Abstract
Sedentary lifestyles and increased pollution brought about by industrialization pose major challenges to the prevention of both obesity and chronic respiratory diseases such as chronic obstructive pulmonary disease (COPD), asthma, obstructive sleep apnea and obesity hypoventilation syndrome. Obesity has emerged as an important risk factor for these respiratory diseases, and in many instances weight loss is associated with important symptomatic improvement. Moreover, obesity may influence the development and presentation of these diseases. In this article, we review the current understanding of the influence of obesity on chronic respiratory diseases and the clinical management of obesity concurrent with asthma, COPD, obstructive sleep apnea or obesity hypoventilation syndrome.
More than one billion people around the world are overweight or obese with a body mass index (BMI) of 25 kg/m2 or more.1 Obesity is a major cause of morbidity; for example, in the United States in 2000 it was responsible for approximately 400 000 deaths and accounted for about 7% of health care expenditures.2 Obesity, particularly abdominal obesity, is a significant risk factor for cardiovascular disease, type 2 diabetes, rheumatoid arthritis and cancer.3 A link between obesity and chronic respiratory diseases is also increasingly recognized.
The number of patients with a chronic respiratory disease such as chronic obstructive pulmonary disease (COPD), asthma or obstructive sleep apnea is also increasing. Physicians are therefore routinely challenged by the presentation of obesity concurrently with chronic respiratory disease. Under certain conditions, obesity may be causally linked to the respiratory disorder, as with obstructive sleep apnea and obesity hypoventilation syndrome. Obesity is also commonly found in association with COPD and asthma, although the nature of this association has not been fully elucidated.
Definition of obesity
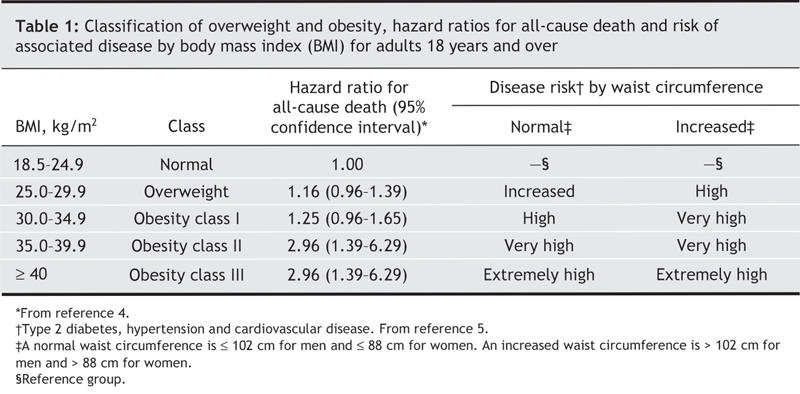
In the classification of body weight, BMI, an index of weight to height (kg/m2), is generally recognized as the most useful indicator of health risk among people who are over-or underweight (Table 1).5 It is also acknowledged that BMI provides no information about the distribution of fat in the body. The health risks related to obesity, including its effects on respiratory function, are linked not only to the magnitude of obesity but also to the presence of abdominal fat.6,7 Waist circumference is highly correlated with visceral adipose tissue,8 and thus is used in combination with BMI to further refine the assessment of the level of cardiovascular risk associated with obesity (Table 1).5,9 The recommended use and limitations of this body weight classification system are reviewed at www.hc-sc.gc.ca/hpfb-dgpsa/onpp-bppn/cg_bwc_introduction_e.html.
Table 1
Effect of obesity on respiratory function
Fat tissue accumulation impairs ventilatory function in adults7,10 and children.11 Increasing BMI is typically associated with a reduction in forced expiratory volume in one second (FEV1), forced vital capacity (FVC),7,10 total lung capacity, functional residual capacity and expiratory reserve volume.12 Thoracic restriction associated with obesity is usually mild and is attributed to the mechanical effects of fat on the diaphragm and the chest wall: diaphragm excursion is impeded and thoracic compliance reduced. A clinically significant restrictive pattern (total lung capacity < 85% predicted) is usually seen only in massive obesity, when the patient's weight-to-height ratio is 0.9–1.0 kg/cm or greater.13,14 However, a restrictive disorder may still be attributed to obesity when the weight-to-height ratio is less than 0.9 kg/cm. This typically occurs in the presence of central fat deposition, which is indicated by a waist-to-hip ratio of 0.95 or greater.7,15 When obesity is less than massive, a restrictive defect should not be attributed to fat accumulation until other causes of restrictive impairment, such as interstitial lung disease or neuromuscular disease, have been excluded.
A low FEV1/FVC ratio (< 70%), the spirometric signature of airflow obstruction, is not a feature of respiratory diseases associated with obesity,7,16 although evidence of small-airway diseases has been reported in this context.12 Diffusion capacity may be increased in obesity,13 but this is not a universal finding.14 Last, respiratory muscle strength may be compromised in obesity, as indicated by reduced maximal inspiratory pressure in obese subjects compared with control subjects with normal body weight.17,18 Respiratory muscle weakness in obesity has been attributed to muscle inefficiency, a consequence of reduced chest wall compliance or lower operating lung volumes or both.17,18 Not surprisingly, exercise capacity is often impaired in obese patients. Although cardiorespiratory fitness as assessed by maximal oxygen consumption is generally preserved in obese patients, functional status during exercise such as walking is reduced because of the higher metabolic cost of carrying the extra body weight.19
There is a clear association between dyspnea and obesity.16 Obesity increases the work of breathing because of the reductions in both chest wall compliance18,20 and respiratory muscle strength.17,18 This creates an imbalance between the demand on the respiratory muscles and their capacity to generate tension,18 which leads to the perception of increased breathing effort.21 Furthermore, dyspnea in obese patients could unmask other associated conditions, such as respiratory and heart diseases. Among these diseases, asthma deserves a special mention. Patients with obesity frequently report dyspnea and wheezing and are therefore often given therapy for asthma without objective diagnostic confirmation by pulmonary function testing.16 An accurate diagnosis is important because dyspnea related to other mechanisms or diseases may require a different therapeutic strategy. Thus, the diagnosis of asthma or COPD should not be based solely on symptoms but should include spirometric confirmation.
COPD and asthma
COPD is currently the fourth leading cause of death in the world, and a further increase in its prevalence and related rates of death is expected in the coming decades.22 COPD is a progressive disorder that encompasses chronic bronchitis and emphysema and is characterized by airflow limitation that is not fully reversible. It is also associated with an abnormal inflammatory response of the lungs to noxious particles or gases, among which cigarette smoking is by far the most important.22 COPD, particularly in the form of emphysema, is typically associated with weight loss and muscle wasting; these phenomena usually occur in advanced disease and are associated with increased rates of death.23,24 The factors responsible for weight loss and muscle wasting in emphysema are currently being investigated. A state of low-grade systemic inflammation, oxidative stress, negative energy balance and hypoxemia are among those proposed.25
Overweight or obesity is usually seen with chronic bronchitis rather than with emphysema, although this distinction is not always the rule.26 There is evidence that patients with COPD lead a more sedentary lifestyle, which would contribute to the development of obesity: a case–control study revealed that elderly patients with COPD walked an average of 44 minutes per day, whereas healthy control patients walked 81 minutes per day (p < 0.001).27 Alternatively, obesity may modify the clinical picture of COPD, although there is little research on this complex issue. For example, exercise intolerance, a hallmark of COPD, is likely to be worsened by fat accumulation. However, based on the observation that a BMI greater than 24 kg/m2 is associated with better survival, it has been proposed that obesity could be protective in COPD.28,29 In one large epidemiologic study, overweight and obesity in patients with COPD was associated with a decreased risk of death compared with normal weight (hazard ratio 0.9, 95% confidence interval [CI] 0.7–1.0).30 Although in the general population excess weight increases the risk of death, overweight and obesity are, paradoxically, associated with better outcomes in some chronic diseases. This phenomenon is well characterized in patients with heart failure31 and is referred to as the obesity paradox. However, the notion that fat accumulation is protective in COPD may be an oversimplification that neglects the potential consequences of obesity in this disease. It is worth noting that an increased BMI does not protect against fat-free mass depletion in COPD, since there is a preferential loss of muscle tissue in this disease.32 In fact, muscle mass may be reduced despite a normal BMI.32 Furthermore, a reduced muscle mass is associated with increased rates of death, irrespective of BMI.24,30,33 In a further compounding of the potentially significant interactions between COPD and obesity, COPD is now recognized as a risk factor for cardiovascular disease, increasing the risk of these diseases 2–3-fold, independent of traditional risk factors such as hypertension, dyslipidemia and smoking.34,35 Given the current epidemics of obesity and COPD, their concurrent association will increase in the future. Further studies are required to provide a better understanding of the impact of obesity in COPD and vice versa.
Asthma, a chronic inflammatory disorder of airways, is associated with reversible airway obstruction and increased airway responsiveness to a variety of stimuli. However, longstanding asthma can also lead to airflow limitation that is not fully reversible.36 The presence of atopy, a greater reversibility of the airflow limitation after inhalation of β2-agonists, and smoking history are helpful in differentiating asthma from COPD.
Obesity was shown to be epidemiologically associated with respiratory symptoms in the 1980s.37 Although parallel increases in the prevalence of obesity and asthma in Western countries have been reported, it remains to be determined whether this association is causal or by chance.38 Although obesity may simply be a marker of recently adopted lifestyle habits also associated with asthma, several specific mechanisms can be proposed for their association,39 and it is now recognized that a causal relation may exist between them.40–42 The association between asthma and obesity is particularly worrisome in children.43 The obesity epidemic may lead to an increase in the number of young adults with severe asthma since obesity is a predictor of unremitting asthma after puberty44 and worse asthma control.45
Conflicting results regarding the nature of the association between obesity and asthma may be attributed to different study designs46 and misclassification of wheeze or asthma. For instance, Schachter and colleagues reported an association between obesity and a clinical diagnosis of asthma, whereas no such relation was found between obesity and increased airway responsiveness.47 The perception of dyspnea is often increased in obesity, and a diagnosis of asthma may be erroneously made in this setting.16
In the presence of obesity, the mechanical properties of the respiratory system are profoundly modified. Reduced tidal lung expansion compromises the dilating forces that maintain the patency of the airways and may lead to greater contractile responses of the airway smooth muscle.11,48 This can potentially cause increased airway responsiveness. Supporting this contention is our recent report of a suppression of the protective effect of deep inspiration on airway closure in nonasthmatic obese patients.49
Fat tissue produces a plethora of inflammatory mediators, which suggests an immunologic link between obesity and asthma.50,51 This hypothesis is substantiated by the presence of increased concentrations of C-reactive protein, tumour necrosis factor-α and interleukin-6 in the serum of obese subjects. Increased leptin secretion in obesity may be specifically involved in the development of asthma by modulating airway inflammation.52 Conversely, reduction in adiponectin secretion, an anti-inflammatory cytokine, is another potential link between obesity and asthma.53
The interaction between BMI and asthma is stronger in women than men, and thus it has been suggested that increased levels of female sex hormones may play a role in the increased prevalence of asthma among obese women.41,54 Estrogens may modulate the immune response and increase the risk of asthma.42 These findings are particularly relevant in obesity, where enhanced aromatization of testosterone to estrogens by the adipose tissue and decreased sex hormone-binding globulin levels may result in greater estrogen tissue availability.42 However, the interaction between estrogens and airway inflammation in asthma has yet to be fully explored.
Genetic factors may also play a role in the relation between obesity and asthma. Obesity genes may influence a patient's susceptibility to asthma in several ways: several candidate genes have been linked to both asthma and obesity; candidate obesity genes are clustered in proximity to chromosomal regions that have been associated with asthma; and candidate genes for obesity may encode protein products such as inflammatory mediators that could be directly involved in the pathogenesis of asthma.42 This topic also requires further study.
Evaluation of COPD and asthma in obesity
COPD and asthma should be considered in any patient presenting with chronic cough, sputum production, wheeze or dyspnea, especially if the person has risk factors such as smoking or having frequent exposure to allergens. In this situation, spirometric testing should be part of the clinical assessment. Patients with COPD typically show a persistent decrease in FEV1 (e.g., FEV1 < 80% predicted) and FVC together with an FEV1/FVC ratio less than 70%. Asthma is characterized by airflow obstruction that varies in severity either spontaneously or with treatment. In contrast to COPD, in which airflow obstruction is persistent, most patients with asthma have normal lung function when adequate treatment is received. Methacholine challenges can be used to demonstrate airway hyperresponsiveness and to confirm clinical suspicion of asthma when spirometry test results are normal.
Therapeutic implications of obesity for COPD and asthma
Weight reduction is undoubtedly the optimal health strategy for obese patients with chronic respiratory disease. Approaches to weight loss and weight-loss maintenance have been summarized in various evidence-based reports.5 In general, therapy comprising diet, physical activity and medication promote a moderate degree of weight or fat loss in the short term. However, the results of studies with long-term follow-up are disappointing because most patients regain some or most of their excess weight. No specific recommendations exist about weight-loss strategies in the presence of respiratory disease. This is not a trivial issue, since patients with chronic respiratory disease are often inactive and therefore often do not comply with the recommendation to increase their levels of physical activity. Incorporating pulmonary rehabilitation and exercise training strategies may therefore help patients begin to exercise, but the efficacy of these approaches in achieving weight loss has not been documented.
The impact of weight reduction on dyspnea, exercise tolerance and quality of life in obese or overweight patients with COPD has not been examined in the literature. In fact, most studies focus on undernourished patients with COPD. With asthma, weight loss has been shown to improve lung function and symptoms, independent of changes in airway hyper-responsiveness.55–57 One study that involved 58 obese women, 24 of whom had asthma, demonstrated that for every 10% relative loss of weight, FVC improved by 92 mL (p = 0.05) and the FEV1 improved by 73 mL (p = 0.04).55 A small randomized trial of the effects of a weight reduction program on obese patients with asthma also showed that an 11% reduction in body weight was associated with a 7.6% improvement in FEV1 compared with the control group (p = 0.02).56 There is no published evidence of whether therapeutic approaches to COPD or asthma (e.g., inhalation therapy, education and rehabilitation) should be modified in the presence of obesity. However, our experience suggests that the response of obese patients with asthma to pharmacotherapy is often suboptimal, a clinical impression supported by the association between obesity and poor asthma control.58 Additional studies are required to confirm whether obesity modulates the response to inhalation therapy.
Obstructive sleep apnea
The prevalence of obstructive sleep apnea in the general population is highly variable, ranging from 25% to 58% among men and from 10% to 37% among women, depending on ethnicity and the geographic area studied.59 Obstructive sleep apnea is characterized by intermittent upper airway obstruction due to the inabililty of pharyngeal musculature to maintain upper airway patency in the presence of alterations in airway shape and diameter.60 This results in a fall in arterial oxygen content, a rise in carbon dioxide levels and increased inspiratory efforts that lead to abrupt awakenings as the person struggles to breathe.61 The result is profoundly disturbed sleep.
Obesity is a well-recognized risk factor for obstructive sleep apnea. Increased fat tissue deposition in the pharyngeal region and reduced operating lung volumes in obesity act together to reduce upper airway caliber, modify airway configuration and increase their collapsibility; airways are thus predisposed to repetitive closures during sleep.62 About 70% of people with obstructive sleep apnea are obese, and, conversely, the prevalence of the disorder among obese people is approximately 40%.63 Indeed, almost all men with class III obesity also have obstructive sleep apnea.64 Obstructive sleep apnea is associated with excess mortality from accidents related to daytime sleepiness and to the high incidence of cardiovascular disorders reported in this condition.65–68 Therefore, obstructive sleep apnea is one of the life-threatening sequelae of obesity.
Evaluation of obstructive sleep apnea in obesity
Nocturnal polysomnography is the “gold standard” diagnostic test for sleep apnea.59 This test allows the identification of complete cessation of airflow (apnea) and of reduction of airflow associated with a decrease in oxygen saturation and arousal or both (hypopnea). Diagnosis of obstructive sleep apnea is made when symptomatic patients have an apnea– hypopnea index — the number of episodes of apnea and hypopnea per hour of total sleep time — greater than 5.69 However, nocturnal polysomnography is time-consuming and costly, and its availability is limited because it requires sophisticated sleep laboratories and highly trained technicians.59 Therefore, other diagnostic strategies have been developed to determine whether sleep apnea is present.70–72 One such strategy involves abbreviated recordings such as nocturnal oximetry or cardiorespiratory monitoring, which are frequently considered first-line tests for patients with high pretest probability of sleep apnea.73 Another strategy involves clinical prediction rules that use simple parameters such as neck circumference, the presence of hypertension and habitual snoring, and bed-partner reports of nocturnal gasping or choking.74 Although these prediction rules can help in estimating the probability of obstructive sleep apnea and in determining which patients should have priority for sleep studies, they do not provide sufficient diagnostic precision to replace sleep recordings.
Therapeutic implications of obesity
Weight loss improves the symptoms of obstructive sleep apnea75 and reduces breathing disturbances during sleep.76 Weight loss was demonstrated to improve symptoms as measured by the apnea–hypopnea index (66.5 [standard deviation (SD) 28.7] to 50.3 [SD 38.4] per hour, p < 0.05) in a cohort of obese patients who lost an average of 9 kg.75 In another study, a 10% increase in weight predicted a 6-fold (95% CI 2.2–17.0) increase in the odds of developing moderate to severe obstructive sleep apnea.76 Unfortunately, most patients are unable or unwilling to maintain a healthy body weight. Therefore, nasal continuous positive airway pressure (CPAP) is the treatment of choice for obese patients with obstructive sleep apnea.77 CPAP corrects sleep-related breathing disorders, improves daytime symptoms, decreases rates of death,78 reduces heart rate variability and blood pressure during the day and night79 and improves autonomic function.80 Mandibular positioner devices, uvulopalatopharingoplasty or more complex maxillofacial surgeries81,82 have limited applicability in the context of obesity. Bariatric surgery is currently the only definitive treatment for class III obesity. It markedly improves the symptoms of obstructive sleep apnea, and CPAP can usually be discontinued after significant weight loss.57 Use of CPAP should be part of the preoperative preparation of these patients, not only to initiate treatment for the sleeping disorder before significant weight loss occurs, but also to minimize the occurrence of postoperative cardiopulmonary complications.83
Obesity hypoventilation syndrome
Hypercapnic respiratory failure and cor pulmonale are frequently observed in obesity. In the absence of other known causes of respiratory failure, this syndrome, which was first described 50 years ago,84 is now termed obesity hypoventilation syndrome.85 Respiratory failure, severe hypoxemia, hypercapnia and pulmonary hypertension represent the syndrome's most common symptoms.85,86 Most patients with obesity hypoventilation syndrome also have obstructive sleep apnea,85 but that some patients have obesity hypoventilation syndrome but not obstructive sleep apnea suggests that obesity alone can lead to chronic hypoventilation. The diagnostic criteria for the syndrome are provided in Box 1.
Box 1.

Evaluation
Arterial blood gas analysis must be obtained to document daytime hypercapnia (PaCO2 > 45 mm Hg) and hypoxemia, the gas exchange abnormalities characteristic of obesity hypoventilation syndrome.86 Abbreviated recordings such as nocturnal oximetry may also be used to determine whether nonapneic desaturation is present. However, a formal attended sleep study may be required when concomitant obstructive sleep apnea cannot be ruled out or when the obstructive or nonobstructive nature of nocturnal breathing disturbances has not been ascertained.
Because about one-fifth of patients with obstructive sleep apnea also have obesity hypoventilation syndrome,85 it is recommended that physicians measure daytime arterial blood gas when obstructive sleep apnea is diagnosed, particularly if nocturnal cardiorespiratory monitoring results suggest the occurrence of nonapneic desaturation. Pulmonary function testing will also be useful to rule out the presence of other specific causes of hypoventilation such as COPD or neuromuscular disease. Clinicians should also suspect obesity hypoventilation syndrome when obese patients have unexplained pulmonary hypertension or daytime hypoxemia.86
Therapeutic implications
A return to normal body weight is the only cure for obesity hypoventilation syndrome, and it is associated with improvements in blood gases, sleep-related breathing disorders and pulmonary hypertension.86 Unfortunately, weight loss is often difficult to achieve by dietary means, and in many cases bariatric surgery may be necessary. CPAP is the most effective treatment of obstructive breathing disturbances. However, nocturnal noninvasive ventilation may be required for most patients. In patients with persisting hypoxemia, oxygen supplementation may be needed in addition to ventilatory support.
Assessment of body composition in patients with a respiratory disease
Body composition is not routinely assessed when patients with respiratory disorders are evaluated. BMI should be calculated in order to classify the level of obesity (Table 1). This will significantly aid proper therapeutic intervention, the goals of which are not only to reduce body weight but also to improve respiratory symptoms. Assessing body composition to differentiate fat mass from fat-free mass (muscle mass) may also be useful, since muscle mass may be reduced despite a normal or even increased BMI.32 A corollary of this is that the prevalence of muscle atrophy in chronic respiratory diseases will be underestimated if body weight only is measured.
The measurement of skinfold thickness at 4 sites — the triceps, biceps, below the scapula and above the iliac bone — is a simple and widely used method for assessing fat mass and body composition. Total fat mass is estimated using equations or tables (Appendix 1, available online at www.cmaj.ca/cgi/content/full/174/9/1293/DC1), and fat-free mass is obtained by subtracting fat mass from body weight.87 However, anthropometric estimates of fat mass may not be valid in elderly people, in whom fat is preferentially located in central and internal parts of the body. In elderly people, fat mass tends to be underestimated and fat-free mass overestimated when anthropometric estimates are compared with “gold standard” methods such as deuterium dilution.88
Bioelectrical impedance is another simple method to assess body composition, and it can be done easily using commercially available equipment. Numerous bioelectrical impedance formulas have been derived from different populations to calculate fat-free mass, and care has to be taken to use a formula that is appropriate for the subject being evaluated.89 Two formulas have been specifically developed for chronic respiratory diseases.88,90 From these measurements, a fat-free mass index can be calculated by dividing fat-free mass in kilograms by height in metres squared. Cut-off points of 16 kg/m2 and 15 kg/m2 have been associated with poor survival in men and women with COPD respectively.30,33
Dual-energy x-ray absorptiometry (DEXA) is a newer method that uses a double photon beam generated by a radiography source. This method requires sophisticated equipment but is simple to perform, very reproducible, noninvasive, and has been validated against independent reference methods.91 DEXA is best suited to assess body composition changes either over time or after a therapeutic intervention.
Conclusion
The obesity epidemic poses a new challenge to health professionals caring for patients with chronic respiratory diseases. The influence of obesity on asthma and obstructive sleep apnea has been well documented, and weight loss has been associated with improved symptomatic control in these diseases. The impact of obesity on COPD has been much less studied. It is likely that obesity modifies the clinical picture of COPD because of its effects on the perception of dyspnea and exercise tolerance. An important challenge will be to find efficacious weight-loss strategies for obese patients with chronic respiratory diseases. The usual recommendation to increase physical activity is difficult for these patients to implement, since they are prone to a sedentary lifestyle imposed by shortness of breath. Further studies are therefore necessary to address the rapidly growing problem of obesity in chronic respiratory diseases.
Supplementary Material
Acknowledgments
Magali Poulain was supported in part by a grant from the Groupe de recherche en santé respiratoire de l'Université Laval. Frédéric Sériès and François Maltais are research scholars of the Fonds de la recherche en santé du Québec.
Footnotes
This article has been peer reviewed.
Contributors: Magali Poulain and François Maltais were responsible for the conception of the article and for writing the manuscript. All of the authors contributed substantially to the scientific content of the manuscript, reviewed it critically for important intellectual content and approved the final version.
Competing interests: None declared for Magali Poulain, Mariève Doucet, Frédéric Sériès, Angelo Tremblay and Geneviève C. Major. Louis-Philippe Boulet has been on the advisory boards of ALTANA Pharma, AstraZeneca, GlaxoSmithKline, Merck Frosst and Novartis; he has received speaker fees from AstraZeneca, GlaxoSmithKline, Merck Frosst, 3M Pharmaceutical and Novartis, sponsorship for basic research from ALTANA Pharma, AstraZeneca, Merck Frosst and 3M Pharmaceutical and additional funding for participation in multicentre studies of the pharmacotherapy of asthma from ALTANA Pharma, AstraZeneca, Asthmatx, Boehringer Ingelheim, IVAX Pharmaceuticals, Dynavax, Genentech, GlaxoSmithKline, Merck Frosst, Novartis, Roche, Topigen, Schering and 3M Pharmaceutical. François Maltais has been on the advisory boards of ALTANA Pharma, Boehringer Ingelheim and GlaxoSmithKline; he has received speaker fees from Boehringer Ingelheim, GlaxoSmithKline and Pfizer, travel assistance from AstraZeneca, unrestricted research grants from Boehringer Ingelheim and GlaxoSmithKline and funding for participation in multicentre studies from ALTANA Pharma, Boehringer Ingelheim, GlaxoSmithKline and Merck Frosst.
Correspondence to: Dr. François Maltais, Centre de Pneumologie, Hôpital Laval, 2725 chemin Ste-Foy, Québec QC G1V 4G5; francois.maltais@med.ulaval.ca
REFERENCES
- 1.International obesity taskforce [IOTF]. Available: www.iotf.org/indes.asp (accessed 20 Mar 2006).
- 2.Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States, 2000. JAMA 2004;291:1238-45. [DOI] [PubMed]
- 3.Conway B, Rene A. Obesity as a disease: no lightweight matter. Obes Rev 2004;5:145-51. [DOI] [PubMed]
- 4.Katzmarzyk PT, Craig CL, Bouchard C. Original article underweight, overweight and obesity: relationships with mortality in the 13-year follow-up of the Canada Fitness Survey. J Clin Epidemiol 2001;54:916-20. [DOI] [PubMed]
- 5.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults — The Evidence Report. National Institutes of Healt. Obes Res 1998;6(Suppl 2):51S-209S. [PubMed]
- 6.Shinohara E, Kihara S, Yamashita S, et al. Visceral fat accumulation as an important risk factor for obstructive sleep apnoea syndrome in obese subjects. J Intern Med 1997;241:11-8. [DOI] [PubMed]
- 7.Lazarus R, Sparrow D, Weiss ST. Effects of obesity and fat distribution on ventilatory function: the normative aging study. Chest 1997;111:891-8. [DOI] [PubMed]
- 8.Després JP, Lemieux I, Prud'homme D. Treatment of obesity: need to focus on high risk abdominally obese patients. BMJ 2001;322:716-20. [DOI] [PMC free article] [PubMed]
- 9.Douketis JD. Body weight classification. CMAJ 2005;172:1274-5. [DOI] [PMC free article] [PubMed]
- 10.Chinn DJ, Cotes JE, Reed JW. Longitudinal effects of change in body mass on measurements of ventilatory capacity. Thorax 1996;51:699-704. [DOI] [PMC free article] [PubMed]
- 11.Inselma LS, Milanese A, Deurloo A. Effect of obesity on pulmonary function in children. Pediatr Pulmonol 1993;16:130-7. [DOI] [PubMed]
- 12.Rubinstein I, Zamel N, DuBarry L, et al. Airflow limitation in morbidly obese, nonsmoking men. Ann Intern Med 1990;112:828-32. [DOI] [PubMed]
- 13.Ray CS, Sue DY, Bray G, et al. Effects of obesity on respiratory function. Am Rev Respir Dis 1983;128:501-6. [DOI] [PubMed]
- 14.Biring MS, Lewis MI, Liu JT, et al. Pulmonary physiologic changes of morbid obesity. Am J Med Sci 1999;318:293-7. [DOI] [PubMed]
- 15.Canoy D, Luben R, Welch A, et al. Abdominal obesity and respiratory function in men and women in the EPIC-Norfolk Study, United Kingdom. Am J Epidemiol 2004;159:1140-9. [DOI] [PubMed]
- 16.Sin DD, Jones RL, Man SF. Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch Intern Med 2002;162:1477-81. [DOI] [PubMed]
- 17.Weiner P, Waizman J, Weiner M, et al. Influence of excessive weight after gastroplasty for morbid obesity on respiratory muscle performance. Thorax 1998;53:39-42. [DOI] [PMC free article] [PubMed]
- 18.Chlif M, Keochkerian D, Mourlhon C, et al. Noninvasive assessment of the tension-time index of inspiratory muscles at rest in obese male subjects. Int J Obes (Lond) 2005;29:1478-83. [DOI] [PubMed]
- 19.Norman AC, Drinkard B, McDuffie JR, et al. Influence of excess adiposity on exercise fitness and performance in overweight children and adolescents. Pediatrics 2005;115:e690-6. [DOI] [PMC free article] [PubMed]
- 20.Sharp JT, Henry JP, Sweany SK, et al. The total work of breathing in normal and obese men. J Appl Physiol 1964;43:728-39. [DOI] [PMC free article] [PubMed]
- 21.LeBlanc P, Bowie DM, Summers E, et al. Breathlessness and exercise in patients with cardiopulmonary disease. Am Rev Respir Dis 1986;133:21-5. [DOI] [PubMed]
- 22.Global Initiative for Chronic Obstructive Lung Disease. Workshop report, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. September 2005. Available: www.goldcopd.org/Guidelineitem.asp?l1=2&l2=1&intId=989 (accessed 2006 Mar 20).
- 23.Wilson DO, Rogers RM, Wright EC, et al. Body weight in chronic obstructive pulmonary disease. The National Institutes of Health Intermittent Positive-Pressure Breathing Trial. Am Rev Respir Dis 1989;139:1435-8. [DOI] [PubMed]
- 24.Marquis K, Debigaré R, LeBlanc P, et al. Mid-thigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with COPD. Am J Respir Crit Care Med 2002;166:809-13. [DOI] [PubMed]
- 25.Agusti AG. Systemic effects of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005;2:367-70. [DOI] [PubMed]
- 26.Guerra S, Sherrill DL, Bobadilla A, et al. The relation of body mass index to asthma, chronic bronchitis, and emphysema. Chest 2002;122:1256-63. [DOI] [PubMed]
- 27.Pitta F, Troosters T, Spruit MA, et al. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;171:972-7. [DOI] [PubMed]
- 28.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350:1005-12. [DOI] [PubMed]
- 29.Schols AMW, Slangen J, Volovics L, et al. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1791-7. [DOI] [PubMed]
- 30.Vestbo J, Prescott E, Almdal T, et al. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen city heart study. Am J Respir Crit Care Med 2006;173:79-83. [DOI] [PubMed]
- 31.Curtis JP, Selter JG, Wang Y, et al. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med 2005;165:55-61. [DOI] [PubMed]
- 32.Schols AMWJ, Soeters PB, Dingemans MC, et al. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis 1993;147:1151-6. [DOI] [PubMed]
- 33.Schols AM, Broekhuizen R, Weling-Scheepers CA, et al. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr 2005;82:53-9. [DOI] [PubMed]
- 34.Hole DJ, Watt GC, Vey-Smith G, et al. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ 1996;313:711-5. [DOI] [PMC free article] [PubMed]
- 35.Sin DD, Man SFP. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 2003;107:1514-9. [DOI] [PubMed]
- 36.Lange P, Parner J, Vestbo J, et al. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med 1998;339:1194-200. [DOI] [PubMed]
- 37.Somerville SM, Rona RJ, Chinn S. Obesity and respiratory symptoms in primary school. Arch Dis Child 1984;59:940-4. [DOI] [PMC free article] [PubMed]
- 38.Chinn S, Rona RJ. Can the increase in body mass index explain the rising trend in asthma in children? Thorax 2001;56:845-50. [DOI] [PMC free article] [PubMed]
- 39.Weiss ST, Shore S. Obesity and asthma: directions for research. Am J Respir Crit Care Med 2004;169:963-8. [DOI] [PubMed]
- 40.Shaheen SO, Sterne JA, Montgomery SM, et al. Birth weight, body mass index and asthma in young adults. Thorax 1999;54:396-402. [DOI] [PMC free article] [PubMed]
- 41.Camargo CA Jr., Weiss ST, Zhang S, et al. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med 1999;159:2582-8. [DOI] [PubMed]
- 42.Tantisira KG, Weiss ST. Complex interactions in complex traits: obesity and asthma. Thorax 2001;56(Suppl 2):ii64-73. [PMC free article] [PubMed]
- 43.Gilliland FD, Berhane K, Islam T, et al. Obesity and the risk of newly diagnosed asthma in school-age children. Am J Epidemiol 2003;158:406-15. [DOI] [PubMed]
- 44.Guerra S, Wright AL, Morgan WJ, et al. Persistence of asthma symptoms during adolescence: role of obesity and age at the onset of puberty. Am J Respir Crit Care Med 2004;170:78-85. [DOI] [PubMed]
- 45.Lavoie KL, Bacon SL, Labrecque M, et al. Higher BMI is associated with worse asthma control and quality of life but not asthma severity. Respir Med 2005. [DOI] [PubMed]
- 46.Schaub B. von ME. Obesity and asthma, what are the links? Curr Opin Allergy Clin Immunol 2005;5:185-93. [DOI] [PubMed]
- 47.Schachter LM, Salome CM, Peat JK, et al. Obesity is a risk for asthma and wheeze but not airway hyperresponsiveness. Thorax 2001;56:4-8. [DOI] [PMC free article] [PubMed]
- 48.Hakala K, Stenius-Aarniala B, Sovijarvi A. Effects of weight loss on peak flow variability, airways obstruction, and lung volumes in obese patients with asthma. Chest 2000;118:1315-21. [DOI] [PubMed]
- 49.Boulet LP, Turcotte H, Boulet G, et al. Deep inspiration avoidance and airway response to methacholine: influence of body mass index. Can Respir J 2005;12:371-6. [DOI] [PubMed]
- 50.Weiss ST. Obesity: insight into the origins of asthma. Nat Immunol 2005;6:537-9. [DOI] [PubMed]
- 51.Bergeron C, Boulet LP, Hamid Q. Obesity, allergy and immunology. J Allergy Clin Immunol 2005;115:1102-4. [DOI] [PubMed]
- 52.Maffei M, Halaas J, Ravussin E, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1995;1:1155-61. [DOI] [PubMed]
- 53.Shore SA, Fredberg JJ. Obesity, smooth muscle, and airway hyperresponsiveness. J Allergy Clin Immunol 2005;115:925-7. [DOI] [PubMed]
- 54.Kim S, Camargo CA Jr. Sex-race differences in the relationship between obesity and asthma: the behavioral risk factor surveillance system, 2000. Ann Epidemiol 2003;13:666-73. [DOI] [PubMed]
- 55.Aaron SD, Fergusson D, Dent R, et al. Effect of weight reduction on respiratory function and airway reactivity in obese women. Chest 2004;125:2046-52. [DOI] [PubMed]
- 56.Stenius-Aarniala B, Poussa T, Kvarnstrom J, et al. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. BMJ 2000;320:827-32. [DOI] [PMC free article] [PubMed]
- 57.Simard B, Turcotte H, Marceau P, et al. Asthma and sleep apnea in patients with morbid obesity: outcome after bariatric surgery. Obes Surg 2004;14:1381-8. [DOI] [PubMed]
- 58.Saint-Pierre P, Bourdin A, Chanez P, et al. Are overweight asthmatics more difficult to control? Allergy 2006;61:79-84. [DOI] [PubMed]
- 59.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230-5. [DOI] [PubMed]
- 60.Deegan PC, McNicholas WT. Pathophysiology of obstructive sleep apnoea. Eur Respir J 1995;8:1161-78. [DOI] [PubMed]
- 61.Parish JM, Somers VK. Obstructive sleep apnea and cardiovascular disease. Mayo Clin Proc 2004;79:1036-46. [DOI] [PubMed]
- 62.Sériès F. Upper airway muscles awake and asleep. Sleep Med Rev 2002;6:229-42. [DOI] [PubMed]
- 63.Resta O, Foschino-Barbaro MP, Legari G, et al. Sleep-related breathing disorders, loud snoring and excessive daytime sleepiness in obese subjects. Int J Obes Relat Metab Disord 2001;25:669-75. [DOI] [PubMed]
- 64.Valencia-Flores M, Orea A, Castano VA, et al. Prevalence of sleep apnea and electrocardiographic disturbances in morbidly obese patients. Obes Res 2000;8:262-9. [DOI] [PubMed]
- 65.Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000;342:1378-84. [DOI] [PubMed]
- 66.Hu FB, Willett WC, Manson JE, et al. Snoring and risk of cardiovascular disease in women. J Am Coll Cardiol 2000;35:308-13. [DOI] [PubMed]
- 67.Wolk R, Kara T, Somers VK. Sleep-disordered breathing and cardiovascular disease. Circulation 2003;108:9-12. [DOI] [PubMed]
- 68.Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353:2034-41. [DOI] [PubMed]
- 69.The Report of an American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep 1999;22:667-89. [PubMed]
- 70.Viner S, Szalai JP, Hoffstein V. Are history and physical examination a good screening test for sleep apnea? Ann Intern Med 1991;115:356-9. [DOI] [PubMed]
- 71.Stoohs R, Guilleminault C. Investigations of an automatic screening device (MESAM) for obstructive sleep apnoea. Eur Respir J 1990;3:823-9. [PubMed]
- 72.Gyulay S, Olson LG, Hensley MJ, et al. A comparison of clinical assessment and home oximetry in the diagnosis of obstructive sleep apnea. Am Rev Respir Dis 1993;147:50-3. [DOI] [PubMed]
- 73.Sériès F, Marc I, Cormier Y, et al. Utility of nocturnal home oximetry for case finding in patients with suspected sleep apnea hypopnea syndrome. Ann Intern Med 1993;119:449-53. [DOI] [PubMed]
- 74.Flemons WW, Whitelaw WA, Brant R, et al. Likelihood ratios for a sleep apnea clinical prediction rule. Am J Respir Crit Care Med 1994;150:1279-85. [DOI] [PubMed]
- 75.Noseda A, Kempenaers C, Kerkhofs M, et al. Sleep apnea after 1 year domiciliary nasal-continuous positive airway pressure and attempted weight reduction. Potential for weaning from continuous positive airway pressure. Chest 1996;109:138-43. [DOI] [PubMed]
- 76.Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000;284:3015-21. [DOI] [PubMed]
- 77.Sullivan CE, Issa FG, Berthon-Jones M, et al. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet 1981;1:862-5. [DOI] [PubMed]
- 78.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046-53. [DOI] [PubMed]
- 79.Dursunoglu N, Dursunoglu D, Cuhadaroglu C, et al. Acute effects of automated continuous positive airway pressure on blood pressure in patients with sleep apnea and hypertension. Respiration 2005;72:150-5. [DOI] [PubMed]
- 80.Veale D, Pepin JL, Wuyam B, et al. Abnormal autonomic stress responses in obstructive sleep apnoea are reversed by nasal continuous positive airway pressure. Eur Respir J 1996;9:2122-6. [DOI] [PubMed]
- 81.Riley RW, Powell NB, Guilleminault C. Maxillofacial surgery and nasal CPAP. A comparison of treatment for obstructive sleep apnea syndrome. Chest 1990;98:1421-5. [DOI] [PubMed]
- 82.Gotsopoulos H, Chen C, Qian J, et al. Oral appliance therapy improves symptoms in obstructive sleep apnea: a randomized, controlled trial. Am J Respir Crit Care Med 2002;166:743-8. [DOI] [PubMed]
- 83.Herder Cd. Schmeck J, Appelboom DJK, de Vries N. Risks of general anaesthesia in people with obstructive sleep apnoea. BMJ 2004;329:955-9. [DOI] [PMC free article] [PubMed]
- 84.Bickelmann AG, Burwell CS, Robin ED, et al. Extreme obesity associated with alveolar hypoventilation; a Pickwickian syndrome. Am J Med 1956;21:811-8. [DOI] [PubMed]
- 85.Kessler R, Chaouat A, Schinkewitch P, et al. The obesity-hypoventilation syndrome revisited: a prospective study of 34 consecutive cases. Chest 2001;120:369-76. [DOI] [PubMed]
- 86.Olson AL, Zwillich C. The obesity hypoventilation syndrome. Am J Med 2005;118:948-56. [DOI] [PubMed]
- 87.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr 1974;32:77-97. [DOI] [PubMed]
- 88.Schols AMWJ, Wouters EFM, Soeters PB, et al. Body composition by bioelectrical-impedance analysis compared with deuterium dilution and skinfold anthropometry in patients with chronic obstructive pulmonary disease. Am J Clin Nutr 1991;53:421-4. [DOI] [PubMed]
- 89.Pichard C, Kyle UG, Janssens JP, et al. Body composition by X-ray absorptiometry and bioelectrical impedance in chronic respiratory insufficiency patients. Nutrition 1997;13:952-8. [DOI] [PubMed]
- 90.Kyle UG, Pichard C, Rochat T, et al. New bioelectrical impedance formula for patients with respiratory insufficiency: comparison to dual-energy X-ray absorptiometry. Eur Respir J 1998;12:960-6. [DOI] [PubMed]
- 91.Slosman DO, Casez JP, Pichard C, et al. Assessment of whole-body composition with dual-energy x-ray absorptiometry. Radiology 1992;185:593-8. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


