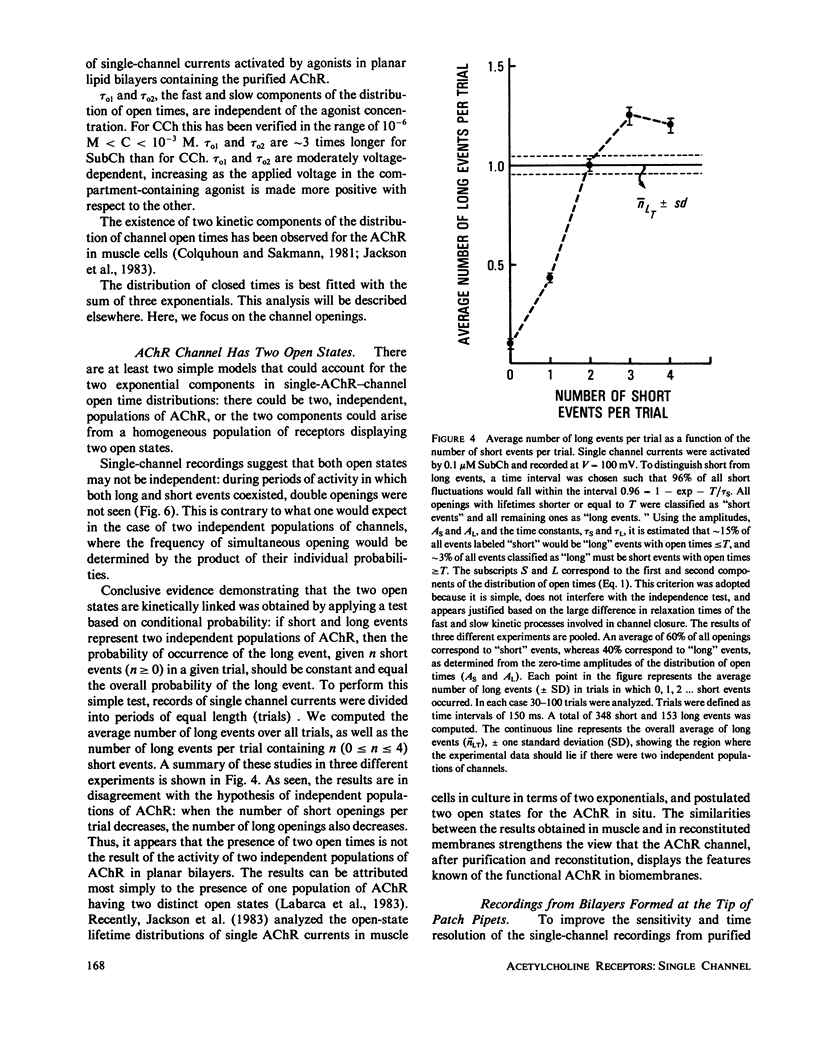
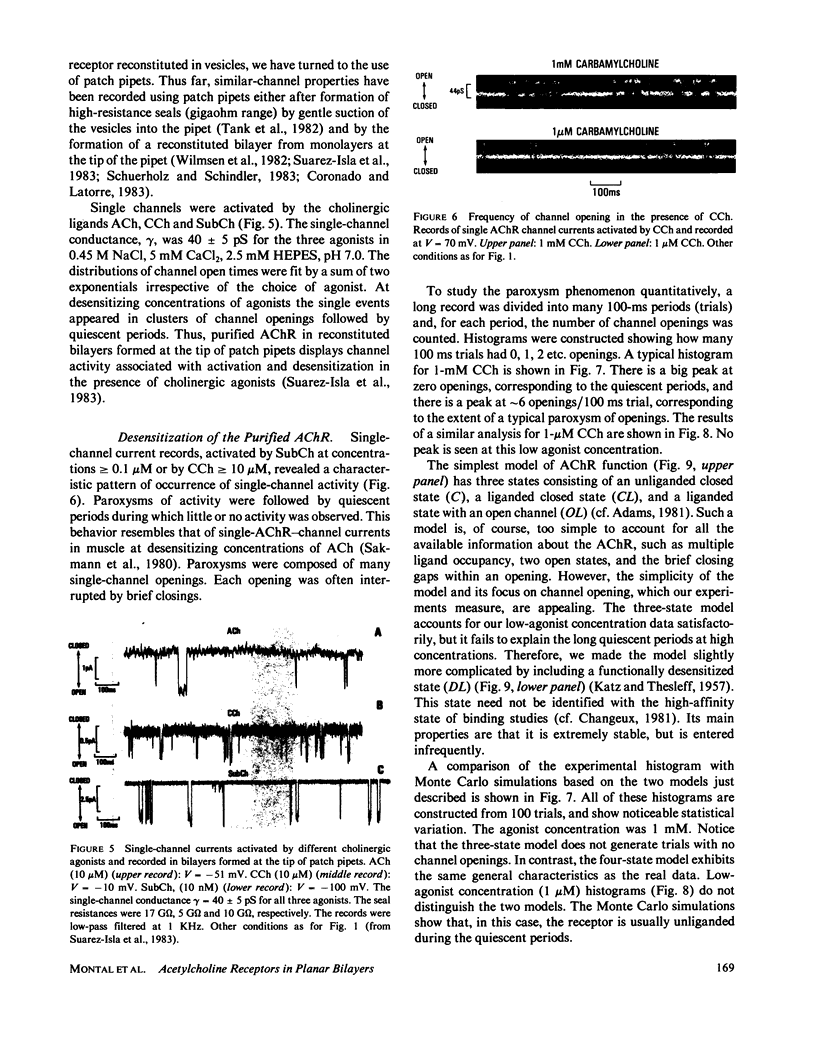
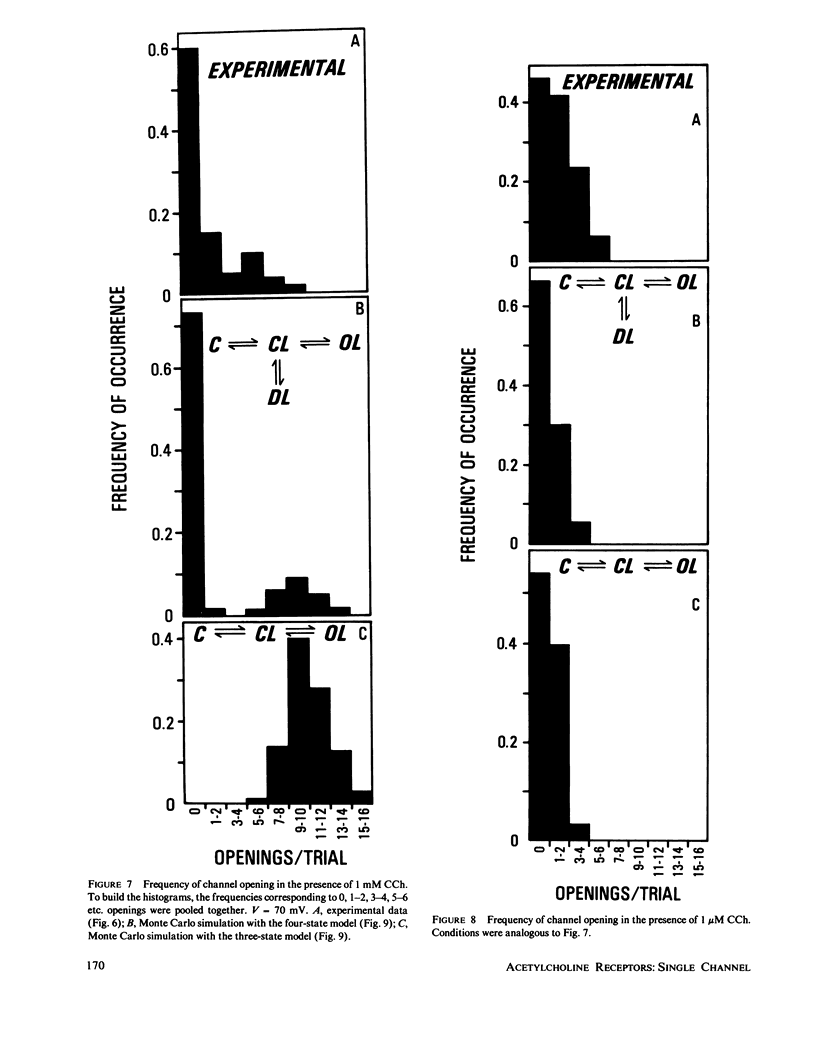
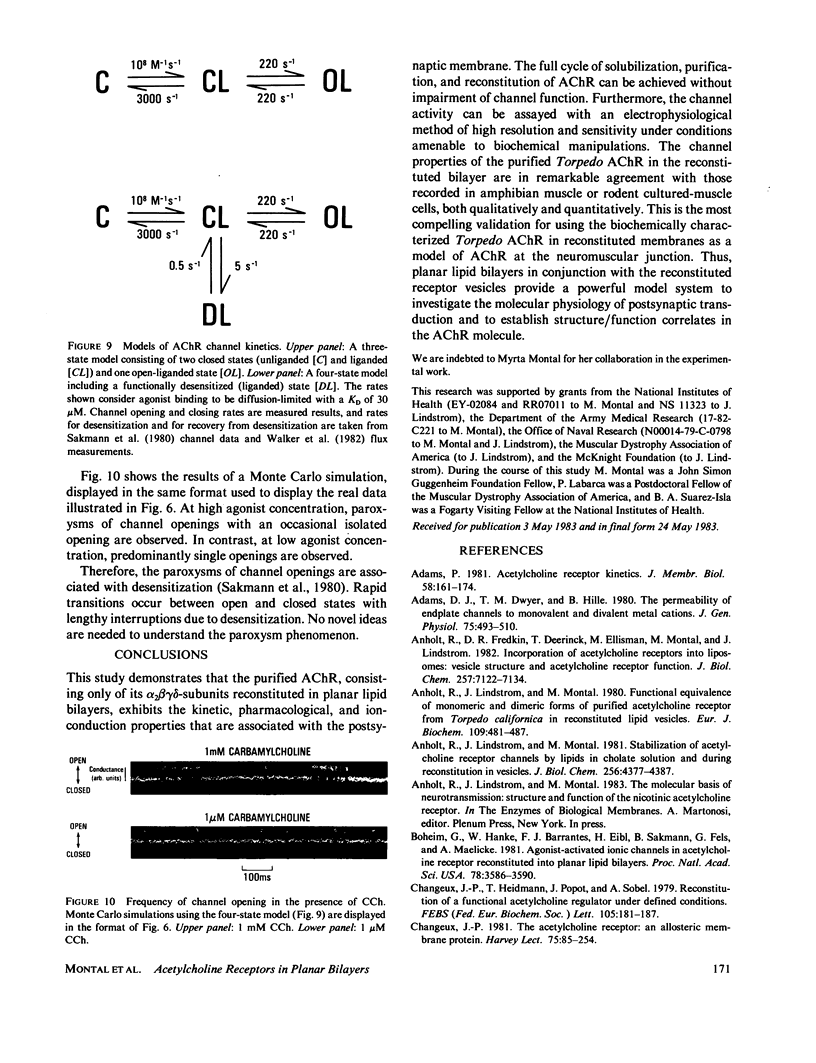
Abstract
The electrophysiological properties of the cation channel of the purified nicotinic acetylcholine receptor (AChR) reconstituted in planar lipid bilayers were characterized. Single-channel currents were activated by acetylcholine, carbamylcholine and suberyldicholine. The single channel conductance (28 pS in 0.3 M NaCl) was ohmic and independent of the agonist. Single channel currents increased with Na+ concentration to a maximum conductance of 95 pS and showed a half-saturation point of 395 mM. The apparent ion selectivity sequence, derived from single-channel current recordings, is: NH+4 greater than Cs+ greater than Rb+ greater than or equal to Na+ Cl-, F-, SO2-(4). The distribution of channel open times was fit by a sum of two exponentials, reflecting the existence of at least two distinct open states. The time constants depend on the choice of agonist, being consistently longer for suberyldicholine than for carbamylcholine. Similar channel properties were recorded in bilayers formed from monolayers at the tip of patch pipets . Single-channel currents occur in paroxysms of channel activity followed by quiescent periods. This pattern is more pronounced as the agonist concentration increases, and is reflected in histograms of channel-opening frequencies. Computer simulations with a three-state model, consisting of two closed (unliganded and liganded) and one open state, do not resemble the recorded pattern of channel activity, especially at high agonist concentration. Inclusion of a desensitized liganded state reproduces the qualitative features of channel recordings. The occurrence of paroxysms of channel activity thus seems to result from the transit of AChR through its active conformation, from which it can open several times before desensitizing.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Dwyer T. M., Hille B. The permeability of endplate channels to monovalent and divalent metal cations. J Gen Physiol. 1980 May;75(5):493–510. doi: 10.1085/jgp.75.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Acetylcholine receptor kinetics. J Membr Biol. 1981 Feb 28;58(3):161–174. doi: 10.1007/BF01870902. [DOI] [PubMed] [Google Scholar]
- Anholt R., Fredkin D. R., Deerinck T., Ellisman M., Montal M., Lindstrom J. Incorporation of acetylcholine receptors into liposomes. Vesicle structure and acetylcholine receptor function. J Biol Chem. 1982 Jun 25;257(12):7122–7134. [PubMed] [Google Scholar]
- Anholt R., Lindstrom J., Montal M. Functional equivalence of monomeric and dimeric forms of purified acetylcholine receptors from Torpedo californica in reconstituted lipid vesicles. Eur J Biochem. 1980 Aug;109(2):481–487. doi: 10.1111/j.1432-1033.1980.tb04819.x. [DOI] [PubMed] [Google Scholar]
- Anholt R., Lindstrom J., Montal M. Stabilization of acetylcholine receptor channels by lipids in cholate solution and during reconstitution in vesicles. J Biol Chem. 1981 May 10;256(9):4377–4387. [PubMed] [Google Scholar]
- Boheim G., Hanke W., Barrantes F. J., Eibl H., Sakmann B., Fels G., Maelicke A. Agonist-activated ionic channels in acetylcholine receptor reconstituted into planar lipid bilayers. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3586–3590. doi: 10.1073/pnas.78.6.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Heidmann T., Popot J. L., Sobel A. Reconstitution of a functional acetylcholine regulator under defined conditions. FEBS Lett. 1979 Sep 1;105(1):181–187. doi: 10.1016/0014-5793(79)80913-8. [DOI] [PubMed] [Google Scholar]
- Changeux J. P. The acetylcholine receptor: an "allosteric" membrane protein. Harvey Lect. 1979 1980;75:85–254. [PubMed] [Google Scholar]
- Claudio T., Ballivet M., Patrick J., Heinemann S. Nucleotide and deduced amino acid sequences of Torpedo californica acetylcholine receptor gamma subunit. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1111–1115. doi: 10.1073/pnas.80.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature. 1981 Dec 3;294(5840):464–466. doi: 10.1038/294464a0. [DOI] [PubMed] [Google Scholar]
- Coronado R., Latorre R. Phospholipid bilayers made from monolayers on patch-clamp pipettes. Biophys J. 1983 Aug;43(2):231–236. doi: 10.1016/S0006-3495(83)84343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein M., Racker E. Reconstitution of carbamylcholine-dependent sodium ion flux and desensitization of the acetylcholine receptor from Torpedo californica. J Biol Chem. 1978 Oct 10;253(19):6660–6662. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Sakmann B. Multiple conductance states of single acetylcholine receptor channels in embryonic muscle cells. Nature. 1981 Dec 3;294(5840):462–464. doi: 10.1038/294462a0. [DOI] [PubMed] [Google Scholar]
- Horn R., Patlak J. Single channel currents from excised patches of muscle membrane. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6930–6934. doi: 10.1073/pnas.77.11.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir R. L., Schell M. A., Racker E. Reconstitution of the purified acetylcholine receptor from Torpedo californica. FEBS Lett. 1979 Dec 1;108(1):155–160. doi: 10.1016/0014-5793(79)81199-0. [DOI] [PubMed] [Google Scholar]
- Jackson M. B., Wong B. S., Morris C. E., Lecar H., Christian C. N. Successive openings of the same acetylcholine receptor channel are correlated in open time. Biophys J. 1983 Apr;42(1):109–114. doi: 10.1016/S0006-3495(83)84375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A., Weill C. L., McNamee M. G., Valderrama R. Facets of the structures of acetylcholine receptors from Electrophorus and Torpedo. Cold Spring Harb Symp Quant Biol. 1976;40:203–210. doi: 10.1101/sqb.1976.040.01.022. [DOI] [PubMed] [Google Scholar]
- Kistler J., Stroud R. M., Klymkowsky M. W., Lalancette R. A., Fairclough R. H. Structure and function of an acetylcholine receptor. Biophys J. 1982 Jan;37(1):371–383. doi: 10.1016/S0006-3495(82)84685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J., Anholt R., Einarson B., Engel A., Osame M., Montal M. Purification of acetylcholine receptors, reconstitution into lipid vesicles, and study of agonist-induced cation channel regulation. J Biol Chem. 1980 Sep 10;255(17):8340–8350. [PubMed] [Google Scholar]
- Lindstrom J., Merlie J., Yogeeswaran G. Biochemical properties of acteylcholine receptor subunits from Torpedo californica. Biochemistry. 1979 Oct 16;18(21):4465–4470. doi: 10.1021/bi00588a003. [DOI] [PubMed] [Google Scholar]
- Montal M. Formation of bimolecular membranes from lipid monolayers. Methods Enzymol. 1974;32:545–554. doi: 10.1016/0076-6879(74)32053-8. [DOI] [PubMed] [Google Scholar]
- Montal M., Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau M., Changeux J. P. Studies on the electrogenic action of acetylcholine with Torpedo marmorata electric organ. I. Pharmacological properties of the electroplaque. J Mol Biol. 1976 Sep 25;106(3):457–467. doi: 10.1016/0022-2836(76)90246-1. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Nelson N., Anholt R., Lindstrom J., Montal M. Reconstitution of purified acetylcholine receptors with functional ion channels in planar lipid bilayers. Proc Natl Acad Sci U S A. 1980 May;77(5):3057–3061. doi: 10.1073/pnas.77.5.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Furutani Y., Hirose T., Asai M., Inayama S., Miyata T., Numa S. Primary structure of alpha-subunit precursor of Torpedo californica acetylcholine receptor deduced from cDNA sequence. Nature. 1982 Oct 28;299(5886):793–797. doi: 10.1038/299793a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Hirose T., Asai M., Takashima H., Inayama S., Miyata T. Primary structures of beta- and delta-subunit precursors of Torpedo californica acetylcholine receptor deduced from cDNA sequences. Nature. 1983 Jan 20;301(5897):251–255. doi: 10.1038/301251a0. [DOI] [PubMed] [Google Scholar]
- Oswald R., Changeux J. P. Ultraviolet light-induced labeling by noncompetitive blockers of the acetylcholine receptor from Torpedo marmorata. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3925–3929. doi: 10.1073/pnas.78.6.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popot J. L., Cartaud J., Changeux J. P. Reconstitution of a functional acetylcholine receptor. Incorporation into artificial lipid vesicles and pharmacology of the agonist-controlled permeability changes. Eur J Biochem. 1981 Aug;118(2):203–214. [PubMed] [Google Scholar]
- Raftery M. A., Hunkapiller M. W., Strader C. D., Hood L. E. Acetylcholine receptor: complex of homologous subunits. Science. 1980 Jun 27;208(4451):1454–1456. doi: 10.1126/science.7384786. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Karlin A. Molecular weight in detergent solution of acetylcholine receptor from Torpedo californica. Biochemistry. 1978 May 30;17(11):2035–2038. doi: 10.1021/bi00604a001. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Schindler H. Formation of planar bilayers from artificial or native membrane vesicles. FEBS Lett. 1980 Dec 15;122(1):77–79. doi: 10.1016/0014-5793(80)80405-4. [DOI] [PubMed] [Google Scholar]
- Schindler H., Quast U. Functional acetylcholine receptor from Torpedo marmorata in planar membranes. Proc Natl Acad Sci U S A. 1980 May;77(5):3052–3056. doi: 10.1073/pnas.77.5.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Isla B. A., Wan K., Lindstrom J., Montal M. Single-channel recordings from purified acetylcholine receptors reconstituted in bilayers formed at the tip of patch pipets. Biochemistry. 1983 May 10;22(10):2319–2323. doi: 10.1021/bi00279a003. [DOI] [PubMed] [Google Scholar]
- Tank D. W., Miller C., Webb W. W. Isolated-patch recording from liposomes containing functionally reconstituted chloride channels from Torpedo electroplax. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7749–7753. doi: 10.1073/pnas.79.24.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. W., Takeyasu K., McNamee M. G. Activation and inactivation kinetics of Torpedo californica acetylcholine receptor in reconstituted membranes. Biochemistry. 1982 Oct 26;21(22):5384–5389. doi: 10.1021/bi00265a001. [DOI] [PubMed] [Google Scholar]
- Weill C. L., McNamee M. G., Karlin A. Affinity-labeling of purified acetylcholine receptor from Torpedo californica. Biochem Biophys Res Commun. 1974 Dec 11;61(3):997–1003. doi: 10.1016/0006-291x(74)90254-x. [DOI] [PubMed] [Google Scholar]
- Wolosin J. M., Lyddiatt A., Dolly J. O., Barnard E. A. Stoichiometry of the ligand-binding sites in the acetylcholine-receptor oligomer from muscle and from electric organ. Measurement by affinity alkylation with bromoacetylcholine. Eur J Biochem. 1980 Aug;109(2):495–505. doi: 10.1111/j.1432-1033.1980.tb04821.x. [DOI] [PubMed] [Google Scholar]
- Wu W. C., Moore H. P., Raftery M. A. Quantitation of cation transport by reconstituted membrane vesicles containing purified acetylcholine receptor. Proc Natl Acad Sci U S A. 1981 Feb;78(2):775–779. doi: 10.1073/pnas.78.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingsheim H. P., Barrantes F. J., Frank J., Hänicke W., Neugebauer D. C. Direct structural localization of two toxin-recognition sites on an ACh receptor protein. Nature. 1982 Sep 2;299(5878):81–84. doi: 10.1038/299081a0. [DOI] [PubMed] [Google Scholar]


