Abstract
The stability of the genetic structure of rhizobial populations nodulating Phaseolus vulgaris cultivated in a traditionally managed milpa plot in Mexico was studied over three consecutive years. The set of molecular markers analyzed (including partial rrs, glnII, nifH, and nodB sequences), along with host range experiments, placed the isolates examined in Rhizobium etli bv. phaseoli and Rhizobium gallicum bv. gallicum. Cluster analysis of multilocus enzyme electrophoresis and plasmid profile data separated the two species and identified numerically dominant clones within each of them. Population genetic analyses showed that there was high genetic differentiation between the two species and that there was low intrapopulation differentiation of the species over the 3 years. The results of linkage disequilibrium analyses are consistent with an epidemic genetic structure for both species, with frequent genetic exchange taking place within conspecific populations but not between the R. etli and R. gallicum populations. A subsample of isolates was selected and used for 16S ribosomal DNA PCR-restriction fragment length polymorphism analysis, nifH copy number determination, and host range experiments. Plasmid profiles and nifH hybridization patterns also revealed the occurrence of lateral plasmid transfer among distinct multilocus genotypes within species but not between species. Both species were recovered from nodules of the same plants, indicating that mechanisms other than host, spatial, or temporal isolation may account for the genetic barrier between the species. The biogeographic implications of finding an R. gallicum bv. gallicum population nodulating common bean in America are discussed.
Rhizobia are soil bacteria that are capable of inducing the formation of nitrogen-fixing nodules on the roots or stems of particular legume host plants (51). Rhizobial species seem to have coevolved with their hosts at their centers of diversification (30). Common bean (Phaseolus vulgaris) originated in America; this plant was exported to the rest of the world starting in the early 16th century, and it is currently an important crop worldwide (17, 18). At least five species have been reported to nodulate common bean. Rhizobium etli bv. phaseoli is the predominant P. vulgaris-nodulating species in Mexico, Colombia, and Argentina (1, 11, 42). R. etli bv. phaseoli is found in regions where common bean has been introduced, such as Spain, France, Austria, Senegal, Gambia, and Tunisia (10, 21, 24, 33, 34, 44). However, in these countries other Rhizobium species also nodulate this legume. Rhizobium leguminosarum bv. phaseoli is commonly found in Europe, and it has also been reported to be present in Tunisia and Colombia (11, 21, 33, 34). Rhizobium tropici is present in acid soils of South America (31) and has been found in France (2) and several African countries (4, 10). Rhizobium giardinii has been found only in European and Tunisian soils (3, 21, 34). Rhizobium gallicum has been found nodulating beans in Europe (3, 21) and Tunisia (33, 34), and one Mexican strain (strain FL27) has been identified as a member of this species (44).
In Rhizobium a large proportion of the genome is composed of plasmids (16), which contribute significantly to the ecological fitness and symbiotic performance of rhizobial strains (6, 8, 16). The symbiotic genes for nodulation (nod) and nitrogen fixation (nif, fix) are located on the symbiotic plasmid (pSym). The pSym plasmids of biovar phaseoli strains that nodulate P. vulgaris have multiple copies of the nitrogenase reductase gene (nifH) and confer a restricted host range (28). In contrast, R. tropici and R. gallicum bv. gallicum pSym plasmids carry a single nifH copy and confer a broader host range that includes Leucaena spp. (3, 9). Segovia and collaborators (41) suggested that R. leguminosarum bv. phaseoli is the result of pSym transfer from R. etli bv. phaseoli in historic times. The R. gallicum and R. giardinii bv. phaseoli strains that nodulate P. vulgaris harbor pSym plasmids similar to those found in R. leguminosarum bv. phaseoli and R. etli bv. phaseoli, which led Amarger and colleagues (3) to propose that the acquisition of R. etli bv. phaseoli pSym took place via R. leguminosarum strains. The presence of viable R. etli bv. phaseoli strains on the testa of P. vulgaris seeds has been demonstrated (36). This finding could explain the geographical spread of R. etli bv. phaseoli and provides a scenario for the lateral transfer of pSym among indigenous Rhizobium species in historic and recent times along with the introduction of bean crops worldwide.
Besides the strong evidence for interspecies pSym transfer, population level analyses of Rhizobium species have also revealed the existence of lateral transfer of pSym within species in agricultural fields and pastures (25, 27, 40, 60). However, Wernegreen and colleagues (57) found a limited pattern of pSym transfer (48) in R. leguminosarum bv. trifolii populations associated with native Trifolium species growing in mountain meadows of California.
The effects of agricultural practices and plant host domestication on the genetic structure of Rhizobium populations have scarcely been addressed (30, 47). The domestication of wild P. vulgaris plants began around 4,000 years ago in Mesoamerica and the Andean region of South America (22). Beans were probably codomesticated with maize in Mesoamerica, since these two crops are grown in association in a traditional agrosystem called milpa (29). The milpa system is a prehispanic cultivation method, in which beans are intercropped with maize and squash, together with diverse other plant species that are locally used for medicinal and nutritional purposes (47). This cultural practice promotes bean nitrogen fixation, and its advantages have been recognized (5, 29). In a previous study we compared the genetic structures of R. etli bv. phaseoli strains associated with beans under different degrees of domestication (47). We found that the bacteria associated with milpa beans had a genetic structure intermediate between the genetic structures of the bacteria isolated from wild beans and monocultured beans (47). In a more recent study, we analyzed the genetic structure of a Rhizobium population obtained from nodules of P. vulgaris and Phaseolus coccineus plants from milpa plots in San Miguel, Puebla, Mexico, with the aim of understanding its spatial variation (45). To do this, six plots were sampled in a single year, and a hierarchical analysis of multilocus enzyme electrophoresis (MLEE) data revealed (i) the coexistence of two distantly related genetic groups, designated genetic divisions I and III, and (ii) the existence of numerically dominant genotypes within each division; in addition, linkage disequilibrium analyses indicated that recombination is frequent within each genetic division but not between the divisions, which led us to propose a reticulated and epidemic genetic structure (32, 45). In the present study, P. vulgaris plants from one of the previously studied milpa plots were sampled over three consecutive years to determine (i) the temporal stability of the population genetic structure, (ii) the structure and dynamics of the plasmidic compartment, and (iii) the taxonomic affiliations of the genetic groups identified.
MATERIALS AND METHODS
Description of the sampling site and procedure.
San Miguel Acuexcomac is a village with a semiarid climate (annual rainfall, 600 mm) and an alkaline soil (pH 8.4) and is located in the state of Puebla, Mexico (45). This area has a long history of bean cultivation that extends for centuries and has never been inoculated with rhizobial strains (47). Agricultural plots are traditionally managed as typical milpas, in which bean, maize, and squash are cultivated together. Low levels of fertilizer, minimal tillage, and hand weeding practices are used. The main P. vulgaris variety grown at this site is the climbing landrace called mantequilla. The germplasm is actively maintained by the indigenous community, and each year seeds from the previous crop are sown (47). One plot (plot B of Silva et al. [45]) was sampled in three consecutive years (1994, 1995, and 1996). Rhizobium isolates were obtained in the field from root nodules of P. vulgaris plants. One isolate was obtained from each nodule, as previously described (45). All isolates were tested for growth on plates coated with PY medium (per liter, 5 g of peptone, 3 g of yeast extract, and 1 g of calcium chloride) supplemented with nalidixic acid (60 μg/ml) and on Luria-Bertani plates. Isolates were deposited in the collection of the Instituto de Ecología, Universidad Nacional Autónoma de México.
MLEE.
Cell lysates of the isolates were obtained as previously described (45), and electrophoresis was performed on cellulose acetate membranes (20, 45). The following six enzymes were assessed: isocitrate dehydrogenase (EC 1.1.1.42), peptidase (EC 3.4.13), phosphoglucomutase (EC 5.4.2.2), glucose-6-phosphate dehydrogenase (EC 1.1.1.49), xanthine dehydrogenase (EC 1.1.1.204), and malate dehydrogenase (EC 1.1.1.37). Isocitrate dehydrogenase, peptidase, and phosphoglucomutase each exhibited one band of activity, glucose-6-phosphate dehydrogenase and xanthine dehydrogenase each exhibited two bands, and malate dehydrogenase exhibited three bands, which yielded a total of 10 loci for analysis.
Genetic diversity and cluster analysis.
Distinctive mobility variants of each enzyme, numbered in order of decreasing anodal mobility, were considered alleles at the corresponding locus (43). In the case of enzymes that produced more than one band, each band was considered a locus. The absence of enzyme activity was scored as a null allele and was treated as an ordinary allele. The combined allele profiles were defined as multilocus genotypes (electrophoretic types [ETs]). Based on allele frequencies for ETs, the genetic diversity for an enzyme locus (h) was calculated as follows: h = (1 − Σxi2)[n/(n − 1)], where xi is the frequency of the ith allele and n is the number of ETs (43). The total mean genetic diversity (H) is the arithmetic mean of h values for all loci and represents the proportion of loci at which two randomly chosen genotypes can be expected to differ. To compute the H values, we used the program ETDIV, version 2.2 (58).
The genetic distance between each pair of different ETs was estimated by determining the mean character differences, and a similarity matrix was constructed by using the PAUP* program (53); the data were clustered by the unweighted pair group method with arithmetic averages (UPGMA) (46).
Genetic differentiation.
To estimate the relative genetic differentiation (Gst), we used Nei's equation, Gst = (Ht − Hs)/Ht (49), where Ht is the expected diversity in an equivalent randomly mating total population and Hs is the average diversity of the subpopulations. To compute the Gst values, we used the program ETDIV, version 2.2 (58). The indices range from 0, if there is no genetic differentiation at a given level, to 1, if there is maximal genetic differentiation (35). To test if the Gst values were significantly different from 0, we performed a chi-square test of independence as follows: χ2 = nGst(a − 1), where n is the number of individuals and a is the total number of alleles. The degrees of freedom are (k − 1)(a − 1), where k is the number of subdivisions. The degrees of freedom and χ2 values were summed across loci, and significance was examined at a P value of <0.05 (19, 59).
Linkage disequilibrium analyses.
To determine the extent to which populations exhibited nonrandom associations of alleles between loci, we used a multilocus index based on the distribution of allelic mismatches between pairs of isolates for all loci. The ratio of the variance in mismatches observed in a population (Vo) to the expected variance of the corresponding population at linkage equilibrium (random association of alleles) (Ve) provides a measure of linkage disequilibrium. If there is no linkage disequilibrium, Vo/Ve is 1. The significance of the difference between Vo and Ve was calculated by using a Monte Carlo procedure with 1,000 iterations, which was carried out with the LDV program (50).
Visualization and cluster analysis of plasmid profiles.
The plasmid contents of the isolates were visualized by using the Eckhardt procedure (12). Plasmid mobilities were determined in 0.7% agarose gels by using plasmids of R. etli bv. phaseoli CFN42 and Sinorhizobium meliloti 1021 as molecular size references. A plasmid profile similarity matrix was constructed by using the PAUP* program (53), and data were clustered by using the UPGMA algorithm (46).
PFGE of plasmids and pSym determination.
Pulsed-field gel electrophoresis (PFGE) was used to obtain accurate size estimates for plasmids from selected isolates and to identify the pSym plasmids. Intact genomic DNA was prepared in 0.8% agarose plugs and subjected to PFGE in a contour-clamped homogeneous electric field apparatus (CHEF-DRII; Bio-Rad) by following the manufacturer's instructions. Electrophoresis was carried out at 13°C with a constant voltage of 4.5 V cm−1 by using a two-block program as follows: block 1, 30-s initial switch, 60-s final switch, and 8-h run time; and block 2, 50-s initial switch, 100-s final switch, and 28-h run time. Saccharomyces cerevisiae chromosomes (Bio-Rad) were used as molecular size markers. Gels were stained with ethidium bromide, photographed, and transferred to nylon filters. Membrane hybridization with a nifH probe was performed as described below.
Analysis of nifH gene organization.
Genomic DNA from 34 isolates was digested with endonuclease BamHI, subjected to 1% agarose gel electrophoresis, stained with ethidium bromide, photographed, and transferred to nylon filters. An internal nifH fragment (∼450 bp) from strain CFN42 was amplified with primers o1 and o3 (37), as described below. This fragment was labeled with digoxigenin-dUTP by using random primers, and detection was performed with anti-digoxigenin-alkaline phosphatase Fab fragments by using the chemiluminescence system and following the instructions of the manufacturer (Roche). Membrane hybridization and washing were performed under high-stringency conditions (65°C, 0.5× SSC [1× SSC is 0.15 M NaCl plus 0.015 sodium citrate]). Some membranes were also hybridized with a lambda probe to obtain accurate estimates of the hybridization signal sizes on gels normalized with HindIII-digested lambda DNA.
Plant nodulation tests and acetylene reduction assay.
Seeds of P. vulgaris cv. Negro Jamapa, Macroptilium atropurpureum, and Leucaena leucocephala cv. Peruvian were surface sterilized with sodium hypochlorite (54). Pregerminated seeds were placed in flasks filled with vermiculite, watered with an N-free plant nutrient solution (13), and inoculated with each of the 34 selected isolates. Plants were maintained in a growth chamber at 28°C with a photoperiod of 15 h. After 4 weeks, the numbers of nodules were counted, and nitrogen fixation was measured by the acetylene reduction assay (54).
PCR-restriction fragment length polymorphism (RFLP) analysis of the 16S rRNA gene.
The 16S rRNA genes of 34 isolates were PCR amplified by using primers fD1 and rD1 (55). The amplification products were digested with endonucleases Sau3AI, MspI, and PstI and visualized in agarose gels as described elsewhere (23). Type and reference strains of the five recognized species that nodulate common bean (R. etli bv. phaseoli CFN42, R. tropici CIAT899 and CFN299, R. leguminosarum bv. phaseoli USDA2671, R. gallicum bv. gallicum R602sp and FL27, and R. giardinii H152) were included for comparison.
PCR amplification and nucleotide sequencing.
Two isolates were selected for partial DNA sequencing of two chromosomal genes, rrs coding for 16S rRNA and glnII coding for glutamine synthetase, and two pSym genes, nifH and nodB, coding for the dinitrogenase reductase and N-acetylglucosamine deacetylase, respectively. For PCR amplification, a reaction mixture (50 μl) containing 1× PCR buffer (Gibco BRL), 1.5 mM MgCl2, each deoxynucleotide triphosphate at a concentration of 200 μM, each primer at a concentration of 0.2 μM, and 2 U of Taq polymerase was used. The following temperature profile was used for all amplifications: 3 min at 94°C, followed by 30 cycles of 1 min at 94°C, 1 min at the appropriate annealing temperature, and 1 min of extension at 72°C and a final extension cycle consisting of 72°C for 5 min. The annealing temperatures for the different primer pairs are indicated below. rrs gene fragments were amplified with primers PF2 (TACTGTCGATCTGGAGTATG) and PR1 (ATTGTAGCACGTGTGTAGCC) (annealing temperature, 60°C); glnII gene fragments were amplified with primers GSF1B and GSR2seq (56) (annealing temperature, 70°C); nifH sequences were amplified with primers o1 and o3 (37) (annealing temperature, 58°C); and almost complete nodB genes were amplified with primers nodB3F (56) and nodCRR (GAGACGGCGRCRRTGCTGGTTG) (annealing temperature, 65°C). The same primers were used for sequencing reactions with ABI Prism Big Dye chemistry, and the products were analyzed with an ABI377 sequencer (ABI, Foster City, Calif.).
Nucleotide sequence accession numbers.
The sequences obtained in this study have been deposited in the GenBank sequence database under accession numbers AF529011 to AF529022.
RESULTS
Plant sampling and bacterial isolation.
In 1994, 1995, and 1996 six, seven, and eight plants were sampled and 30, 38, and 58 isolates were obtained, respectively (Table 1). Over the 3 years a total of 21 plants were sampled and 126 isolates were obtained. All isolates grew on PY plates supplemented with nalidixic acid (60 μg/ml) but were unable to grow on Luria-Bertani plates. A total of 108 isolates formed gummy colonies on PY plates (classified as R. etli [see below]), and the remaining 18 isolates exhibited a rough colony appearance (classified as R. gallicum [see below]). Ten of the 21 plants sampled harbored R. etli isolates exclusively, whereas 11 plants harbored both R. etli and R. gallicum isolates.
TABLE 1.
Distribution of ETs and plasmid profiles among 18 R. gallicum and 108 R. etli isolates from nodules of P. vulgaris plants over three consecutive yearsa
| ET | Sampling year
|
Total no. of isolates | ||
|---|---|---|---|---|
| 1994 | 1995 | 1996 | ||
| R. gallicum | ||||
| ET4 | P13 (2)b | P12 (2), P21, P22 | 6 | |
| ET8 | P4, P31 | 2 | ||
| ET12 | P4 | 1 | ||
| ET15 | P4 | 1 | ||
| ET16 | P12 | 1 | ||
| ET19 | P14 | 1 | ||
| ET26 | P4 | 1 | ||
| ET29 | P4 | 1 | ||
| ET30 | P4 | 1 | ||
| ET38 | P4 | 1 | ||
| ET39 | P14 | 1 | ||
| ET40 | P4 | 1 | ||
| R. etli | ||||
| ET1 | P1 (2), P2 (5), P5 (2), P6, P11, P19 | P1 (4), P2 | P1 (9), P2 (2), P3, P5 (2), P6 (3), P8, P11, P26, P27 | 38 |
| ET2 | P3, P9, P10, P17, P18, P20 | P1, P3 (2), P7, P9, P10, P23 | P1, P16, P25, P29, P32 | 18 |
| ET3 | P2 (4), P3 (3) | P3 (3) | P1, P2 (2), P3, P15 | 15 |
| ET5 | P9 | P9, P10 | 3 | |
| ET6 | P1 (3) | 3 | ||
| ET7 | P1 (2), P2 | 3 | ||
| ET9 | P1, P30 | 2 | ||
| ET10 | P1, P8 | 2 | ||
| ET11 | P2 | P3 | 2 | |
| ET13 | P3 | 1 | ||
| ET14 | P11 | 1 | ||
| ET17 | P2 | 1 | ||
| ET18 | P7 | 1 | ||
| ET20 | P3 | 1 | ||
| ET21 | P5 | 1 | ||
| ET22 | P16 | 1 | ||
| ET23 | P15 | 1 | ||
| ET24 | P7 | 1 | ||
| ET25 | P7 | 1 | ||
| ET27 | P24 | 1 | ||
| ET28 | P1 | 1 | ||
| ET31 | P3 | 1 | ||
| ET32 | P5 | 1 | ||
| ET33 | P28 | 1 | ||
| ET34 | P2 | 1 | ||
| ET35 | P8 | 1 | ||
| ET36 | P1 | 1 | ||
| ET37 | P3 | 1 | ||
| ET41 | P1 | 1 | ||
| ET42 | P8 | 1 | ||
| ET43 | P33 | 1 | ||
A total of 126 isolates were obtained (30 isolates in 1994, 38 isolates in 1995, and 58 isolates in 1996).
Plasmid profiles found in multiple isolates are indicated by boldface type, and the number of isolates is given in parentheses when there was more than one isolate.
Genetic diversity, genetic differentiation, and cluster analysis.
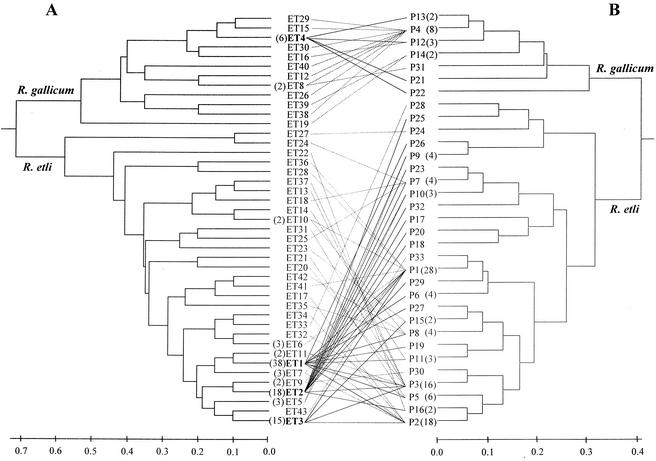
The MLEE survey yielded 43 different multilocus genotypes for the 126 isolates. Cluster analysis of the MLEE data revealed two genetic divisions separated at a mean genetic distance of 0.7 (Fig. 1), corresponding to genetic divisions I and III described previously (45). On the basis of the nucleotide sequence, plasmid profiling, nifH copy number, and host range analyses described below, the 18 division I isolates were classified as R. gallicum bv. gallicum, and the 108 division III isolates were confirmed to be R. etli bv. phaseoli. The R. gallicum and R. etli isolates had similar mean genetic diversities (Table 2), and both populations displayed ET dominance, since a few ETs were represented by many isolates (Table 1 and Fig. 1). For the R. gallicum population, ET4 was recovered at the highest frequency and ET4 isolates comprised 33% of the R. gallicum isolates, although it was not found in the 1996 samples (Table 1). In the R. etli population, ET1, ET2, and ET3 (with 38, 18, and 15 isolates, respectively) were the most abundant ETs, were found in all 3 years and comprised 66% of the R. etli isolates (Table 1 and Fig. 1). A chi-square test showed that the frequencies of these three ETs did not vary significantly over the 3 years (χ2 = 7.14; df = 4; P = 0.129), suggesting that there was temporally stable genotype dominance in the population. These dominant R. etli genotypes grouped in a tight cluster, as shown in Fig. 1 (mean genetic distance, <0.2), indicating that they form a clonal complex (14).
FIG. 1.
Dendrograms showing the genetic relatedness among chromosomal and plasmidic genotypes of R. etli and R. gallicum and the chromosome-plasmid profile combinations. (A) Genetic relatedness of the multilocus genotypes based on 10 isoenzymatic loci. The ET designations are indicated. (B) Genetic relatedness of the plasmid profiles based on the presence or absence of the different plasmid size classes. The plasmid profile designations are indicated. The number of isolates for each multiple ET or plasmid profile is given in parentheses. The genetic distance between each pair of ETs or plasmid profiles was estimated by determining mean character differences, and data were clustered by the UPGMA. The four most abundant ETs and their plasmid profile combinations are indicated by boldface type and solid lines; the other combinations are indicated by dashed lines.
TABLE 2.
Genetic diversity, genetic differentiation, and linkage disequilibrium estimates for R. gallicum and R. etli populations associated with P. vulgaris plants in San Miguel Acuexcomac, Mexico
| Population | No. of isolates | No. of ETs | Mean genetic diversity | Mean no. of alleles | Gst | Isolates
|
ETs
|
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean no. of mismatches | Vo/Vea | Pb | Mean no. of mismatches | Vo/Vea | Pb | ||||||
| R. gallicum | 18 | 12 | 0.388 (0.086)c | 2.5 | 0.045 | 3.3 | 1.66 | <0.001 | 3.9 | 0.96 | NSd |
| R. etli | 108 | 31 | 0.351 (0.059) | 2.9 | 0.073 | 1.9 | 1.72 | <0.001 | 3.5 | 1.06 | NS |
| Total | 126 | 43 | 0.501 (0.060) | 3.7 | 0.285e | 3.3 | 3.90 | <0.001 | 5.0 | 2.18 | <0.001 |
Observed variance/expected variance of the mismatch distribution.
Probability of rejecting by chance the null hypothesis that Vo = Ve.
The values in parentheses are standard errors.
NS, not significant.
Gst is significantly different from 0.
The level of genetic differentiation between the R. etli and R. gallicum populations was high and significant (Gst = 0.285; P < 0.001), whereas the levels of intrapopulation genetic differentiation over the 3 years for R. etli (Gst = 0.073; P = 0.243) and R. gallicum (Gst = 0.045; P = 0.999) were not significant (Table 2). Furthermore, of the 37 alleles found in the 126 isolates, 8 were detected exclusively in the R. gallicum population, while 12 were unique to the R. etli population, and the 17 shared alleles were found at disparate frequencies in the two species (the MLEE data set is available from the corresponding author upon request). These results indicate that the two species do not share the main part of their alleles and that within the species the levels of genetic variability remained constant during the 3 years sampled.
Linkage disequilibrium analyses.
A hierarchical linkage disequilibrium analysis was performed with the MLEE data set to estimate the extents of genetic exchange within and between the R. etli and R. gallicum populations. As shown in Table 2, both populations showed linkage equilibrium when only ETs were included in the analysis. When all isolates of each species were considered, the populations appeared to be in linkage disequilibrium, due to the presence of numerically dominant ETs (epidemic clones [32]). These results revealed an epidemic genetic structure for both species, in which the frequency of a few dominant genotypes increases to produce epidemic clones, but frequent genetic exchange occurs among the members of the population. Linkage disequilibrium was detected when the analysis was performed with either all 126 isolates or 43 ETs (Table 2), indicating that the extent of genetic exchange between R. etli and R. gallicum is negligible, if there is any exchange at all.
Diversity and cluster analysis of plasmid profiles.
Eleven plasmid size classes were identified among the 18 R. gallicum isolates; the sizes ranged from ∼50 to >1,500 kb and represented seven distinct profiles (Table 1 and Fig. 1). R. gallicum isolates harbored two to four plasmids (average, 3.1 plasmids), and all isolates contained a >1,500-kb megaplasmid, which was absent in the R. etli population (Table 3). Profile P4 was the dominant profile; it was observed in 44% of the isolates (Table 1 and Fig. 1) and was indistinguishable from the profile of the type strain of R. gallicum bv. gallicum R602sp (Table 3). Fifteen plasmid size classes were detected among the population of 108 R. etli isolates, and the plasmid sizes ranged from ∼125 to ∼700 kb. Twenty-six plasmid profiles were found, consisting of two to five plasmids (average, 3.5 plasmids). All of the R. etli profiles included the ∼700-kb plasmid, which was not found in the R. gallicum population (Table 3). The R. etli population displayed a high degree of plasmid profile dominance, with many isolates sharing a few plasmid profiles. Profiles P1, P2, and P3 were found in 57% of the R. etli isolates (Table 1 and Fig. 1); these profiles were the predominant profiles and were recovered in all 3 years. A chi-square test indicated that the frequencies of these three profiles varied significantly over the 3 years (χ2= 19.32; df = 4; P < 0.001). Profile P1 was the most abundant profile in 1996, P2 was the most abundant profile in 1994, and P3 was the most abundant profile in 1995. These apparent temporal changes in plasmid profile dominance in the R. etli population could have been due simply to the small sample size or could reflect actual changes in the plasmid combinations selected in the crop seasons (for example, due to fluctuations in environmental conditions).
TABLE 3.
Multilocus genotypes (ETs), plasmid profiles, 16S ribosomal DNA RFLP patterns, nifH hybridization patterns, and estimated sizes of plasmids and nifH hybridization bands for 34 selected P. vulgaris isolates and reference strains
| Straina | ET | 16S ribosomal DNA RFLP patternb | Plasmid
|
nifH
|
||
|---|---|---|---|---|---|---|
| Profile | Sizes (kb)c | Pattern | Size(s) (kb) | |||
| R. gallicum isolates | ||||||
| IE992 | ET4 | AAB | P13 | >1,500, 550, 350, 250 | C1 | 8.3 |
| IE2703 | ET4 | AAB | P22 | >1,500, 650, 125, 50 | C1 | 8.3 |
| IE4868 | ET40 | ABA | P4 | >1,500, 550, 250 | C1 | 8.3 |
| IE4845 | ET38 | AAA | P4 | >1,500, 550, 250 | C1 | 8.3 |
| IE2729 | ET15 | AAB | P4 | >1,500, 550, 250 | C2 | 9.5 |
| IE988 | ET12 | AAA | P4 | >1,500, 550, 250 | C2 | 9.5 |
| IE4770 | ET29 | AAB | P4 | >1,500, 550, 250 | C1 | 8.3 |
| IE4872 | ET8 | AAA | P31 | >1,500, 550, 180, 125 | C1 | 8.3 |
| IE2735 | ET16 | ABA | P12 | >1,500, 550 | C1 | 8.3 |
| IE2751 | ET19 | ABA | P14 | >1,500, 525, 250 | C3 | 12.0 |
| R. etli isolates | ||||||
| IE4810 | ET6 | BAB | P1 | 700, 550, 450 | A1 | 4.6, 12.4, 16.6 |
| IE4813 | ET32 | BAB | P5 | 700, 450, 390, 125 | B1 | 5.6, 9.8 |
| IE4794 | ET31 | BAB | P3 | 700, 450, 390, 250 | A2 | 2.6, 12.4, 16.6 |
| IE4803 | ET5 | BAB | P9 | 700, 500, 425 | A1 | 4.6, 12.4, 16.6 |
| IE4815 | ET33 | BAB | P28 | 700, 425, 350, 270 | A2 | 2.6, 12.4, 16.6 |
| IE4876 | ET43 | BAB | P33 | 700, 550, 450, 225 | A1 | 4.6, 12.4, 16.6 |
| IE954 | ET1 | BAB | P1 | 700, 550, 450 | A1 | 4.6, 12.4, 16.6 |
| IE4777 | ET1 | BAB | P1 | 700, 550, 450 | A1 | 4.6, 12.4, 16.6 |
| IE963 | ET1 | BAB | P2 | 700, 450, 390 | B1 | 5.6, 9.8 |
| IE2730 | ET1 | BAB | P2 | 700, 450, 390 | B1 | 5.6, 9.8 |
| IE4804 | ET1 | BAB | P3 | 700, 450, 390, 250 | A2 | 2.6, 12.4, 16.6 |
| IE4877 | ET1 | BAB | P5 | 700, 450, 390, 125 | B1 | 5.6, 9.8 |
| IE994 | ET1 | BAB | P5 | 700, 450, 390, 125 | B1 | 5.6, 9.8 |
| IE1006 | ET1 | BAB | P6 | 700, 550, 450, 125 | A1 | 4.6, 12.4, 16.6 |
| IE1009 | ET1 | BAB | P11 | 700, 450 | A1 | 4.6, 12.4, 16.6 |
| IE2737 | ET2 | BAB | P1 | 700, 550, 450 | A1 | 4.6, 12.4, 16.6 |
| IE4795 | ET2 | BAB | P1 | 700, 550, 450 | A1 | 4.6, 12.4, 16.6 |
| IE950 | ET2 | BAB | P3 | 700, 450, 390, 250 | A2 | 2.6, 12.4, 16.6 |
| IE2704 | ET2 | BAB | P3 | 700, 450, 390, 250 | A2 | 2.6, 12.4, 16.6 |
| IE1004 | ET2 | BAB | P10 | 700, 450, 350, 300, 250 | A2 | 2.6, 12.4, 16.6 |
| IE4874 | ET3 | BAB | P1 | 700, 550, 450 | A1 | 4.6, 12.4, 16.6 |
| IE951 | ET3 | BAB | P2 | 700, 450, 390 | B2 | 4, 5.6, 9.8 |
| IE4837 | ET3 | BAB | P2 | 700, 450, 390 | B2 | 4, 5.6, 9.8 |
| IE4771 | ET3 | BAB | P3 | 700, 450, 390, 250 | B1 | 5.6, 9.8 |
| Reference strains | ||||||
| CFN42 | ET44 | BAB | P34 | 650, 510, 390, 270, 180, 150 | B2 | 4, 5.6, 9.8 |
| FL27 | NDd | AAB | P35 | >1,500, 600, 390 | C1 | 8.3 |
| R602sp | ND | AAA | P4 | >1,500, 550, 250 | C1 | 8.3 |
The underlined isolates were used for determination of rrs, glnII, nifH, and nodB gene sequences.
Each letter refers to a restriction pattern obtained with the enzymes Sau3AI, MspI, and PstI. Restriction sites for each enzyme were mapped on the rrs sequence of R. gallicum R602sp (accession number U86343), which had an AAA pattern. The type B pattern for Sau3AI contained additional restriction sites at nucleotides 501, 505, and 561. The MspI type B pattern lacked the site at position 951. For PstI digestion, the type B pattern had an additional restriction site at nucleotide 926.
pSym plasmids are indicated by boldface type.
ND, not determined.
Figure 1 shows the separation of R. gallicum and R. etli populations on the basis of both MLEE and plasmid profiles. It is noteworthy that within species isolates belonging to the same ET could have different plasmid profiles and, conversely, that a particular plasmid profile could be found in isolates with different ETs, suggesting that plasmid transfer occurs within conspecific populations but not between species, as graphically shown in Fig. 1.
Based on the MLEE and plasmid profile data, 34 isolates were selected for further molecular analyses; this selection included 10 isolates from the R. gallicum population and 24 isolates from the R. etli population. This selection included isolates from dominant ETs and plasmid profiles, as well as isolates displaying unique ETs or plasmid profiles (Table 3).
PFGE and pSym identification.
PFGE was used to obtain accurate plasmid size estimates for the 34 isolates selected for molecular analyses, and the values are shown in Table 3. These values were used to estimate the plasmid sizes for the whole sample of 126 isolates, based on Eckhardt plasmid profiles.
The pSym plasmids of 20 isolates (4 R. gallicum and 16 R. etli isolates) were identified by Southern analysis of plasmid profiles resolved by PFGE by using a nifH probe and are indicated in Table 3. Most R. gallicum isolates harbored 550-kb pSym plasmids; the single exception was isolate IE2751, which harbored a 525-kb pSym. The sizes of the pSym plasmids of R. etli isolates ranged from 350 to 550 kb. One-half of the R. etli isolates contained a 390-kb pSym, like the R. etli type strain CFN42. In general, pSym plasmids of the same size were associated with identical or similar plasmid profiles; for example, isolates with profiles P2 and P3 harbored 390-kb pSym plasmids. The only exception was isolate IE4874, which contained a 450-kb pSym, whereas the other two isolates with profile P1 contained 550-kb pSym plasmids (Table 3).
Determination of nifH copy number by Southern hybridization.
For the 34 isolates analyzed, six different hybridization patterns were recorded (Table 3). All R. gallicum isolates exhibited a single hybridization signal, and most of them shared an ∼8.3-kb hybridizing band (pattern C1) with R. gallicum bv. gallicum strains R602sp and FL27 (Table 3), supporting classification of our isolates as members of R. gallicum bv. gallicum. All R. etli isolates contained multiple (two or three) nifH copies, and two groups of related patterns were observed. Patterns A1 and A2 had two bands with sizes of ∼12.4 and ∼16.6 kb, while patterns B1 and B2 had two bands with sizes of ∼5.6 and ∼9.8 kb; the latter pattern was identical to that displayed by type strain R. etli CFN42 (Table 3).
Within the R. gallicum and R. etli populations, nifH hybridization patterns could be found scattered throughout the different branches of the MLEE and plasmid profile dendrograms, further supporting the conclusion derived from the comparative cluster analyses of MLEE and plasmid profile data that lateral transfer of plasmids occurs within each species but not between the species. However, in some instances an association between plasmid profile and nifH pattern within species was observed. For example, all the R. etli isolates that had a P1 plasmid profile displayed a type A1 nifH hybridization pattern, as summarized in Table 3. Notable associations between pSym size and nifH hybridization pattern were also found. For example, R. etli isolates with the A1 nifH pattern harbored 450- to 550-kb pSym plasmids, the A2 nifH patterns were associated with 390- to 425-kb pSym plasmids, and the B1 and B2 nifH patterns were strictly associated with 390-kb pSym plasmids (Table 3). R. gallicum isolates with 550-kb pSym plasmids yielded only the C1 nifH pattern, while a 525-kb pSym was associated with the distinct C3 nifH pattern. A 550-kb pSym was detected in the R. etli and R. gallicum populations, but the nifH hybridization patterns revealed that these plasmids were distinct.
Plant nodulation tests and symbiotic effectiveness.
All 34 selected isolates (Table 3) nodulated and fixed nitrogen with P. vulgaris plants (Table 4). However, R. gallicum isolates induced only one-third of the nodules induced by R. etli isolates. All R. gallicum isolates formed effective nodules on M. atropurpureum, whereas R. etli isolates induced less than one-third of the nodules induced by R. gallicum isolates on this host and were ineffective (Table 4). All R. gallicum isolates nodulated and fixed nitrogen on L. leucocephala plants, further supporting classification of these isolates as R. gallicum bv. gallicum, while all R. etli isolates were unable to nodulate this host (Table 4).
TABLE 4.
Average numbers of nodules formed by 10 R. gallicum and 24 R. etli isolates on three leguminous hostsa
| Organism | No. of nodules formed on:
|
||
|---|---|---|---|
| P. vulgaris | M. atropurpureum | L. leucocephala | |
| R. gallicum | 12.6 ± 3.1 (F)b | 15.1 ± 1.2 (F) | 5.3 ± 1.1 (F) |
| R. etli | 36.6 ± 3.4 (F) | 4.5 ± 0.75 | 0.0 |
See Table 3.
Average ± standard error. (F), nitrogen fixation (acetylene reduction was detected in nodules).
PCR-RFLP analysis of the 16S rRNA gene.
Almost complete 16S rRNA genes (∼1.5 kb) for 34 isolates were PCR amplified and digested with endonucleases Sau3AI, MspI, and PstI. Four different composite restriction patterns were found (Table 3). R. gallicum isolates displayed three different patterns (AAA, AAB, and ABA); pattern AAB was identical to the pattern of R. gallicum FL27, while pattern AAA was identical the pattern of R. gallicum R602sp. All R. etli isolates displayed the same restriction pattern (BAB), which was identical to the pattern of R. etli CFN42 (Table 3). The restriction sites for each enzyme were mapped on the rrs sequence of R. gallicum R602sp (accession number U86343) and are indicated in Table 3. The patterns displayed by other Rhizobium reference species were different from those of our isolates (data not shown).
Nucleotide sequence analyses.
Partial sequences of two chromosomally encoded genes (rrs and glnII) and two pSym-encoded genes (nifH and nodB) were determined for R. gallicum isolate IE988 (ET12 in Table 1 and Fig. 1) and R. etli isolate IE2730 (ET1 in Table 1 and Fig. 1) and, when the sequences were not available in public sequence databases, also for R. gallicum strains R602sp and FL27.
The 558-bp rrs sequence segment of isolate IE988 was very similar to that of strain R602sp (Table 5). However, the level of similarity with the R602sp and FL27 sequences reported by Sessitsch et al. (44) was lower, due to the presence of several insertions which are absent in the R602sp sequence reported by Amarger et. al. (3) and in our IE988 sequence. The rrs sequence of isolate IE2730 differed only at two nucleotides from the rrs sequence of R. etli CFN42 (Table 5).
TABLE 5.
Numbers of pairwise nucleotide differences and percentages of similarity for aligned rrs, glnII, nifH, and nodB gene segments of the R. gallicum IE988 and R. etli IE2730 isolates compared with reference strains
| Strain | No. of nucleotide differences (% of similarity)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
rrs gene
|
glnII gene
|
nifH gene
|
nodB gene
|
||||||||||
| R. gallicum R602sp (U86343)a | R. gallicum R602sp (AF008130)a | R. gallicum FL27 (AF008129)a | R. etli CFN42 (U28916)a | R. gallicum R602sp (AF529015)b | R. gallicum FL27 (AF529016)b | R. etli CFN42 (AF169585)b | R. gallicum R602sp (AF218126)a | R. gallicum FL27 (M55226)a | R. etli CFN42 (M15942)a | R. gallicum R602sp (AF529022)b | R. gallicum FL27 (AF529021)b | R. etli CFN42 (M58626)a | |
| IE988c | 5 (99.1)e | 15 (97.3) | 15 (97.3) | 11 (98.0) | 10 (98.1) | 7 (98.7) | 68 (86.4) | 5 (98.7) | 10 (97.1) | 13 (96.7) | 4 (99.3) | 6 (98.9) | 109 (80.9) |
| IE2730d | 12 (97.8) | 20 (96.4) | 18 (96.8) | 2 (99.6) | 73 (86.4) | 70 (87.0) | 25 (96.0) | 15 (97.2) | 14 (96.1) | 0 (100) | 108 (81.1) | 109 (80.9) | 0 (100) |
The number in parentheses is the accession number of the sequences retrieved from GenBank.
The number in parentheses is the accession number of the sequence generated in this study.
The accession numbers for rrs, glnII, nifH, and nodB gene segments of R. gallicum IE988 are AF529011, AF529013, AF529017, and AF529019, respectively.
The accession numbers for rrs, glnII, nifH, and nodB gene segments of R. etli IE2730 are AF529012, AF529014, AF529018, and AF529020, respectively.
Pairwise nucleotide difference, including gaps (percentage of similarity).
A 540-bp glnII sequence segment was obtained for isolates IE988 and IE2730 and for strains R602sp and FL27. The highest levels of similarity were found between isolate IE988 and strains FL27 and R602sp, while isolate IE2730 showed the highest level of similarity with R. etli CFN42 (Table 5).
The 389-bp nifH sequence segment obtained for isolate IE988 was most similar to the nifH sequence segments of strains R602sp and FL27, while the nifH sequence of isolate IE2730 was identical to the sequence of the three paralogous nifH copies of R. etli CFN42 (Table 5).
An almost complete nodB sequence segment (571 bp) was obtained for isolates IE988 and IE2730 and for strains R602sp and FL27. The nodB sequence segment of IE988 was very similar to the nodB sequence segments of strains R602sp and FL27, while the nodB sequence of IE2730 was identical to the nodB sequence of R. etli CFN42 (Table 5).
Taken together, these results strongly support the taxonomic placement of isolate IE988 in R. gallicum bv. gallicum and the taxonomic placement of isolate IE2730 in R. etli bv. phaseoli.
DISCUSSION
In this study we analyzed the genetic structure of rhizobial populations nodulating P. vulgaris plants growing in a traditionally managed milpa plot in San Miguel Acuexcomac, Mexico, over three consecutive years. This site was selected for our study because of its long history of bean cultivation that extends back to pre-Columbian times (47). In a previous study of the genetic structure of rhizobial populations associated with beans grown in six milpa plots at this site, low levels of genetic differentiation among the plots (Gst = 0.072) were found, and five dominant ETs were recovered from all six plots, indicating that the population structure was stable at the spatial scale analyzed (45). Based on these findings, we decided to sample a single representative plot to study the temporal stability of the genetic structure, as well as to gain insight into the structure and dynamics of the plasmidic compartment, an issue that has not been addressed previously.
The three dominant R. etli ETs identified in the present work correspond to those found in the previous study (45), indicating that the genetic composition of the populations at this site is stable both in terms of space (six plots sampled in a single year [45]) and in terms of time (one plot sampled over 3 years [this study]). Furthermore, the two distinct lineages that were consistently recovered over the 3 years sampled correspond to the previously described genetic divisions I and III (45). Linkage disequilibrium analyses confirmed that recombination takes place within but not between these lineages. Importantly, the set of molecular markers analyzed in this study, along with the host range experiments, unambiguously placed our division I population in R. gallicum bv. gallicum and the division III population in R. etli bv. phaseoli. Therefore, the two lineages correspond to two distinct species and are not compartments within an R. etli population, as suggested previously (45). As a consequence, we concluded that the genetic structure of the R. etli and R. gallicum populations at this site is epidemic but not reticulated, as defined by Maynard Smith and colleagues (32).
Strong evidence that there is genetic exchange within but not between the R. etli and R. gallicum populations was also provided by the analysis of the plasmid compartment. Both the plasmid profiles and the nifH hybridization patterns provided evidence that there is plasmid transfer among distinct multilocus genotypes within each species (Fig. 1 and Table 3). The panmictic plasmid transfer pattern (48) found for both species could have been the result of the selective pressure imposed by agricultural practices, as suggested by Wernegreen and colleagues (57), or may have been an intrinsic feature of these Rhizobium species. The evidence that recombination took place also in the chromosomal compartment, as demonstrated by the linkage disequilibrium analyses, favors the latter possibility. Taken together, these results indicate that genetic exchange is an important source of variation and cohesion in the ecology and evolution of the two species, although it is not great enough to prevent the emergence of epidemic clones that are recovered from nodules at a high frequency. This finding may be interpreted as evidence that there is strong selective pressure imposed by the host, which favors the maintenance of particular chromosome-plasmid associations. It will be interesting to test whether the epidemic clones are particularly competitive for nodulation or if they are simply numerically dominant clones in the soil. It is important that both species nodulate the same individual plants, indicating that mechanisms other than host, spatial, or temporal isolation may account for the genetic barrier between them.
The 34 strains selected for molecular and host range analyses revealed that the subsample of 10 R. gallicum isolates constitutes a diverse lineage that displays three of the four 16S PCR-RFLP patterns detected, three different nifH hybridization patterns, and two pSym sizes (Table 3). In addition to P. vulgaris, these isolates effectively nodulated the other two hosts tested (Table 4). On the other hand, the 24 R. etli isolates effectively nodulated only P. vulgaris. All of the isolates displayed the same 16S PCR-RFLPs, but they harbored six pSym size classes and displayed four nifH hybridization patterns. The comparison of plasmid profiles, nifH hybridization patterns, and pSym sizes revealed the complex and dynamic structure of the plasmidic compartment within species. Generally, pSym plasmids that were the same size and had identical nifH hybridization patterns were associated with identical plasmid profiles. However, there were some exceptions that might indicate the existence of genetic rearrangements which affect the plasmidic compartment. These rearrangements can operate within particular strains, as extensively documented for CFN42 and NGR234 (7, 15, 38, 39), and may be coupled with lateral transfer within populations, as proposed in the present study.
Only 14% of all of the isolates examined in this study are R. gallicum isolates. This is in good agreement with the results of a previous study (45), in which it was reported that 10% of the isolates obtained from P. vulgaris corresponded to genetic division I (R. gallicum). In that study, however, R. gallicum isolates were recovered from P. coccineus nodules at nearly the same frequency (54%) as R. etli isolates. These results indicate that R. gallicum and R. etli nodulate both hosts, although the latter species is clearly more competitive for P. vulgaris nodulation. Thus, although R. gallicum was originally isolated from P. vulgaris nodules in France (3), this plant may not be its primary host. This possibility is further supported by the report that R. gallicum strains were isolated in Canada from Onobrychis viciifolia and Oxytropis riparia (tribe Galegae), suggesting that not all potential hosts for this species have been identified yet (26).
Besides its broad host range, R. gallicum has a wide geographic distribution, which raises a question about its biogeography. R. gallicum populations have been isolated from bean nodules in several European countries and Tunisia (3, 21, 33), and only one American R. gallicum bean isolate (strain FL27) has been reported previously (44). The present study provides the first report of an R. gallicum population nodulating beans in America, and it is the first population genetic analysis for the species that was performed. Sessitsch and colleagues (44) reported that the European R. gallicum strains can be distinguished from the Mexican FL27 strain by an RFLP resulting from a single nucleotide difference in the 16S rRNA gene, which created a PstI restriction site that was absent in all the French and Austrian isolates which they analyzed but was present in the Mexican strain FL27. However, this restriction site was absent in 60% of our R. gallicum isolates (Table 3) and hence cannot be used to distinguish European R. gallicum strains from American R. gallicum strains. Furthermore, the plasmid profiles and nucleotide sequences of chromosomal and plasmidic genes of the Mexican population were very similar to those of R. gallicum type strain R602sp (in some instances even more similar than they were to those of Mexican strain FL27). These observations suggest that if R. gallicum was imported from America to Europe, as previously suggested (44), the lineages that gave rise to the European isolates remain in America and that the divergence between intercontinental populations is less pronounced than the divergence suggested by Sessitsch et al. (44). The presence of R. etli and R. gallicum in Tunisian soils recently cropped with beans and the absence of these organisms in fields which had not been cultivated with beans (33) further support the hypothesis that P. vulgaris microsymbionts were introduced by being carried along with bean seeds (36). Interestingly, R. gallicum bv. phaseoli was not present in the isolates from San Miguel Acuexcomac, even though R. etli bv. phaseoli and R. gallicum bv. gallicum may have coexisted at this site for centuries. One possible explanation for this is that R. gallicum bv. phaseoli may be outcompeted by R. etli bv. phaseoli and R. gallicum bv. gallicum strains. An alternative explanation is that since R. leguminosarum has not been found in Mexico, the hypothesized bridge for the conjugal transfer of pSym from R. etli bv. phaseoli to R. gallicum is missing (3). Perhaps the introduction of common beans along with their seed-borne symbionts to other continents exerted selective pressure and provided the ecological opportunity for lateral transfer of symbiotic information to resident Rhizobium populations, as in the case of the symbiotic island acquired by saprophytic Mesorhizobium spp. native soil populations in New Zealand (52).
In conclusion, our data are consistent with the biogeographical hypothesis of an American origin of the R. gallicum lineage (44), a view that is also consistent with the report of Canadian R. gallicum isolates obtained from O. viciifolia and O. riparia (26). Alternatively, however, R. gallicum could have a wide geographical distribution and a long evolutionary history of adaptation to different environments and leguminous hosts. Further research is clearly needed to examine these alternative hypotheses. We are currently analyzing the sequences of several chromosomal and plasmidic genes of R. gallicum isolates from different continents and hosts in order to elucidate the phylogeographic origin and dispersal pathways of this species.
Acknowledgments
This research was supported by CONACyT grant 27557-M to V.S. and by a Ph.D. fellowship to C.S.
We thank the community of San Miguel, especially the Aguilar family, for their kindness and help with fieldwork. B. Gaut is gratefully acknowledged for providing sequencing facilities at the University of California Irvine, and René Hernández is acknowledged for sequencing at IBT, UNAM. We thank Marco A. Rogel, Aldo Valera, Laura Espinosa, and Rafael Díaz for providing technical assistance and P. Burgos for designing primers PF2 and PR1. We acknowledge David Romero and Susana Brom for providing critical comments on the manuscript.
REFERENCES
- 1.Aguilar, O. M., M. V. López, P. M. Riccillo, R. A. González, M. Pagano, D. H. Grasso, A. Puhler, and G. Favelukes. 1998. Prevalence of the Rhizobium etli-like allele in genes coding for 16S rRNA among the indigenous rhizobial populations found associated with wild beans from the southern Andes in Argentina. Appl. Environ. Microbiol. 64:3520-3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amarger, N., M. Bours, F. Revoy, M. R. Allard, and G. Laguerre. 1994. Rhizobium tropici nodulates field-grown Phaseolus vulgaris in France. Plant Soil 161:147-156. [Google Scholar]
- 3.Amarger, N., V. Macheret, and G. Laguerre. 1997. Rhizobium gallicum sp. nov. and Rhizobium giardinii sp. nov., from Phaseolus vulgaris nodules. Int. J. Syst. Bacteriol. 47:996-1006. [DOI] [PubMed] [Google Scholar]
- 4.Anyango, B., K. J. Wilson, J. L. Beynon, and K. E. Giller. 1995. Diversity of Rhizobium nodulating Phaseolus vulgaris L. in two Kenyan soils with contrasting pHs. Appl. Environ. Microbiol. 61:4016-4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brockwell, J., P. J. Bottomley, and J. E. Thies. 1995. Manipulation of rhizobia microflora for improving legume productivity and soil fertility: a critical assessment. Plant Soil 174:143-180. [Google Scholar]
- 6.Brom, S., A. García de los Santos, L. Cervantes, R. Palacios, and D. Romero. 2000. In Rhizobium etli symbiotic plasmid transfer, nodulation competitivity and cellular growth require interaction among different replicons. Plasmid 44:34-43. [DOI] [PubMed] [Google Scholar]
- 7.Brom, S., A. García de los Santos, M. de Lourdes Girard, G. Dávila, R. Palacios, and D. Romero. 1991. High-frequency rearrangements in Rhizobium leguminosarum bv. phaseoli plasmids. J. Bacteriol. 173:1344-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brom, S., L. Girard, A. García-de los Santos, J. M. Sanjuan-Pinilla, J. Olivares, and J. Sanjuan. 2002. Conservation of plasmid-encoded traits among bean-nodulating Rhizobium species. Appl. Environ. Microbiol. 68:2555-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brom, S., E. Martínez, G. Dávila, and R. Palacios. 1988. Narrow- and broad-host-range symbiotic plasmids of Rhizobium spp. strains that nodulate Phaseolus vulgaris. Appl. Environ. Microbiol. 54:280-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diouf, A., P. de Lajudie, M. Neyra, K. Kersters, M. Gillis, E. Martínez-Romero, and M. Gueye. 2000. Polyphasic characterization of rhizobia that nodulate Phaseolus vulgaris in West Africa (Senegal and Gambia). Int. J. Syst. E vol. Microbiol. 50:159-170. [DOI] [PubMed] [Google Scholar]
- 11.Eardly, B. D., F. S. Wang, T. S. Whittam, and R. K. Selander. 1995. Species limits in Rhizobium populations that nodulate the common bean (Phaseolus vulgaris). Appl. Environ. Microbiol. 61:507-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckhardt, T. 1978. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid 1:584-588. [DOI] [PubMed] [Google Scholar]
- 13.Fahraeus, G. 1957. The infection of clover root hairs by nodule bacteria studied by a single glass slide technique. J. Gen. Microbiol. 16:374-381. [DOI] [PubMed] [Google Scholar]
- 14.Feil, E. J., and B. G. Spratt. 2001. Recombination and the population structures of bacterial pathogens. Annu. Rev. Microbiol. 55:561-590. [DOI] [PubMed] [Google Scholar]
- 15.Flores, M., P. Mavingui, X. Perret, W. J. Broughton, D. Romero, G. Hernández, G. Dávila, and R. Palacios. 2000. Prediction, identification, and artificial selection of DNA rearrangements in Rhizobium: toward a natural genomic design. Proc. Natl. Acad. Sci. USA 97:9138-9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García de los Santos, A., S. Brom, and D. Romero. 1996. Rhizobium plasmids in bacteria-legume interactions. World J. Microbiol. Biotechnol. 12:119-125. [DOI] [PubMed] [Google Scholar]
- 17.Gepts, P. 1990. Biochemical evidence bearing on the domestication of Phaseolus (Fabaceae) beans. Econ. Bot. 44:28-38. [Google Scholar]
- 18.Gepts, P., and F. A. Bliss. 1988. Dissemination pathways of common bean (Phaseolus vulgaris, Fabaceae) deduced from phaseolin electrophoretic variability. II. Europe and Africa. Econ. Bot. 42:86-104. [Google Scholar]
- 19.Hagen, M. J., and J. L. Hamrick. 1996. A hierarchical analysis of population genetic structure in Rhizobium leguminosarum bv. trifolii. Mol. Ecol. 5:177-186. [DOI] [PubMed] [Google Scholar]
- 20.Hebert, P. D. N., and M. J. Beaton. 1993. Methodologies for allozyme analysis using cellulose acetate electrophoresis. Helena Laboratories, Beumont, Tex.
- 21.Herrera-Cervera, J. A., J. Caballero-Mellado, G. Laguerre, H. V. Tichy, N. Requena, N. Amarger, E. Martínez-Romero, J. Olivares, and J. Sanjuan. 1999. At least five rhizobial species nodulate Phaseolus vulgaris in a Spanish soil. FEMS Microbiol. Ecol. 30:87-97. [Google Scholar]
- 22.Kaplan, L., and T. F. Lynch. 1999. Phaseolus (Fabaceae) in archeology: AMS radiocarbon dates and their significance for pre-Columbian agriculture. Econ. Bot. 53:261-272. [Google Scholar]
- 23.Laguerre, G., M. R. Allard, F. Revoy, and N. Amarger. 1994. Rapid identification of rhizobia by restriction fragment length polymorphism analysis of PCR-amplified 16S rRNA genes. Appl. Environ. Microbiol. 60:56-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laguerre, G., M. P. Fernández, V. Edel, P. Normand, and N. Amarger. 1993. Genomic heterogeneity among French Rhizobium strains isolated from Phaseolus vulgaris L. Int. J. Syst. Bacteriol. 43:761-767. [DOI] [PubMed] [Google Scholar]
- 25.Laguerre, G., E. Geniaux, S. I. Mazurier, R. Rodríguez Casartelli, and N. Amarger. 1992. Conformity and diversity among field isolates of Rhizobium leguminusarum bv. viciae, bv. trifolii, and bv. phaseoli revealed by DNA hybridization using chromosome and plasmid probes. Can. J. Microbiol. 39:412-419. [Google Scholar]
- 26.Laguerre, G., P. van Berkum, N. Amarger, and D. Prevost. 1997. Genetic diversity of rhizobial symbionts isolated from legume species within the genera Astragalus, Oxytropis, and Onobrychis. Appl. Environ. Microbiol. 63:4748-4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louvrier, P., G. Laguerre, and N. Amarger. 1996. Distribution of symbiotic genotypes in Rhizobium leguminosarum biovar viciae populations isolated directly from soils. Appl. Environ. Microbiol. 62:4202-4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martínez, E., M. A. Pardo, R. Palacios, and M. A. Cevallos. 1985. Reiteration of nitrogen fixation gene sequences and specificity of Rhizobium in nodulation and nitrogen fixation in Phaseolus vulgaris. J. Gen. Microbiol. 131:1779-1786. [Google Scholar]
- 29.Martínez-Romero, E. Diversity of Rhizobium-Phaseolus vulgaris symbiosis: overview and perspectives. Plant Soil, in press.
- 30.Martínez-Romero, E., and J. Caballero-Mellado. 1996. Rhizobium phylogenies and bacterial genetic diversity. Crit. Rev. Plant Sci. 15:113-140. [Google Scholar]
- 31.Martínez-Romero, E., L. Segovia, F. M. Mercante, A. A. Franco, P. Graham, and M. A. Pardo. 1991. Rhizobium tropici, a novel species nodulating Phaseolus vulgaris L. beans and Leucaena sp. trees. Int. J. Syst. Bacteriol. 41:417-426. [DOI] [PubMed] [Google Scholar]
- 32.Maynard Smith, J., N. H. Smith, M. O'Rourke, and B. G. Spratt. 1993. How clonal are bacteria? Proc. Natl. Acad. Sci. USA 90:4384-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mhamdi, R., M. Jebara, M. E. Aouani, R. Ghrir, and M. Mars. 1999. Genotypic diversity and symbiotic effectiveness of rhizobia isolated from root nodules of Phaseolus vulgaris L. grown in Tunisian soils. Biol. Fertil. Soils 28:313-320. [Google Scholar]
- 34.Mhamdi, R., G. Laguerre, M. E. Aouani, M. Mars, and N. Amarger. 2002. Different species and symbiotic genotypes of field rhizobia can nodulate Phaseolus vulgaris in Tunisian soils. FEMS Microbiol. Ecol. 41:77-84. [DOI] [PubMed] [Google Scholar]
- 35.Nei, M. 1987. Molecular evolutionary genetics. Columbia University Press, New York, N.Y.
- 36.Pérez-Ramírez, N. O., M. A. Rogel, E. Wang, J. Z. Castellanos, and E. Martínez-Romero. 1998. Seeds of Phaseolus vulgaris bean carry Rhizobium etli. FEMS Microbiol. Ecol. 26:289-296. [Google Scholar]
- 37.Rodríguez, C., and D. Romero. 1998. Multiple recombination events maintain sequence identity among members of the nitrogenase multigene family in Rhizobium etli. Genetics 149:785-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romero, D., S. Brom, J. Martínez-Salazar, M. L. Girard, R. Palacios, and G. Dávila. 1991. Amplification and deletion of a nod-nif region in the symbiotic plasmid of Rhizobium phaseoli. J. Bacteriol. 173:2435-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romero, D., J. Martínez-Salazar, L. Girard, S. Brom, G. Dávila, R. Palacios, M. Flores, and C. Rodríguez. 1995. Discrete amplifiable regions (amplicons) in the symbiotic plasmid of Rhizobium etli CFN42. J. Bacteriol. 177:973-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schofield, P. R., A. H. Gibson, W. F. Dudman, and J. M. Watson. 1987. Evidence for genetic exchange and recombination of Rhizobium symbiotic plasmid in a soil population. Appl. Environ. Microbiol. 53:2942-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Segovia, L., D. Piñero, R. Palacios, and E. Martínez-Romero. 1991. Genetic structure of a soil population of nonsymbiotic Rhizobium leguminosarum. Appl. Environ. Microbiol. 57:426-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Segovia, L., J. P. Young, and E. Martínez-Romero. 1993. Reclassification of American Rhizobium leguminosarum biovar phaseoli type I strains as Rhizobium etli sp. nov. Int. J. Syst. Bacteriol. 43:374-377. [DOI] [PubMed] [Google Scholar]
- 43.Selander, R. K., D. A. Caugant, H. Ochman, J. M. Musser, M. N. Gilmour, and T. S. Whittam. 1986. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl. Environ. Microbiol. 51:873-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sessitsch, A., H. Ramírez-Saad, G. Hardarson, A. D. Akkermans, and W. M. de Vos. 1997. Classification of Austrian rhizobia and the Mexican isolate FL27 obtained from Phaseolus vulgaris L. as Rhizobium gallicum. Int. J. Syst. Bacteriol. 47:1097-1101. [DOI] [PubMed] [Google Scholar]
- 45.Silva, C., L. E. Eguiarte, and V. Souza. 1999. Reticulated and epidemic population genetic structure of Rhizobium etli biovar phaseoli in a traditionally managed locality in Mexico. Mol. Ecol. 8:277-287. [Google Scholar]
- 46.Sneath, P. H. A., and R. R. Sokal. 1973. Numerical taxonomy. W. H. Freeman & Co., San Francisco, Calif.
- 47.Souza, V., J. Bain, C. Silva, V. Bouchet, A. Valera, E. Márquez, and L. E. Eguiarte. 1997. Ethnomicrobiology: do agricultural practices modify the population structure of the nitrogen fixing bacteria Rhizobium etli biovar phaseoli? J. Ethnobiol. 17:249-266. [Google Scholar]
- 48.Souza, V., and L. E. Eguiarte. 1997. Bacteria gone native vs. bacteria gone awry: plasmidic transfer and bacterial evolution. Proc. Natl. Acad. Sci. USA 94:5501-5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Souza, V., L. E. Eguiarte, G. Avila, R. Cappello, C. Gallardo, J. Montoya, and D. Piñero. 1994. Genetic structure of Rhizobium etli biovar phaseoli associated with wild and cultivated bean plants (Phaseolus vulgaris and Phaseolus coccineus) in Morelos, Mexico. Appl. Environ. Microbiol. 60:1260-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Souza, V., T. T. Nguyen, R. R. Hudson, D. Piñero, and R. E. Lenski. 1992. Hierarchical analysis of linkage disequilibrium in Rhizobium populations: evidence for sex? Proc. Natl. Acad. Sci. USA 89:8389-8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spaink, H. P., A. Kondorosi, and P. J. J. Hooykaas. 1998. The Rhizobiaceae. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 52.Sullivan, J. T., H. N. Patrick, W. L. Lowther, D. B. Scott, and C. W. Ronson. 1995. Nodulating strains of Rhizobium loti arise through chromosomal and symbiotic gene transfer in the environment. Proc. Natl. Acad. Sci. USA 92:8995-8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swofford, D. L. 2002. PAUP*: phylogenetic analysis using parsimony and other methods, 4.0 ed. Sinuauer Associates, Sunderland, Mass.
- 54.Wacek, T., and W. J. Brill. 1976. Simple, rapid assay for screening nitrogen fixing ability in soybean. Crop Sci. 16:519-522. [Google Scholar]
- 55.Weisburg, W. G., S. M. Barns, D. A. Pelletie, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wernegreen, J., and M. A. Riley. 1999. Comparison of the evolutionary dynamics of symbiotic and housekeeping loci: a case for the genetic coherence of rhizobial lineages. Mol. Biol. Evol. 16:98-113. [DOI] [PubMed] [Google Scholar]
- 57.Wernegreen, J. J., E. E. Harding, and M. A. Riley. 1997. Rhizobium gone native: unexpected plasmid stability of indigenous Rhizobium leguminosarum. Proc. Natl. Acad. Sci. USA 94:5483-5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whittam, T. S. 1990. ETDIV and ETCLUS programs. Pennsylvania State University, University Park.
- 59.Workman, P. L., and J. D. Niswander. 1970. Population studies on southwestern Indian tribes. II. Local genetic differentiation in the Papago. Am. J. Hum. Genet. 22:24-49. [PMC free article] [PubMed] [Google Scholar]
- 60.Young, J. P. W., and M. Wexler. 1988. Sym plasmid and chromosomal genotypes are correlated in field populations of Rhizobium leguminosarum. J. Gen. Microbiol. 134:2731-2739. [Google Scholar]