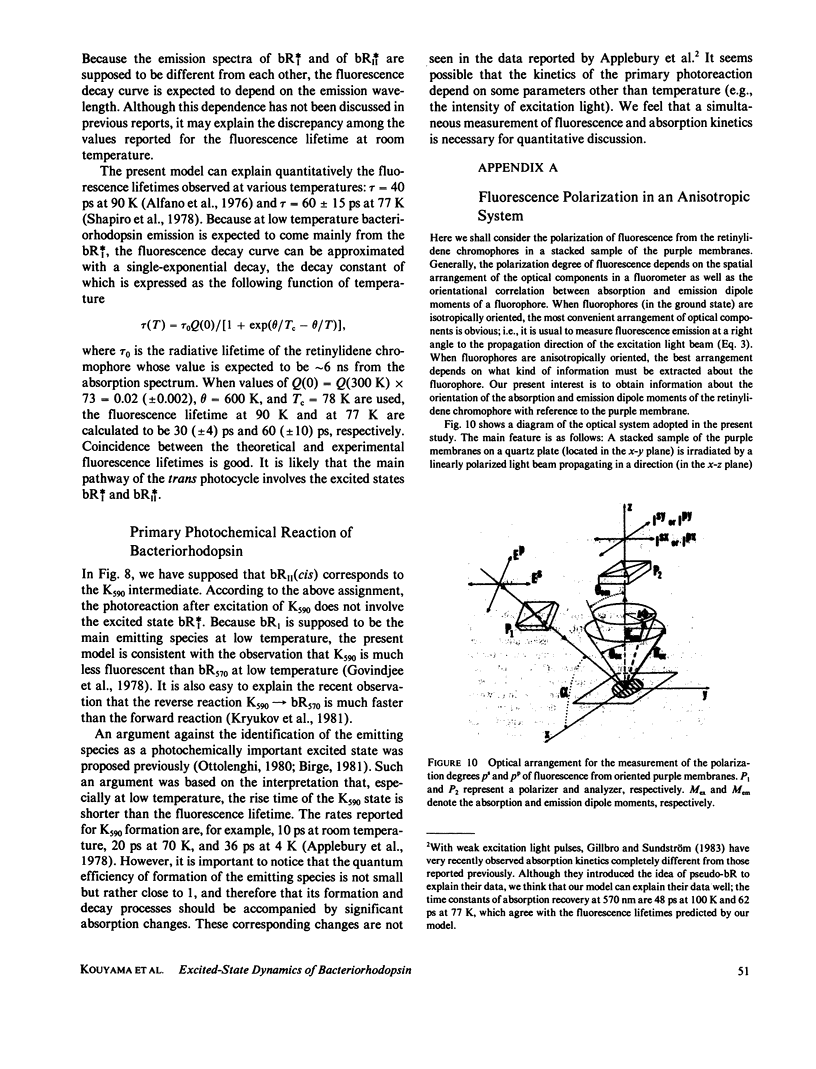
Near infrared emission of bacteriorhodopsin at neutral pH and at room temperature was characterized by a large Stokes shift. This characteristic was lost in an acidic pH (approximately pH 2) where a remarkable enchancement (more than 10 times) in the fluorescence quantum yield accompanied the red shift in the main absorption band. It is suggested from fluorescence polarization measurements that the emission occurs from the first allowed excited state of the retinylidene chromophore, irrespective of pH. We suggest that the large Stokes shift observed at neutral pH is a result of a charge displacement (e.g., proton translocation) that occurs immediately after excitation, and is prevented by protonation (in the ground state) of an amino-acid residue in the protein.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfano R. R., Govindjee R., Becher B., Ebrey T. G. Picosecond kinetics of the fluorescence from the chromophore of the purple membrane protein of Halobacterium halobium. Biophys J. 1976 May;16(5):541–545. doi: 10.1016/S0006-3495(76)85709-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applebury M. L., Peters K. S., Rentzepis P. M. Primary intermediates in the photochemical cycle of bacteriorhodopsin. Biophys J. 1978 Sep;23(3):375–382. doi: 10.1016/S0006-3495(78)85456-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birge R. R. Photophysics of light transduction in rhodopsin and bacteriorhodopsin. Annu Rev Biophys Bioeng. 1981;10:315–354. doi: 10.1146/annurev.bb.10.060181.001531. [DOI] [PubMed] [Google Scholar]
- Fischer U., Oesterhelt D. Chromophore equilibria in bacteriorhodopsin. Biophys J. 1979 Nov;28(2):211–230. doi: 10.1016/S0006-3495(79)85172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillbro T., Kriebel A. N., Wild U. P. On the origin of the red emission of light adapted purple membrane of Halobacterium halobium. FEBS Lett. 1977;78(1):57–60. doi: 10.1016/0014-5793(77)80272-x. [DOI] [PubMed] [Google Scholar]
- Govindjee R., Becher B., Ebrey T. G. The fluorescence from the chromophore of the purple membrane protein. Biophys J. 1978 Apr;22(1):67–77. doi: 10.1016/S0006-3495(78)85471-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R. The structure of the purple membrane from Halobacterium hallobium: analysis of the X-ray diffraction pattern. J Mol Biol. 1975 Apr 5;93(2):123–138. doi: 10.1016/0022-2836(75)90123-0. [DOI] [PubMed] [Google Scholar]
- Hirsch M. D., Marcus M. A., Lewis A., Mahr H., Frigo N. A method for measuring picosecond phenomena in photolabile species: the emission lifetime of bacteriorhodopsin. Biophys J. 1976 Dec;16(12):1399–1409. doi: 10.1016/S0006-3495(76)85783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippen E. P., Shank C. V., Lewis A., Marcus M. A. Subpicosecond spectroscopy of bacteriorhodopsin. Science. 1978 Jun 16;200(4347):1279–1281. doi: 10.1126/science.663607. [DOI] [PubMed] [Google Scholar]
- Isenberg I., Dyson R. D. The analysis of fluorescence decay by a method of moments. Biophys J. 1969 Nov;9(11):1337–1350. doi: 10.1016/S0006-3495(69)86456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann K. J., Rentzepis P. M., Stoeckenius W., Lewis A. Primary photochemical processes in bacteriorhodopsin. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1109–1115. doi: 10.1016/0006-291x(76)90310-7. [DOI] [PubMed] [Google Scholar]
- Kinosita K., Jr, Kataoka R., Kimura Y., Gotoh O., Ikegami A. Dynamic structure of biological membranes as probed by 1,6-diphenyl-1,3,5-hexatriene: a nanosecond fluorescence depolarization study. Biochemistry. 1981 Jul 21;20(15):4270–4277. doi: 10.1021/bi00518a006. [DOI] [PubMed] [Google Scholar]
- Kouyama T., Kimura Y., Kinosita K., Jr, Ikegami A. Location and orientation of the chromophore in bacteriorhodopsin. Analysis by fluorescence energy transfer. J Mol Biol. 1981 Dec 5;153(2):337–359. doi: 10.1016/0022-2836(81)90282-5. [DOI] [PubMed] [Google Scholar]
- Kriebel A. N., Gillbro T., Wild U. P. A low temperature investigation of the intermediates of the photocycle of light-adapted bacteriorhodopsin. Optical absorption and fluorescence measurements. Biochim Biophys Acta. 1979 Apr 11;546(1):106–120. doi: 10.1016/0005-2728(79)90174-9. [DOI] [PubMed] [Google Scholar]
- Lewis A., Spoonhower J. P., Perreault G. J. Observation of light emission from a rhodopsin. Nature. 1976 Apr 22;260(5553):675–678. doi: 10.1038/260675a0. [DOI] [PubMed] [Google Scholar]
- Lewis A. The molecular mechanism of excitation in visual transduction and bacteriorhodopsin. Proc Natl Acad Sci U S A. 1978 Feb;75(2):549–553. doi: 10.1073/pnas.75.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathies R., Stryer L. Retinal has a highly dipolar vertically excited singlet state: implications for vision. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2169–2173. doi: 10.1073/pnas.73.7.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowery P. C., Lozier R. H., Chae Q., Tseng Y. W., Taylor M., Stoeckenius W. Effect of acid pH on the absorption spectra and photoreactions of bacteriorhodopsin. Biochemistry. 1979 Sep 18;18(19):4100–4107. doi: 10.1021/bi00586a007. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Meentzen M., Schuhmann L. Reversible dissociation of the purple complex in bacteriorhodopsin and identification of 13-cis and all-trans-retinal as its chromophores. Eur J Biochem. 1973 Dec 17;40(2):453–463. doi: 10.1111/j.1432-1033.1973.tb03214.x. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Shapiro S. L., Campillo A. J., Lewis A., Perreault G. J., Spoonhower J. P., Clayton R. K., Stoeckenius W. Picosecond and steady state, variable intensity and variable temperature emission spectroscopy of bacteriorhodopsin. Biophys J. 1978 Sep;23(3):383–393. doi: 10.1016/S0006-3495(78)85457-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling W., Carl P., Rafferty Ch, Dencher N. A. Photochemistry and dark equilibrium of retinal isomers and bacteriorhodopsin isomers. Biophys Struct Mech. 1977 Jun 29;3(2):79–94. doi: 10.1007/BF00535798. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]


