Abstract
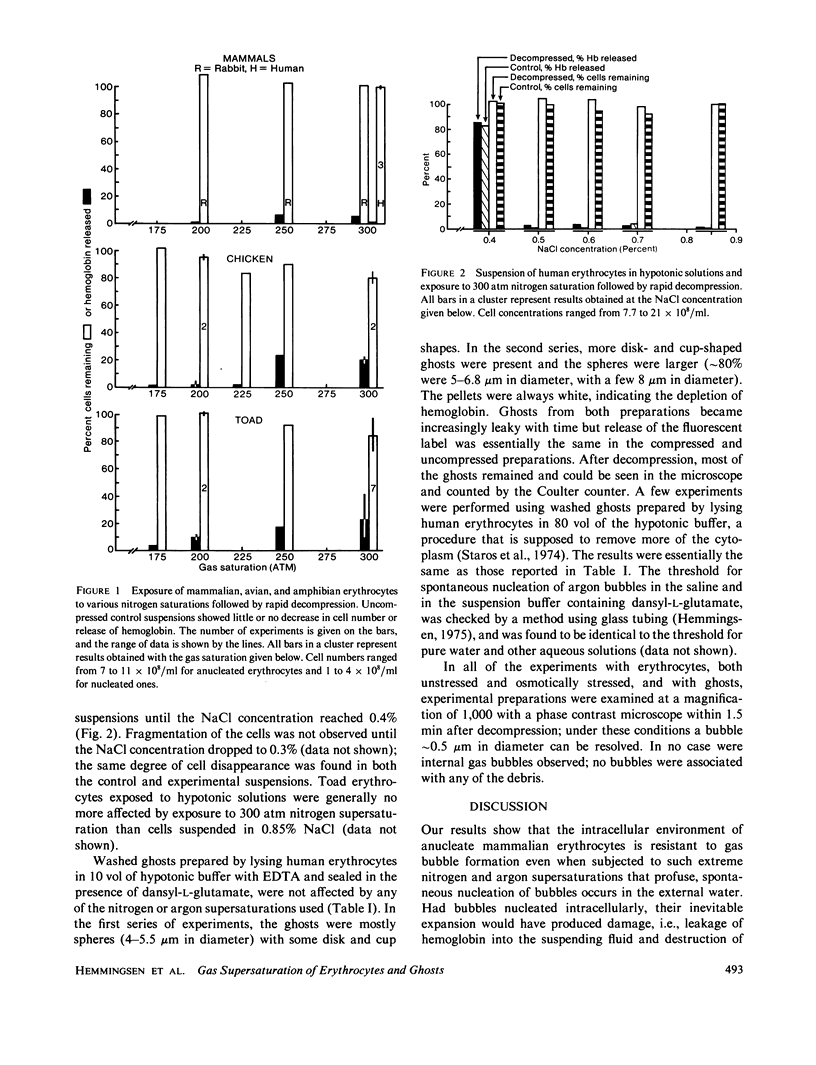
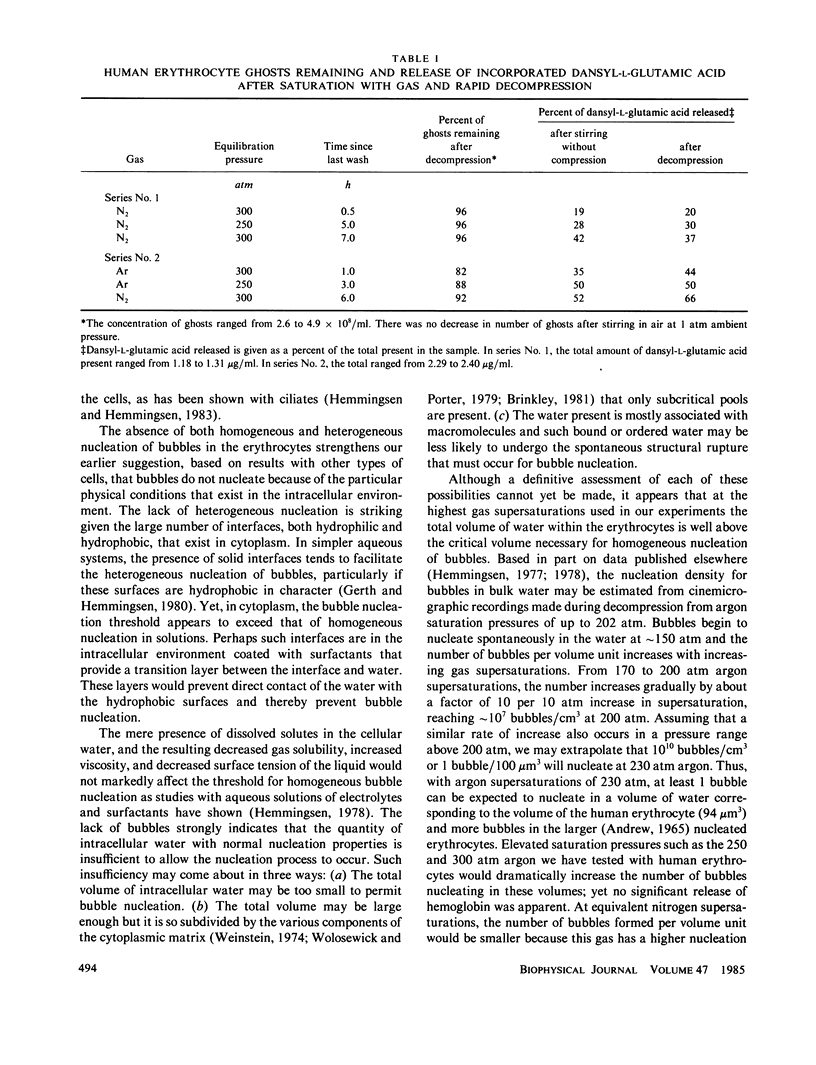
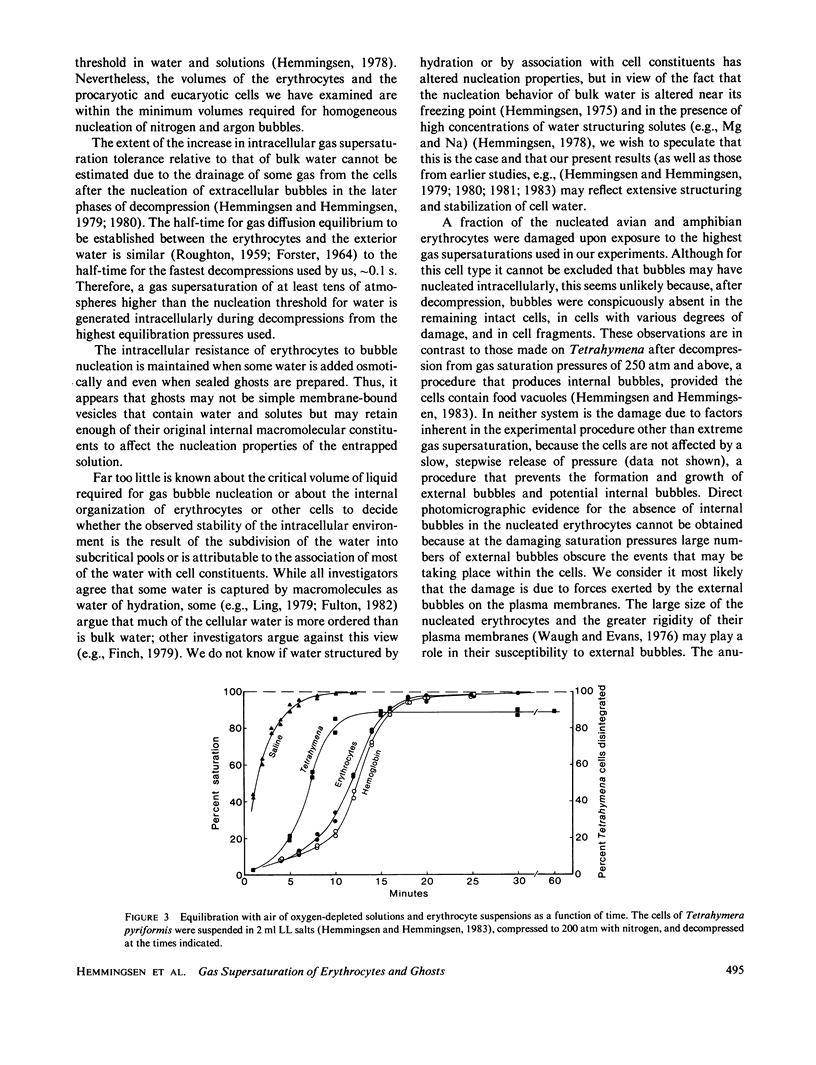
Intact mammalian, avian, and amphibian erythrocytes were saturated with up to 300 atm nitrogen or argon gas and rapidly decompressed. Despite the profuse nucleation of gas bubbles in the suspending fluid, no evidence of intracellular gas bubble nucleation was found; all or most of the cells remained intact and little or no hemoglobin escaped. Internal bubbles were similarly absent from resealed ghosts of human erythrocytes as shown by lack of disintegration and by retention of an entrapped fluorescent compound. The absence of bubbles may indicate that much of the internal water does not have the same nucleation properties as external water.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinkley B. R. Organization of the cytoplasm. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):1029–1040. doi: 10.1101/sqb.1982.046.01.095. [DOI] [PubMed] [Google Scholar]
- Fulton A. B. How crowded is the cytoplasm? Cell. 1982 Sep;30(2):345–347. doi: 10.1016/0092-8674(82)90231-8. [DOI] [PubMed] [Google Scholar]
- Gratzer W. B. The red cell membrane and its cytoskeleton. Biochem J. 1981 Jul 15;198(1):1–8. doi: 10.1042/bj1980001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmingsen B. B., Hemmingsen E. A. Rupture of the cell envelope by induced intracellular gas phase expansion in gas vacuolate bacteria. J Bacteriol. 1980 Aug;143(2):841–846. doi: 10.1128/jb.143.2.841-846.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmingsen B. B., Hemmingsen E. A. Tolerance of bacteria to extreme gas supersaturations. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1379–1384. doi: 10.1016/0006-291x(78)91156-7. [DOI] [PubMed] [Google Scholar]
- Hemmingsen E. A., Hemmingsen B. B. Lack of intracellular bubble formation in microorganisms at very high gas supersaturations. J Appl Physiol Respir Environ Exerc Physiol. 1979 Dec;47(6):1270–1277. doi: 10.1152/jappl.1979.47.6.1270. [DOI] [PubMed] [Google Scholar]
- Hemmingsen E. A. Spontaneous formation of bubbles in gas-supersaturated water. Nature. 1977 May 12;267(5607):141–142. doi: 10.1038/267141a0. [DOI] [PubMed] [Google Scholar]
- Johnson R. M. The kinetics of resealing of washed erythrocyte ghosts. J Membr Biol. 1975 Jul 24;22(3-4):231–253. doi: 10.1007/BF01868173. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T. The red cell membrane skeleton: recent progress. Blood. 1983 Jan;61(1):1–11. [PubMed] [Google Scholar]
- Mohandas N., Clark M. R., Health B. P., Rossi M., Wolfe L. C., Lux S. E., Shohet S. B. A technique to detect reduced mechanical stability of red cell membranes: relevance to elliptocytic disorders. Blood. 1982 Apr;59(4):768–774. [PubMed] [Google Scholar]
- Staros J. V., Haley B. E., Richards F. M. Human erythrocytes and resealed ghosts. A comparison of membrane topology. J Biol Chem. 1974 Aug 10;249(15):5004–5007. [PubMed] [Google Scholar]
- Waugh R., Evans E. A. Viscoelastic properties of erythrocyte membranes of different vertebrate animals. Microvasc Res. 1976 Nov;12(3):291–304. doi: 10.1016/0026-2862(76)90027-3. [DOI] [PubMed] [Google Scholar]
- Wolosewick J. J., Porter K. R. Microtrabecular lattice of the cytoplasmic ground substance. Artifact or reality. J Cell Biol. 1979 Jul;82(1):114–139. doi: 10.1083/jcb.82.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]


