Abstract
A model for random cross-linking of identical monomers diffusing in a membrane was formulated to test whether rhodopsin's cross-linking behavior was quantitatively consistent with a monomeric structure. Cross-linking was performed on rhodopsin both in intact retinas and in isolated rod outer segment (ROS) membranes using the reagent glutaraldehyde. The distribution of covalent oligomers formed was analyzed by SDS-polyacrylamide gel electrophoresis and compared to predictions for the random model. A similar analysis was made for ROS membranes cross-linked by diisocyanatohexane and retinas cross-linked by cupric ion complexed with o-phenanthroline. Patterns of cross-linking produced by these three reagents are reasonably consistent with the monomer model. Glutaraldehyde was also used to cross-link the tetrameric protein aldolase in order to verify that cross-linking of a stable oligomer, under conditions comparable to those used for ROS, yielded the pattern predicted for a tetrameric protein having D2 symmetry. This pattern is markedly different from the one for a random-collision model. Moreover, a comparison of rates showed that aldolase cross-linking with glutaraldehyde is significantly faster than cross-linking of membrane-bound rhodopsin. It is concluded that rhodopsin is monomeric in dark-adapted photoreceptor membranes and that the observed cross-linking results from collisions between diffusing rhodopsin molecules.
Full text
PDF

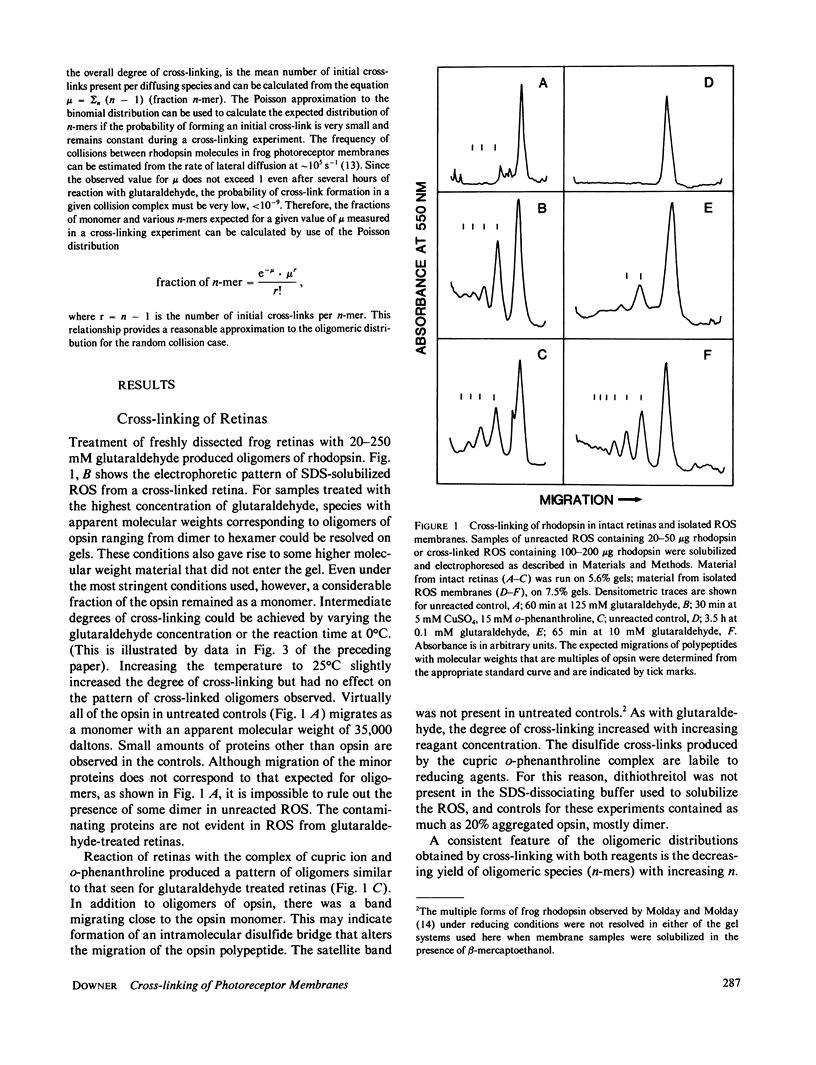
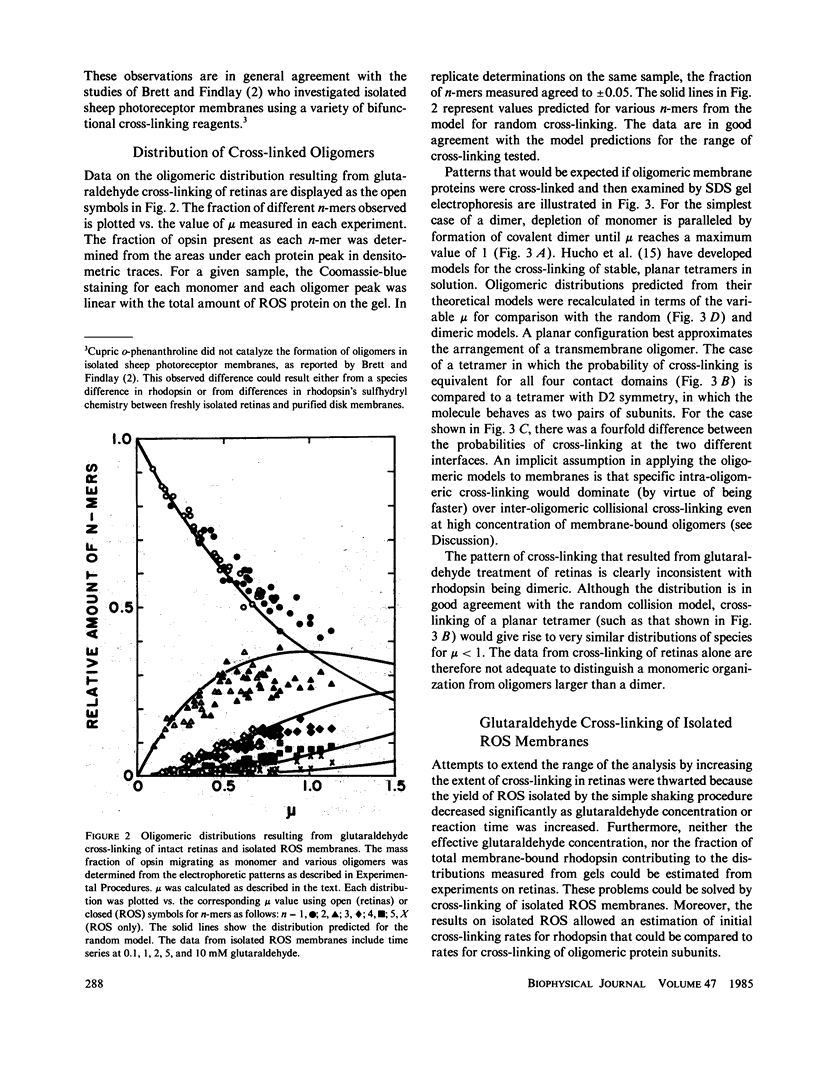
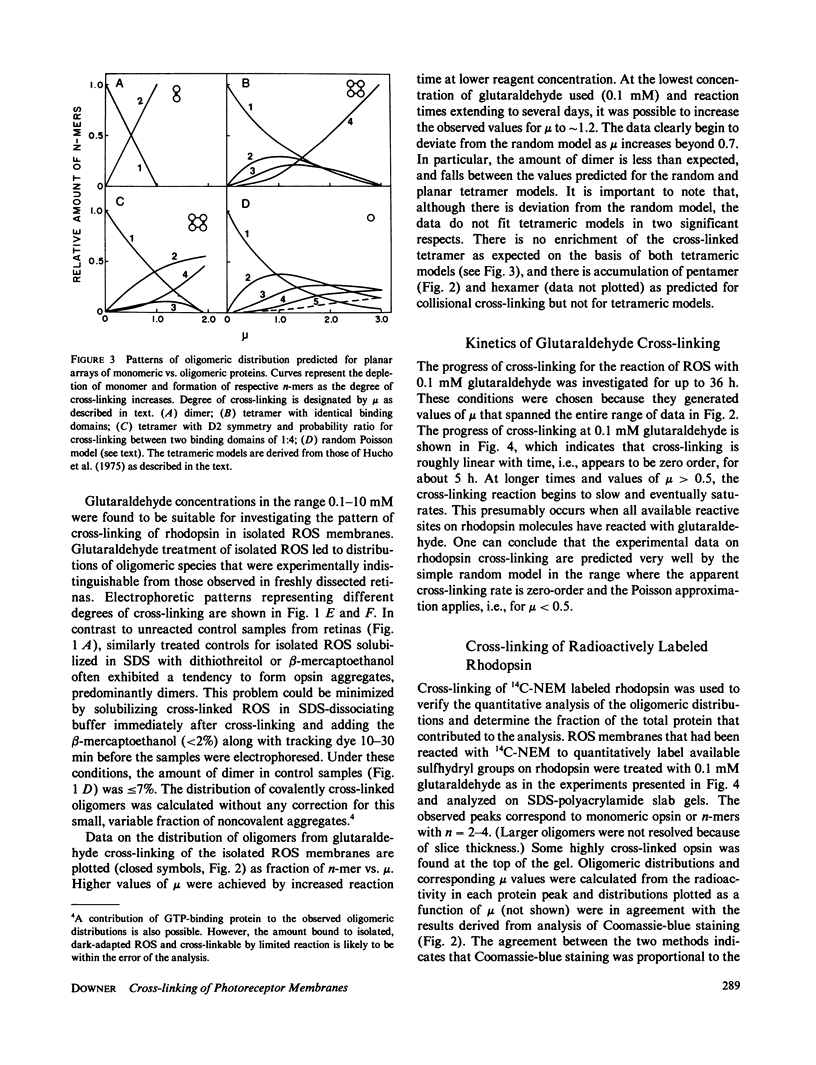
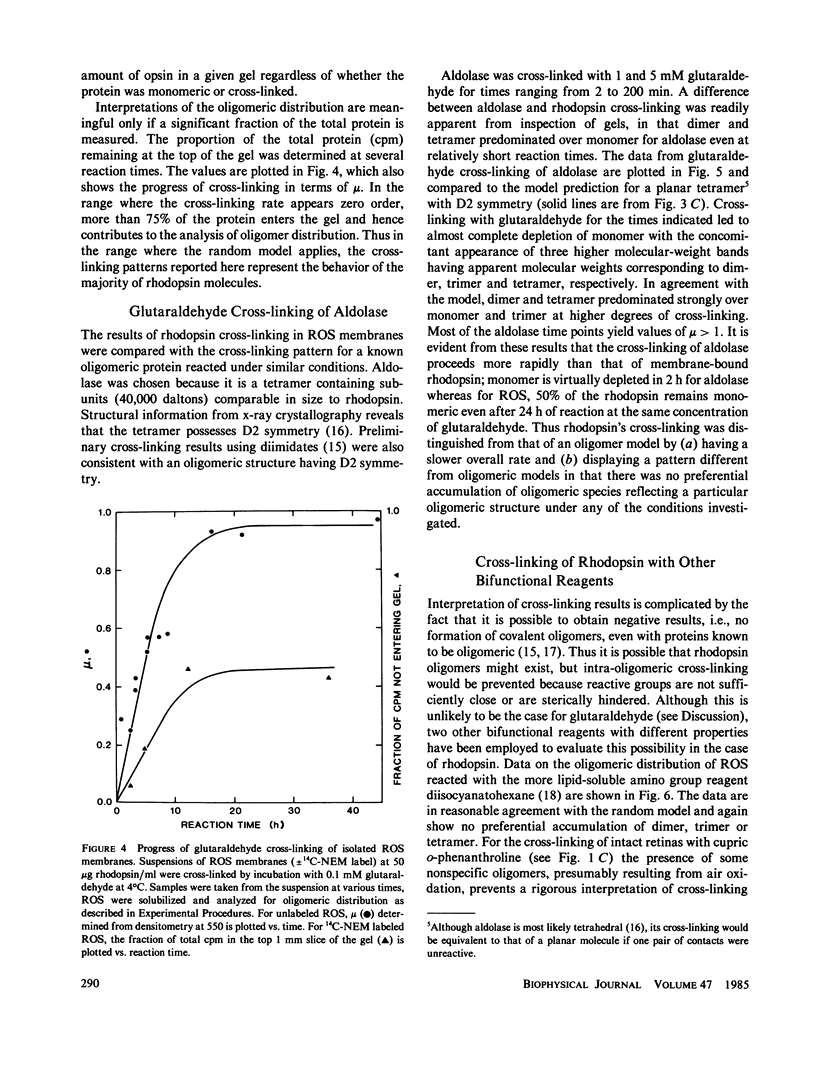


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blasie J. K., Worthington C. R. Molecular localization of frog retinal receptor photopigment by electron microscopy and low-angle X-ray diffraction. J Mol Biol. 1969 Feb 14;39(3):407–416. doi: 10.1016/0022-2836(69)90135-1. [DOI] [PubMed] [Google Scholar]
- Borochov-Neori H., Fortes P. A., Montal M. Rhodopsin in reconstituted phospholipid vesicles. 2. Rhodopsin-rhodopsin interactions detected by resonance energy transfer. Biochemistry. 1983 Jan 4;22(1):206–213. doi: 10.1021/bi00270a030. [DOI] [PubMed] [Google Scholar]
- Brett M., Findlay J. B. Investigation of the organization of rhodopsin in the sheep photoreceptor membrane by using cross-linking reagents. Biochem J. 1979 Jan 1;177(1):215–223. doi: 10.1042/bj1770215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. S., Hubbell W. L. Temperature- and light-dependent structural changes in rhodopsin-lipid membranes. Exp Eye Res. 1973 Dec 24;17(6):517–532. doi: 10.1016/0014-4835(73)90082-1. [DOI] [PubMed] [Google Scholar]
- Corless J. M., McCaslin D. R., Scott B. L. Two-dimensional rhodopsin crystals from disk membranes of frog retinal rod outer segments. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1116–1120. doi: 10.1073/pnas.79.4.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer N. W., Cone R. A. Transient dichroism in photoreceptor membranes indicates that stable oligomers of rhodopsin do not form during excitation. Biophys J. 1985 Mar;47(3):277–284. doi: 10.1016/S0006-3495(85)83917-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer N. W., Englander S. W. Hydrogen exchange study of membrane-bound rhodopsin. I. Protein structure. J Biol Chem. 1977 Nov 25;252(22):8092–8100. [PubMed] [Google Scholar]
- Eagles P. A., Johnson L. N., Joynson M. A., McMurray C. H., Gutfreund H. Subunit structure of aldolase: chemical and crystallographic evidence. J Mol Biol. 1969 Nov 14;45(3):533–544. doi: 10.1016/0022-2836(69)90310-6. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fatt P. Proteins of vertebrate rod outer segments: a possible role for multiple forms of rhodopsin. Exp Eye Res. 1981 Jul;33(1):31–46. doi: 10.1016/s0014-4835(81)80079-6. [DOI] [PubMed] [Google Scholar]
- Fung B. K., Hubbell W. L. Organization of rhodopsin in photoreceptor membranes. 1. Proteolysis of bovine rhodopsin in native membranes and the distribution of sulfhydryl groups in the fragments. Biochemistry. 1978 Oct 17;17(21):4396–4402. doi: 10.1021/bi00614a007. [DOI] [PubMed] [Google Scholar]
- Hucho F., Müllner H., Sund H. Investigation of the symmetry of oligomeric enzymes with bifunctional reagents. Eur J Biochem. 1975 Nov 1;59(1):79–87. doi: 10.1111/j.1432-1033.1975.tb02427.x. [DOI] [PubMed] [Google Scholar]
- Kiehm D. J., Ji T. H. Photochemical cross-linking of cell membranes. A test for natural and random collisional cross-links by millisecond cross-linking. J Biol Chem. 1977 Dec 10;252(23):8524–8531. [PubMed] [Google Scholar]
- Knight P. Hydrolysis of p-NN'-phenylenebismaleimide and its adducts with cysteine. Implications for cross-linking of proteins. Biochem J. 1979 Apr 1;179(1):191–197. doi: 10.1042/bj1790191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight P., Offer G. p-NN'-phenylenebismaleimide, a specific cross-linking agent for F-actin. Biochem J. 1978 Dec 1;175(3):1023–1032. doi: 10.1042/bj1751023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaslin D. R., Tanford C. Different states of aggregation for unbleached and bleached rhodopsin after isolation in two different detergents. Biochemistry. 1981 Sep 1;20(18):5212–5221. doi: 10.1021/bi00521a018. [DOI] [PubMed] [Google Scholar]
- Molday R. S., Molday L. L. Identification and characterization of multiple forms of rhodopsin and minor proteins in frog and bovine rod outer segment disc membranes. Electrophoresis, lectin labeling, and proteolysis studies. J Biol Chem. 1979 Jun 10;254(11):4653–4660. [PubMed] [Google Scholar]
- Monsan P., Puzo G., Mazarguil H. Etude du mécanisme d'établissement des liaisons glutaraldéhyde-protéines. Biochimie. 1975 Nov-Dec;57(11-12):1281–1292. [PubMed] [Google Scholar]
- Montal M. Rhodopsin in model membranes. Biochim Biophys Acta. 1979 Aug 20;559(2-3):231–257. doi: 10.1016/0304-4157(79)90003-0. [DOI] [PubMed] [Google Scholar]
- Peters K., Richards F. M. Chemical cross-linking: reagents and problems in studies of membrane structure. Annu Rev Biochem. 1977;46:523–551. doi: 10.1146/annurev.bi.46.070177.002515. [DOI] [PubMed] [Google Scholar]
- Poo M., Cone R. A. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974 Feb 15;247(5441):438–441. doi: 10.1038/247438a0. [DOI] [PubMed] [Google Scholar]
- Steck T. L. Cross-linking the major proteins of the isolated erythrocyte membrane. J Mol Biol. 1972 May 14;66(2):295–305. doi: 10.1016/0022-2836(72)90481-0. [DOI] [PubMed] [Google Scholar]


