Abstract
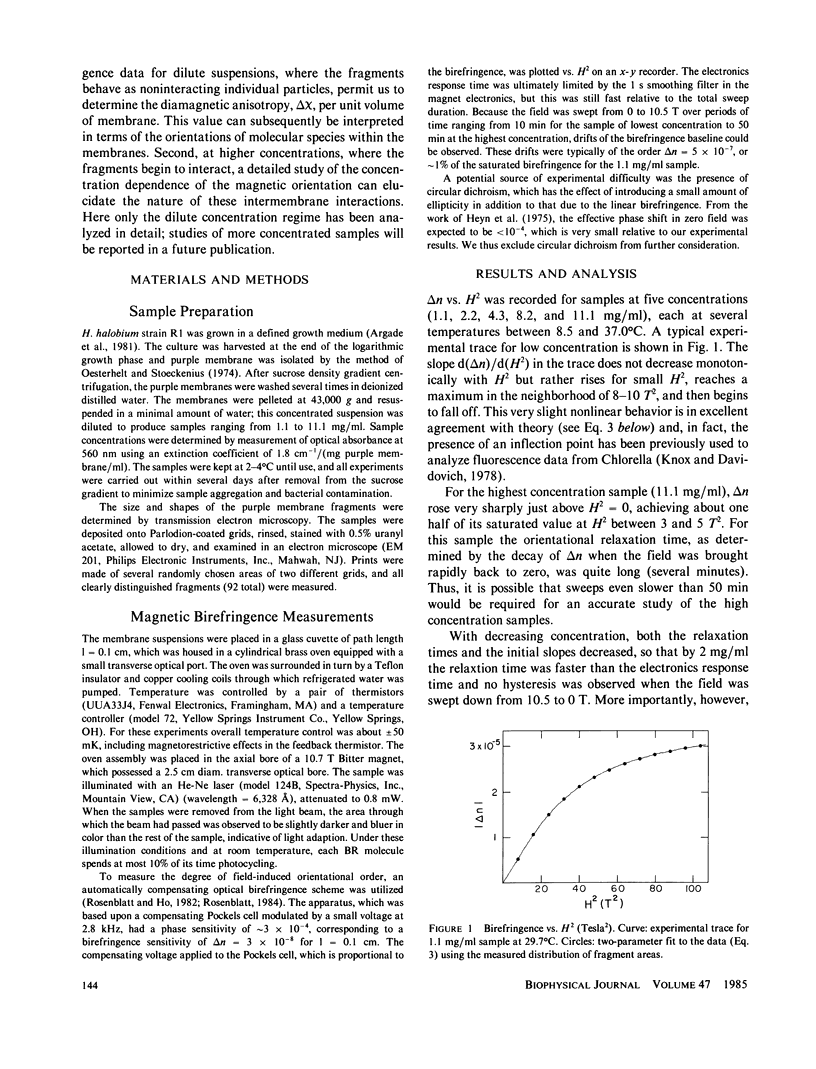
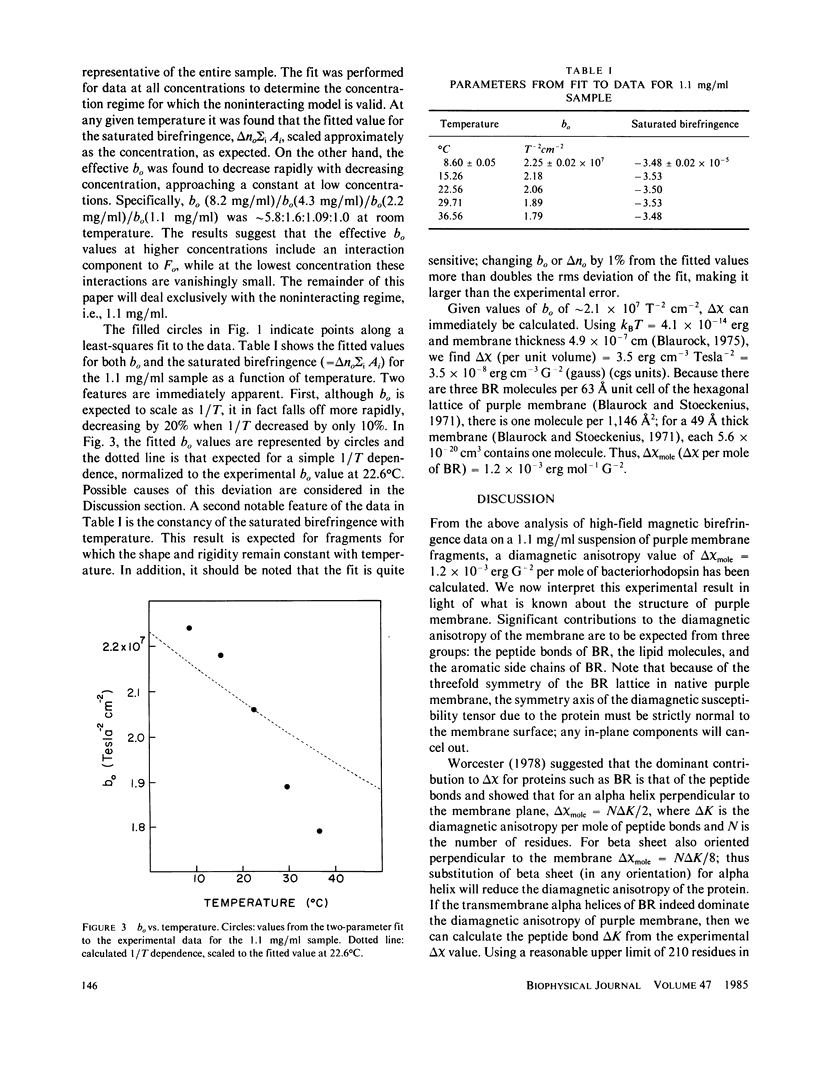
We have observed the magnetically induced orientation of purple membrane suspensions by measuring the birefringence as a function of concentration and temperature at fields up to 10.5 Tesla (T). At these fields, the orientation approaches saturation even in dilute solutions; therefore, the birefringence data, together with an estimate of the membrane size distribution obtained from electron microscopy, permits one to determine the diamagnetic susceptibility anisotropy. We find delta chi mole = 1.2 +/- 0.3 X 10(-3) erg G-2mol-1 of bacteriorhodopsin. If delta chi were due only to the oriented peptide bonds of the transmembrane alpha helices, this experimental value would indicate that delta K, the anisotropy per mole of peptide bonds, is considerably larger than previously suggested. On the other hand, the large value for delta chi mole of bacteriorhodopsin can also be explained by a net orientation of the aromatic amino acid side chains of bacteriorhodopsin with their planes perpendicular to the membrane surface. In addition, the present data analysis demonstrates the critical dependence of the calculated delta chi value on the values for the membrane size distribution.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argade P. V., Rothschild K. J., Kawamoto A. H., Herzfeld J., Herlihy W. C. Resonance Raman spectroscopy of specifically [epsilon-15N]lysine-labeled bacteriorhodopsin. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1643–1646. doi: 10.1073/pnas.78.3.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B., Tokunaga F., Ebrey T. G. Ultraviolet and visible absorption spectra of the purple membrane protein and the photocycle intermediates. Biochemistry. 1978 Jun 13;17(12):2293–2300. doi: 10.1021/bi00605a006. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E. Bacteriorhodospin: a trans-membrane pump containing alpha-helix. J Mol Biol. 1975 Apr 5;93(2):139–158. doi: 10.1016/0022-2836(75)90124-2. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E., Stoeckenius W. Structure of the purple membrane. Nat New Biol. 1971 Sep 29;233(39):152–155. doi: 10.1038/newbio233152a0. [DOI] [PubMed] [Google Scholar]
- Bogomolni R. A., Stubbs L., Lanyi J. K. Illumination-dependent changes in the intrinsic fluorescence of bacteriorhodopsin. Biochemistry. 1978 Mar 21;17(6):1037–1041. doi: 10.1021/bi00599a015. [DOI] [PubMed] [Google Scholar]
- Boroske E., Helfrich W. Magnetic anisotropy of egg lecithin membranes. Biophys J. 1978 Dec;24(3):863–868. doi: 10.1016/S0006-3495(78)85425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brith-Linder M., Rosenheck K. The circular dichroism of bacteriorhodopsin: asymmetry and light-scattering distortions. FEBS Lett. 1977 Apr 1;76(1):41–44. doi: 10.1016/0014-5793(77)80116-6. [DOI] [PubMed] [Google Scholar]
- Clark N. A., Rothschild K. J., Luippold D. A., Simon B. A. Surface-induced lamellar orientation of multilayer membrane arrays. Theoretical analysis and a new method with application to purple membrane fragments. Biophys J. 1980 Jul;31(1):65–96. doi: 10.1016/S0006-3495(80)85041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekiel I., Marsh D., Smallbone B. W., Kates M., Smith I. C. The state of the lipids in the purple membrane of Halobacterium cutirubrum as seen by 31P NMR. Biochem Biophys Res Commun. 1981 May 15;100(1):105–110. doi: 10.1016/s0006-291x(81)80069-1. [DOI] [PubMed] [Google Scholar]
- Henderson R. The purple membrane from Halobacterium halobium. Annu Rev Biophys Bioeng. 1977;6:87–109. doi: 10.1146/annurev.bb.06.060177.000511. [DOI] [PubMed] [Google Scholar]
- Henderson R. The structure of the purple membrane from Halobacterium hallobium: analysis of the X-ray diffraction pattern. J Mol Biol. 1975 Apr 5;93(2):123–138. doi: 10.1016/0022-2836(75)90123-0. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Hess B., Kuschmitz D. Kinetic interaction between aromatic residues and the retinal chromophore of bacteriorhodopsin during the photocycle. FEBS Lett. 1979 Apr 15;100(2):334–340. doi: 10.1016/0014-5793(79)80364-6. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Bauer P. J., Dencher N. A. A natural CD label to probe the structure of the purple membrane from Halobacterium halobium by means of exciton coupling effects. Biochem Biophys Res Commun. 1975 Dec 1;67(3):897–903. doi: 10.1016/0006-291x(75)90761-5. [DOI] [PubMed] [Google Scholar]
- Hoffmann W., Graca-Miguel M., Barnard P., Chapman D. Evidence for conformational transitions in bacteriorhodopsin. FEBS Lett. 1978 Nov 1;95(1):31–34. doi: 10.1016/0014-5793(78)80045-3. [DOI] [PubMed] [Google Scholar]
- Jap B. K., Maestre M. F., Hayward S. B., Glaeser R. M. Peptide-chain secondary structure of bacteriorhodopsin. Biophys J. 1983 Jul;43(1):81–89. doi: 10.1016/S0006-3495(83)84326-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorana H. G., Gerber G. E., Herlihy W. C., Gray C. P., Anderegg R. J., Nihei K., Biemann K. Amino acid sequence of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5046–5050. doi: 10.1073/pnas.76.10.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox R. S., Davidovich M. A. Theory of fluorescence polarization in magnetically oriented photosynthetic systems. Biophys J. 1978 Dec;24(3):689–712. doi: 10.1016/S0006-3495(78)85414-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer D. C., Blaurock A. E. Magnetic orientation of purple membranes demonstrated by optical measurements and neutron scattering. FEBS Lett. 1977;78(1):31–35. doi: 10.1016/0014-5793(77)80266-4. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Abdulaev N. G., Feigina M. Y., Kiselev A. V., Lobanov N. A. The structural basis of the functioning of bacteriorhodopsin: an overview. FEBS Lett. 1979 Apr 15;100(2):219–224. doi: 10.1016/0014-5793(79)80338-5. [DOI] [PubMed] [Google Scholar]
- Pauling L. Diamagnetic anisotropy of the peptide group. Proc Natl Acad Sci U S A. 1979 May;76(5):2293–2294. doi: 10.1073/pnas.76.5.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai I., Kawamura Y., Ikegami A., Iwayanagi S. Magneto-orientation of lecithin crystals. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7232–7236. doi: 10.1073/pnas.77.12.7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz F., Boroske E., Helfrich W. Magnetic anisotropy of lecithin membranes. A new anisotropy susceptometer. Biophys J. 1984 Mar;45(3):589–592. doi: 10.1016/S0006-3495(84)84196-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Torbet J., Maret G. High-field magnetic birefringence study of the structure of rodlike phages Pf1 and fd in solution. Biopolymers. 1981 Dec;20(12):2657–2669. doi: 10.1002/bip.1981.360201212. [DOI] [PubMed] [Google Scholar]
- Worcester D. L. Structural origins of diamagnetic anisotropy in proteins. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5475–5477. doi: 10.1073/pnas.75.11.5475. [DOI] [PMC free article] [PubMed] [Google Scholar]


