Abstract
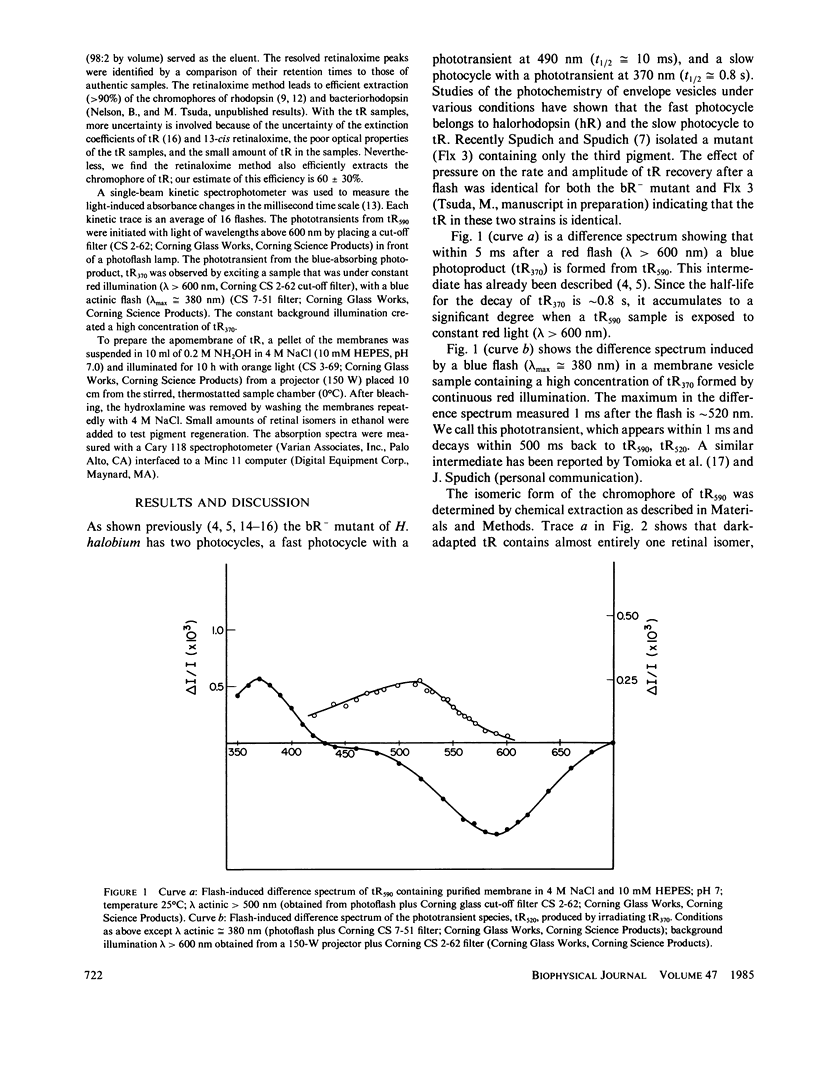
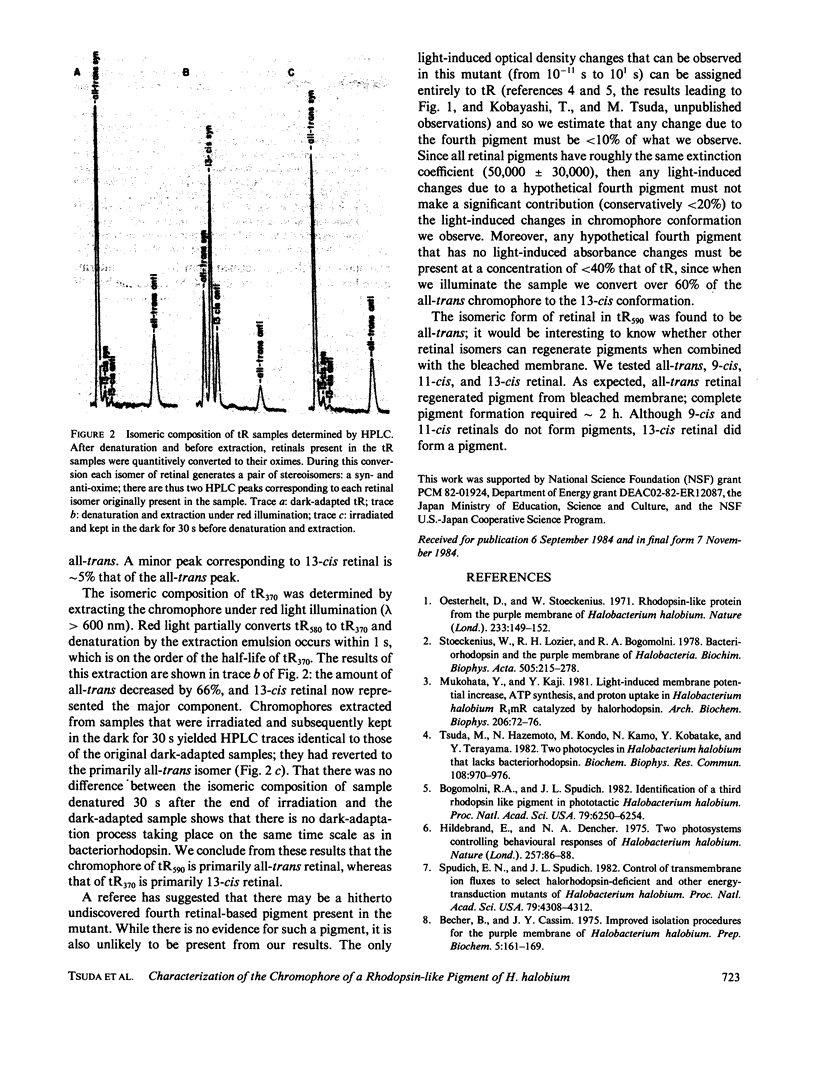
Halobacterium halobium contains at least three retinal-containing pigments: bacteriorhodopsin, halorhodopsin, and a third rhodopsin-like pigment (tR) absorbing at approximately 590 nm, tR590. Illumination of tR590 gives rise to a very long-lived blue absorbing photoproduct, tR370. Using high-performance liquid chromatography we show that the chromophore of tR590 is primarily all-trans retinal and its conversion by light to tR370 causes the chromophore to isomerize primarily to the 13-cis conformation. Irradiation of the tR370 gives rise to a transient photoproduct absorbing at approximately 520 nm that decays back to the initial pigment tR590. In addition to all-trans retinal, the apomembrane of tR can also combine with 13-cis retinal but not with the 9- or 11-cis isomers.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becher B. M., Cassim J. Y. Improved isolation procedures for the purple membrane of Halobacterium halobium. Prep Biochem. 1975;5(2):161–178. doi: 10.1080/00327487508061568. [DOI] [PubMed] [Google Scholar]
- Bogomolni R. A., Spudich J. L. Identification of a third rhodopsin-like pigment in phototactic Halobacterium halobium. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6250–6254. doi: 10.1073/pnas.79.20.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindjee R., Ebrey T. G., Crofts A. R. The quantum efficiency of proton pumping by the purple membrane of Halobacterium halobium. Biophys J. 1980 May;30(2):231–242. doi: 10.1016/S0006-3495(80)85091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenendijk G. W., de Grip W. J., Daemen F. J. Identification and characterization of syn- and anti-isomers of retinaloximes. Anal Biochem. 1979 Nov 1;99(2):304–310. doi: 10.1016/s0003-2697(79)80011-1. [DOI] [PubMed] [Google Scholar]
- Hazemoto N., Kamo N., Kobatake Y., Tsuda M., Terayama Y. Effect of salt on photocycle and ion-pumping of halorhodopsin and third rhodopsinlike pigment of Halobacterium halobium. Biophys J. 1984 Jun;45(6):1073–1077. doi: 10.1016/S0006-3495(84)84254-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazemoto N., Kamo N., Terayama Y., Kobatake Y., Tsuda M. Photochemistry of two rhodopsinlike pigments in bacteriorhodopsin-free mutant of Halobacterium halobium. Biophys J. 1983 Oct;44(1):59–64. doi: 10.1016/S0006-3495(83)84277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukohata Y., Kaji Y. Light-induced membrane-potential increase, ATP synthesis, and proton uptake in Halobacterium halobium, R1mR catalyzed by halorhodopsin: Effects of N,N'-dicyclohexylcarbodiimide, triphenyltin chloride, and 3,5-di-tert-butyl-4-hydroxybenzylidenemalononitrile (SF6847). Arch Biochem Biophys. 1981 Jan;206(1):72–76. doi: 10.1016/0003-9861(81)90067-9. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Spudich E. N., Spudich J. L. Control of transmembrane ion fluxes to select halorhodopsin-deficient and other energy-transduction mutants of Halobacterium halobium. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4308–4312. doi: 10.1073/pnas.79.14.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. L., Bogomolni R. A. Spectroscopic discrimination of the three rhodopsinlike pigments in Halobacterium halobium membranes. Biophys J. 1983 Aug;43(2):243–246. doi: 10.1016/S0006-3495(83)84345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]
- Tomioka H., Kamo N., Takahashi T., Kobatake Y. Photochemical intermediate of third rhodopsin-like pigment in Halobacterium halobium by simultaneous illumination with red and blue light. Biochem Biophys Res Commun. 1984 Sep 28;123(3):989–994. doi: 10.1016/s0006-291x(84)80231-4. [DOI] [PubMed] [Google Scholar]
- Tsuda M., Ebrey T. G. Effect of high pressure on the absorption spectrum and isomeric composition of bacteriorhodopsin. Biophys J. 1980 Apr;30(1):149–157. doi: 10.1016/S0006-3495(80)85083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M., Glaccum M., Nelson B., Ebrey T. G. Light isomerizes the chromophore of bacteriorhodopsin. Nature. 1980 Sep 25;287(5780):351–353. doi: 10.1038/287351a0. [DOI] [PubMed] [Google Scholar]
- Tsuda M., Hazemoto N., Kondo M., Kamo N., Kobatake Y., Terayama Y. Two photocycles in halobacterium halobium that lacks bacteriorhodopsin. Biochem Biophys Res Commun. 1982 Oct 15;108(3):970–976. doi: 10.1016/0006-291x(82)92094-0. [DOI] [PubMed] [Google Scholar]


