Abstract
Many Proteobacteria produce acyl-homoserine lactones (acyl-HSLs) and employ them as dedicated cell-to-cell signals in a process known as quorum sensing. Previously, Variovorax paradoxus VAI-C was shown to utilize diverse acyl-HSLs as sole sources of energy and nitrogen. We describe here the properties of a second isolate, Arthrobacter strain VAI-A, obtained from the same enrichment culture that yielded V. paradoxus VAI-C. Although strain VAI-A grew rapidly and exponentially on a number of substrates, it grew only slowly and aberrantly (i.e., linearly) in media amended with oxohexanoyl-HSL as the sole energy source. Increasing the culture pH markedly improved the growth rate in media containing this substrate but did not abolish the aberrant kinetics. The observed growth was remarkably similar to the known kinetics of the pH-influenced half-life of acyl-HSLs, which decay chemically to yield the corresponding acyl-homoserines. Strain VAI-A grew rapidly and exponentially when provided with an acyl-homoserine as the sole energy or nitrogen source. The isolate was also able to utilize HSL as a sole source of nitrogen but not as energy for growth. V. paradoxus, known to release HSL as a product of quorum signal degradation, was examined for the ability to support the growth of Arthrobacter strain VAI-A in defined cocultures. It did. Moreover, the acyl-HSL-dependent growth rate and yield of the coculture were dramatically superior to those of the monocultures. This suggested that the original coenrichment of these two organisms from the same soil sample was not coincidental and that consortia may play a role in quorum signal turnover and mineralization. The fact that Arthrobacter strain VAI-A utilizes the two known nitrogenous degradation products of acyl-HSLs, acyl-homoserine and HSL, begins to explain why none of the three compounds are known to accumulate in the environment.
Many bacteria control and modulate their physiology in response to increases in their population density by producing and monitoring the accumulation of signal molecules. This phenomenon has become known as quorum sensing (reviewed in references 8 and 26). A variety of Proteobacteria use acyl-homoserine lactones (acyl-HSLs) as quorum signals. Many different acyl-HSL structures have been elucidated, as have the many enzymes and proteins required for their synthesis and perception (10, 27, 29, 35). Although only low nanomolar concentrations of these signals are required for luciferase induction by Vibrio fischeri (16), acyl-HSLs must accumulate to local concentrations in excess of 1 μM to elicit a quorum response in many terrestrial quorum-sensing bacteria (1, 44). Furthermore, acyl-HSLs have been documented to reach concentrations on the order of 10 μM in laboratory cultures (7, 30, 32) and have been reported to accumulate to nearly 1 mM in a biofilm (3). However, little is known about the local concentrations acyl-HSLs achieve in nonlaboratory environments, although presumably they must reach similar concentrations, if at least transiently, for quorum sensing to function in nature. There is no evidence that acyl-HSLs accumulate anywhere over long periods, and there are many reasons why they might not. If they did, quorum-sensing regulatory networks would malfunction during and after downward fluctuations in population density. Moreover, acyl-HSLs are not recalcitrant to chemical and biological decomposition.
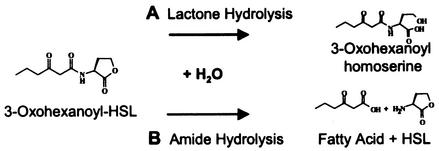
Acyl-HSLs are chemically inactivated via alkaline hydrolysis that yields the cognate acyl-homoserine (42), yet they are considerably more stable in aqueous solutions for weeks or months at pH values of 5 to 6 (34). Inasmuch as environments with a pH of 6 or less are not uncommon, it stands to reason that chemical degradation alone cannot serve to account for why acyl-HSLs do not accumulate in the environment over long periods. Not surprisingly, acyl-HSLs are now known to be subject to biological inactivation (Fig. 1). Analogous to the chemical ring hydrolysis, acyl-homoserine is generated at accelerated rates by acyl-HSL lactonases encoded by Bacillus cereus (and its close relatives) and by Agrobacterium tumefaciens (5, 46). None of these strains have been demonstrated to degrade the molecule further, and no net oxidation occurs during this inactivation reaction. However, there is reason to think that oxidative signal degradation might occur, e.g., during the utilization of acyl-HSL C or N as growth nutrients. The micromolar concentrations of acyl-HSLs often required for quorum sensing to occur equate to ca. 1 mg of organic carbon · liter−1. This is well above the mark that is often cited as being the lower cutoff for growth metabolism by oligotrophic microbes, 100 μg of organic carbon · liter−1 (31).
FIG. 1.
Mechanisms by which a model acyl-HSL quorum signal can be chemically or biochemically degraded. (A) Hydrolytic cleavage of the lactone ring yields the corresponding acyl-homoserine. Chemically, this occurs at rates influenced by half-life kinetics and pH; biochemically, it occurs via the activity of acyl-HSL lactonases encoded by diverse bacteria (see the text). (B) Amide cleavage yields HSL and the corresponding fatty acid. The amide bond of acyl-HSLs is chemically stable under nonextreme temperature and pH but can be cleaved by an acyl-HSL acylase encoded by the bacterium V. paradoxus.
In contrast to the aforementioned ring degradation mechanism, the amide bond is now known to be subject to degradation by acyl-HSL acylases, i.e., during the utilization of quorum signals as growth nutrients by the bacterium Variovorax paradoxus (22). The acyl moiety is then metabolized as an energy substrate, whereas the HSL is released as a product into the culture fluid (20, 22). By a mechanism yet to be elucidated, HSL can very slowly be utilized as a nitrogen source by this bacterium under conditions of N limitation.
Little else is known about the metabolism or environmental fate of either acyl-homoserine or HSL, the aforementioned chemical and biochemical degradation products of acyl-HSL quorum signals. HSL is known to be subject to degradation by a mammalian enzyme, paraoxonase (13, 17). Here, we report that HSL and acyl-homoserine, the two known nitrogenous breakdown products of acyl-HSL quorum signals, are rapidly utilized as energy and nitrogen sources, respectively, by a novel soil isolate.
MATERIALS AND METHODS
Bacterial strains, media, and culture conditions.
The bacterial strains used were Arthrobacter strain VAI-A and V. paradoxus VAI-C (22). For routine maintenance of Arthrobacter strain VAI-A and V. paradoxus VAI-C, we used 5 g (wt/vol) of yeast extract (Difco) · liter−1 in deionized, distilled water. For growth experiments, a defined medium was used as described previously, except that it contained sodium sulfate, not sulfite, which was a typographical error in the reported recipe (22), as the S source. Unless noted otherwise, the medium was buffered to a pH of 5.5 with 5 mM 2-(n-morpholino)-ethanesulfonic acid (MES) (MES 5.5 medium). Growth substrates were added aseptically to sterilized, vitamin-amended medium as indicated.
The stock solution of 100 mM n-3-oxohexanoyl-l-homoserine lactone (3OC6HSL; Sigma) was in ethyl acetate acidified with glacial acetic acid (0.01%, vol/vol) and stored at −20°C. For liquid media, the acyl-HSL was dispensed into sterile tubes, the ethyl acetate was removed by evaporation under a stream of nitrogen gas, and sterile medium was added to the dried acyl-HSL that remained. Cells were grown in 5 ml of medium in 18-mm-diameter tubes with shaking at 30°C unless otherwise noted. Acyl-HSL molecules are stable for 20 to 30 days under the conditions of low pH in our defined medium (34; A. Eberhard, personal communication). A 50 mM acyl-homoserine (N-3-oxohexanoyl-l-homoserine) solution was generated by degrading its parent acyl-HSL via incubation in 250 mM 3-(cyclohexylamino)-2-hydroxy-1-propanesulfonic acid (CAPSO) buffer (pH 9.6) for 24 h. This treatment yielded the expected, corresponding acyl-homoserine as confirmed by reverse-phase liquid chromatography-mass spectrometry (LC-MS). Measurements were performed at the California Institute of Technology's Environmental Analysis Center with a Hewlett Packard 1100 Series LC-MS running a methanol-water-acetic acid (50%:49%:1%, vol/vol/vol) mobile phase isocratically at 0.5 ml · min−1. Stock solutions of HSL (100 mM; Sigma) were prepared, just prior to their use, from well-desiccated reagent stored at −20°C. That no homoserine contamination was present in the stock solution was verified via thin-layer chromatography and ninhydrin staining (15).
Growth studies.
All optical density measurements were performed at 600 nm by using a Spectronic 20 instrument. Viable cell counts were achieved by plating samples from 10-fold serial dilutions of cultures. Molar growth yields attributable to utilization of acyl-homoserine and other substrates as energy sources were determined in NH4Cl-replete MES 5.5 medium containing the indicated substrate at a final concentration of 1 mM. The influence of acyl-homoserine concentration on growth rate was examined in ammonium-replete medium containing 0 to 2 mM concentrations of substrate. Growth yields with nitrogen sources other than ammonium were determined in a medium containing 20 mM sodium succinate as the energy source. The nitrogen sources used in place of NH4Cl were HSL (at concentrations of 0 to 10 mM) or homoserine (at concentrations of 0 to 10 mM) or dl-3OC6-homoserine (0 to 2 mM). A factor for converting optical density to cell dry mass was determined by using cells grown in a medium containing succinate as the energy source and NH4Cl as the nitrogen source, washed with 50 mM ammonium acetate buffer (pH 5.5), and then dried to a constant weight. Experiments were done at least twice.
The effects of HSL and homoserine as potential inhibitors of Arthrobacter strain VAI-A growth were determined in NH4Cl-replete medium containing 2 mM sodium succinate as the energy source. HSL was added in increments of 1 mM between 0 to 15 mM; homoserine was added in identical increments to media. The initial rates of growth as a function of inhibitor concentration were determined. Experiments were performed in duplicate for each concentration.
For initiation of cocultivation studies, monocultures of each strain were first grown overnight in MES 5.5 medium containing 2 mM succinate and 100 μM NH4Cl. Strains were inoculated or coinoculated into 3OC6HSL-containing MES 5.5 medium to an initial optical density of ca. 0.05. Alternatively, established cocultures were transferred (1%, vol/vol) into like media. The characteristics of growth of the mono- and cocultures were examined in both ammonia-replete and ammonia-free MES 5.5 media.
Nucleotide sequence analysis of the 16S rDNA.
The nucleotide sequence (1,443 bp) of a PCR-amplified fragment of the 16S rDNA of strain VAI-A was determined using previously described procedures (21, 22).
Nucleotide sequence accession numbers.
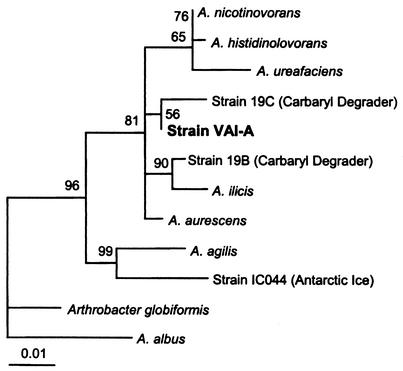
The above-mentioned sequence has been submitted to GenBank and assigned accession no. AY145731. The GenBank accession numbers for the other sequences discussed (see Fig. 3) are as follows: Arthrobacter nicotinovorans, X80743; A. histidinolovorans, X83406; A. ureafaciens, X80744; Arthrobacter strains 19C and 19B, AB017650 and AB017649, respectively; A. ilicis, X83407; A. aurescens, X83405; A. agilis, X80748; Arthrobacter strain IC044, U85895; A. globiformis, X80736; and A. albus, AJ243421 (2, 18, 19, 43).
FIG. 3.
Small-subunit rRNA-based Tree-Puzzle showing the phylogenetic position of strain VAI-A. A total of 1,417 unambiguously aligned nucleotides were used in a 1,000-step Tree-Puzzle 5.0 analysis (36, 41). The bar represents evolutionary distance as 0.01 changes per nucleotide position, determined by measuring the lengths of the horizontal lines connecting the species. The numbers provide support for the robustness of the adjacent nodes. See Materials and Methods for GenBank accession numbers.
RESULTS
General properties of strain VAI-A.
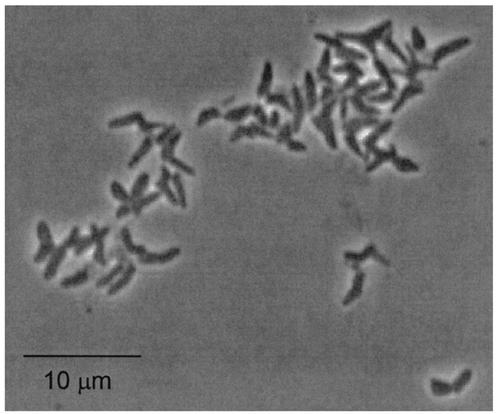
Details of the enrichment and isolation of bacteria from a vitamin-free basal enrichment culture containing 3OC6HSL have been described previously (22). Briefly, three isolates were obtained from a single enrichment culture inoculated with soil. One of these, strain VAI-B, did not grow in medium containing 3OC6-HSL and subsequently was lost from culture. A second strain, VAI-A, grew only very slowly in medium containing 3OC6-HSL as the sole energy and nitrogen source and was chosen here for further study. All experiments reported here were performed at 30°C, as cultures grew only poorly at 37°C. In either yeast extract broth or a defined medium containing succinate as the energy source, strain VAI-A grew exponentially, with an 80- to 90-min doubling time. On yeast extract medium, the isolate formed flat, white colonies that yellowed and produced an aroma of grapes upon aging. Cells from broth cultures were pleomorphic coccobacilli and were often branched (Fig. 2). Cells were observed to be phase bright during exponential growth but phase dark and coccoid after extended incubation. Cells were never observed to be motile. The relationship of optical density to dry weight of cell material was determined to be 418 μg · ml−1 at an optical density at 600 nm of 1.0 (measured on a Spectronic 20 using 18-mm-diameter glass culture tubes). As no members of the phylum Actinobacteria (see below) are known to produce acyl-HSLs, strain VAI-A was not examined for this property.
FIG. 2.
Cell morphology of Arthrobacter strain VAI-A. Shown is a phase-contrast micrograph of cells grown in MES 5.5 defined medium with glucose as the energy source and HSL as the sole nitrogen source.
Phylogenetic analysis of VAI-A.
A nearly complete sequence for the 16S rDNA was obtained. The sequence corresponds to Escherichia coli 16S rRNA nucleotide positions 28 to 1489. Web-based similarity searches against the GenBank and Ribosomal Database Project databases suggested that VAI-A belonged to the Actinobacteria (i.e., high G+C gram positives), clustering with species belonging to the genus Arthrobacter. The 16S rDNA of VAI-A shared 98.5 to 99.4% sequence identities with the 16S rDNAs of A. ilicis, A. histidinolovorans, A. aurescens, A. nicotinovorans, and two less well-characterized, carbaryl (pesticide)-degrading strains. A further phylogenetic analysis supported the conclusion that VAI-A is an Arthrobacter species. Strain VAI-A clustered tightly with the aforementioned species within the A. agilis subgroup when the FastDNAML, Maximum Likelihood, Phylip Maximum Parsimony, and DeSoete Distance treeing algorithms were employed (data not presented). A Tree-Puzzle 5.0 likelihood method was employed for the construction of the phylogram presented in Fig. 3.
Growth in medium containing 3OC6HSL or 3OC6-homoserine as the sole energy source.
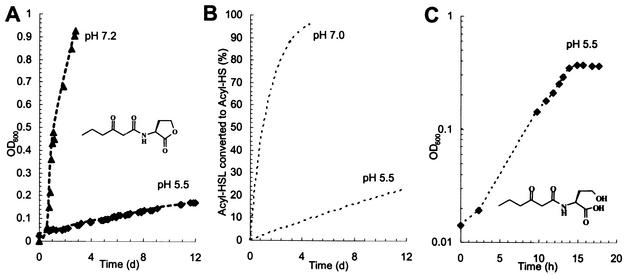
Pure cultures of Arthrobacter strain VAI-A grew poorly in 3OC6HSL-containing medium, even when it was amended with NH4Cl and vitamins. We were unable to ascertain a doubling time for cultures grown on this substrate, as biomass did not accumulate in an exponential manner. Rather, culture biomass accumulated at a decelerating, linear rate (Fig. 4A). Since acyl-HSLs are subject to pH-influenced half-life decay to the corresponding acyl-homoserine, we examined the influence of pH on growth and whether the cells might be using the signal degradation product, not acyl-HSL itself. The growth rate of strain VAI-A in 3OC6HSL-containing medium improved markedly when the pH was increased, but this did not resolve the aberrant kinetics (Fig. 4A). However, the growth kinetics at the two pH values were closely parallel to the expected rates of the generation of acyl-homoserine degradation product from the parent acyl-HSL at similar pH values (Fig. 4B). The growth data fit the following expression: Yt = Y0 + (Ymol)(C0 − C0e−kt), where Yt is the cell yield in grams at time t, t is the time elapsed since substrate addition and culture inoculation, Y0 is the cell inoculum in grams at time zero, Ymol is 152 g of dry biomass · mol of 3OC6-homoserine−1 (see below), C0 is the amount of 3OC6HSL in moles at time zero, and k is 0.693/T1/2 (T1/2 is the half-life, in days, of 3OC6HSL at a given pH, reported to be 10[7 − pH]). The lines fit to the growth data, yielding r2 values of 0.995 and 0.988 when T1/2 values of 22.8 and 0.89, respectively, and pH values of 5.5 and 7.2, respectively, were plugged in.
FIG. 4.
The growth kinetics of Arthrobacter strain VAI-A in media containing 3OC6HSL are influenced by the rate at which the signal decays into 3OC6-homoserine. (A) Growth of strain VAI-A in MES-buffered medium (pH 5.5) containing 1 mM 3OC6HSL and in MOPS (morpholinepropanesulfonic acid)-buffered medium (pH 7.2) containing 3 mM 3OC6HSL. Note that although the growth rates differ, both are linear and appear to decelerate. OD600, optical density at 600 nm; d, day. (B) The theoretical accumulation of 3OC6-homoserine over time as a half-life decay product of 3OC6HSL. Note that, as in panel A, pH influences the rate but not the curvature of the line. (C) Growth of strain VAI-A in media containing 3OC6-homoserine as the sole energy source. In contrast to the first two panels, panel C is in semilog format with time expressed in hours.
When strain VAI-A was incubated in MES 5.5 medium containing 3OC6-homoserine as the sole carbon source, the aberrant kinetics were resolved: strain VAI-A grew exponentially, with a doubling time of 4.1 h (Fig. 4C). The growth rate decreased when the initial 3OC6-homoserine concentration was lowered (data not presented). Strain VAI-A exhibited a batch culture Ks of 166 ± 4 μM 3OC6-homoserine when this substrate was used as the sole energy source. The growth rates and molar yields of strain VAI-A that were obtained using several substrates are listed in Table 1.
TABLE 1.
Growth of Arthrobacter strain VAI-A on 3-oxohexanoyl-homoserine and other compoundsa
| Growth substrate | Yield (g of dry biomass · mol−1) | Doubling time (min) |
|---|---|---|
| 3OC6-l-homoserine | 152 | 245 |
| Glycerol | 24 | 140 |
| Sucrose | 16 | 210 |
| Xylose | 30 | 205 |
| l-Homoserine | 73 | 115 |
| l-Alanine | 69 | 160 |
| Succinate | 58 | 85 |
Data presented are the averages of at least duplicate determinations. Substrates not utilized as energy sources were l-homoserine lactone; N-acetyl-l-aspartate; and dl-C4HSL, dl-C6HSL, dl-C7HSL, dl-C8HSL, dl-C10HSL, dl-C12HSL, l-3OC12HSL, or dl-C14HSL.
Utilization of HSL, homoserine, and acyl-homoserine as nitrogen sources for growth.
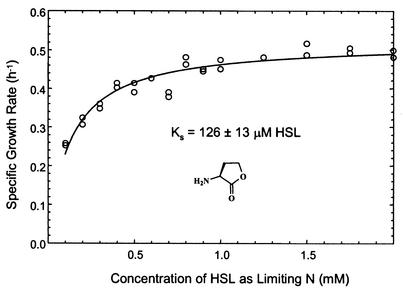
Arthrobacter strain VAI-A, although unable to use acyl-HSL, was capable of growth when free HSL was used as the sole source of nitrogen. When the strain was presented with excess succinate as an energy source and limiting amounts of HSL as the N source, the growth yield varied strictly as a function of HSL concentration (data not presented). The molar growth yield of strain VAI-A in succinate-replete medium with HSL as the sole N source was 437 g of dry biomass · mol of HSL−1. HSL-grown cells often appeared phase bright during microscopic evaluation of wet mounts; thus, they may have been storing excess C intracellularly. The maximal specific growth rate of N-limited cultures when HSL was used was 0.52 ± 0.01 h−1 (doubling time of ca. 80 min). The growth rate decreased as a function of lowered HSL concentration, following a typical Michaelis-Menten relationship (Fig. 5). From this result, it was inferred that Arthrobacter strain VAI-A exhibits a batch culture-derived Ks for HSL of 126 ± 13 μM. The influence of limiting homoserine or ammonium (as sole N nutrients) on growth rate and yield was also examined. The doubling times (80 to 90 min) and molar yields attained were comparable to those seen when HSL was used. For either ammonium or homoserine at the concentrations tested, it was not possible to infer a Ks value from the data. Growth of strain VAI-A using succinate plus 3OC6-homoserine as the sole N source was considerably slower than that seen when the other sources were used (3.1-h doubling time); the impact of decreased acyl-homoserine concentration on N-limited culture growth kinetics was not examined.
FIG. 5.
The influence of HSL concentration as the limiting N source on the growth kinetics of Arthrobacter strain VAI-A. Cultivation was performed in MES 5.5 medium with succinate supplied in gross excess.
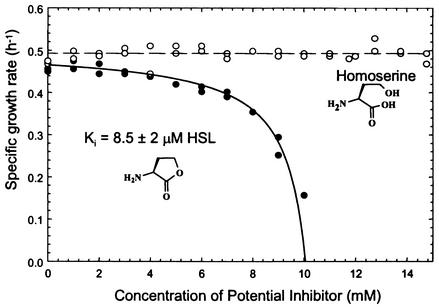
Because both HSL and homoserine (the product of HSL degradation by mammalian enzymes) have been reported to be inhibitors of growth of E. coli (45), we examined the possibility that elevated concentrations of these nutrients might impede the growth of our isolate. Strain VAI-A was grown in ammonium-replete MES 5.5 medium containing 2 mM succinate as the energy source and various concentrations of HSL or homoserine as potential growth inhibitors. HSL, but not homoserine, markedly depressed culture growth rates when amended in concentrations greater than 3 to 4 mM (Fig. 6). Growth was not observed at HSL concentrations in excess of 10 mM. The influence of HSL concentration as an inhibitor of growth followed a Michaelis-Menten kinetic relationship. The apparent Ki of HSL was 8.3 ± 2.1 mM. Homoserine had no apparent influence as an inhibitor of growth at any of the concentrations tested, i.e., ≤15 mM.
FIG. 6.
The influence of HSL and homoserine as potential inhibitors of Arthrobacter strain VAI-A growth. Cultivation was performed in ammonium-replete MES 5.5 medium containing succinate as an energy source.
Consortial degradation of quorum signals.
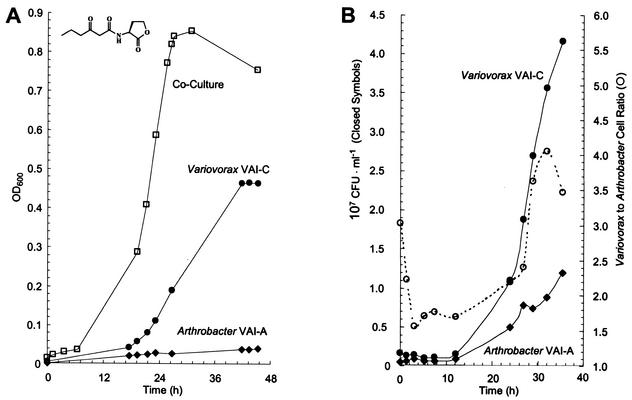
Because they were both isolated from the same original enrichment but were not apparently in competition for the same substrate (3OC6HSL) provided with it, we compared the characteristics of monocultures and defined cocultures of strains VAI-A and VAI-C growing in 3OC6HSL-containing media. When 3OC6HSL was provided to cultures as a sole C source in ammonium-replete MES 5.5 medium, the biomass yield of cocultures markedly surpassed that of either of the monocultures (Fig. 7). As a function of optical density, the growth yields of cocultures were reproducibly 1.8- and 6.3-fold greater than those of V. paradoxus VAI-C and Arthrobacter strain VAI-A, respectively, when grown alone and 1.4-fold greater than the sum of their individual yields. The biomass doubling time of the coculture (ca. 4 h) was nearly identical to that of Variovorax grown alone, although this is not clearly seen in Fig. 7A, which plots optical density increases on an arithmetic scale to underscore the substantial differences in growth yields. The growth rates of both strains growing in coculture were determined via the viable cell counts. The colony morphologies of the two strains were distinct and thus easily differentiated. The growth rate of Arthrobacter strain VAI-A was markedly enhanced during cocultivation (Fig. 7B), improving to a 4.5-h doubling time from its aberrant growth on acyl-HSL as a pure culture, as discussed above. During cocultivation, cells of Variovorax outnumbered those of Arthrobacter by a ratio of 3 to 2 during early exponential growth (Fig. 7B). This ratio increased throughout growth but never exceeded 4 to 1. These coculture attributes were stably maintained after three successive 1% (vol/vol) transfers of the consortium into fresh growth medium.
FIG. 7.
Growth of a model acyl-HSL-degrading consortium compared with that of the individual pure cultures from which it was constructed. (A) Growth of V. paradoxus VAI-C and Arthrobacter strain VAI-A as pure and defined cocultures in identical, ammonium-replete MES 5.5 media containing 1 mM 3OC6HSL as the sole energy source. OD600, optical density at 600 nm. (B) Relative abundances of cells of the two species during consortial growth. Note that the growth of Arthrobacter strain VAI-A in the consortium is exponential, whereas it is linear in the case of a pure culture. The properties of the equally striking consortial utilization of 3OC6HSL as the sole N source are reported in Results.
When 3OC6HSL was provided to cultures as the sole C source and N source, the beneficial effects of cocultivation were even more pronounced (data not shown). Not only were biomass yields stimulated in a fashion similar to that reported above, but the biomass doubling time exhibited by cocultures improved to ca. 4 h, surpassing that of Variovorax grown alone under identical conditions (>18 h). Viable cell counts indicated that, when the consortium utilized 3OC6HSL as the sole C source and N source, both species occurred at nearly equal ratios throughout growth.
DISCUSSION
We isolated a bacterium capable of degrading and utilizing the two known nitrogenous breakdown products of acyl-HSL quorum-sensing signals. Strain VAI-A belongs to the genus Arthrobacter, members of which are commonly encountered soil oligotrophs that are most closely related to other members of the A. agilis subgroup (19). In the contexts of the ribosomal sequence diversity of those species to which it is most closely related and of its novel physiological traits, strain VAI-A may represent a novel species. This isolate was able to utilize 3-oxohexanoyl-homoserine, but none of the tested acyl-HSLs, as both an energy and nitrogen source. 3-Oxohexanoyl-homoserine, like other acyl-homoserines, is generated by the chemical decomposition of its corresponding acyl-HSL under alkaline conditions and also by acyl-HSL lactonases encoded by a variety of bacteria (4, 6, 24, 33, 46). To our knowledge, this is the first demonstration that any acyl-homoserine is degraded by a biological or chemical catalyst.
We were initially puzzled by the slow growth of strain VAI-A in medium containing 3OC6HSL as the sole energy nutrient, especially since the strain grew very rapidly in both yeast extract broth and defined medium supplemented with other energy substrates. Inspection of growth curves revealed that the classical exponential growth equation was a very poor fit for the growth data. Many textbooks discuss nonexponential growth, alternatively known as linear or aberrant growth. However, consider the commonly cited equation for aberrant growth: Yt = Y0 + kt, where Yt and Y0 are the cell yields at time t and time zero, respectively; k is a constant for some relevant, fixed catalytic capacity of the cell population; and t is the time elapsed since the inoculation of the culture. This equation poorly described the observed growth kinetics, which appeared to constantly decelerate. The realization that strain VAI-A was likely utilizing 3OC6-homoserine and not the parent acyl-HSL provided prompted us to examine another model of nonexponential growth: Yt = Y0 + (Ymol)(C0 − C0e−kt) (for definitions of the variables, see Results).
In this model, growth kinetics are influenced by how rapidly a growth substrate is generated via the half-life decay of its nonutilizable chemical precursor, in this case acyl-HSL. This serves to explain the observed decelerating growth rate as a case of the diminishing returns of half-life decay. When the potential doubling time of a cell population supercedes that delivery rate, as was observed in our experiments, then growth rapidly becomes substrate limited and parallels the substrate delivery rate (Fig. 4). In the case of the generation of acyl-homoserine, pH and precursor (acyl-HSL) concentration markedly influence this delivery rate and thus the kinetics of growth. We note that such effects of pH and substrate concentration on growth are very different from what are presented in the commonly cited examples. That is, the effect of pH in this case is chemical and not necessarily biological, i.e., not related to the pH optimum for growth of the given strain. Indeed, Arthrobacter strain VAI-A generally exhibited a rather broad pH optimum, growing exponentially and equally rapidly on succinate and other substrates at pH values of 5.5 and 7.2 (data not presented). Similarly, the effect of the initial precursor concentration on the growth kinetics is not necessarily related to the affinity of the biochemical systems involved in the transport and degradation of the substrate. Rather, the effect is related to how much of the actual growth substrate is yielded as a function of the half-life decay of a chemical precursor. We note that the half-lives of acyl-HSL deduced from these growth experiments (22.3 and 0.9 days, respectively, at pH 5.5 and 7.2) are reasonably similar to the values of 30 and 0.63 days predicted by using the equation T1/2 = 10(7 − pH), with T1/2 in days (34; A. Eberhard, personal communication). We do not know whether the biological consumption of acyl-homoserine influences the half-life kinetics.
The mechanisms by which our isolate or any other biota degrade acyl-homoserine are not yet known. One possibility would involve an initial attack on the amide bond by an acyl-homoserine acylase, i.e., release of the cognate fatty acid and homoserine (both of which are subject to rapid utilization by this isolate). Alternatively, it might first be incompletely oxidized to acyl-aspartate and thereafter degraded by an aspartoacylase, representatives of which are found widely distributed across diverse biota (11, 25). By whatever mechanism, the amino acid from acyl-homoserine appears to be metabolized by strain VAI-A. Cultures of this bacterium did not accumulate ninhydrin-reactive material in the culture fluid. Moreover, this bacterium achieved a molar growth yield of 152 g of dry cell material · mol of 3OC6-homoserine−1. In contrast, the molar yield of V. paradoxus strain VAI-C grown on 3OC6HSL was ca. 40% less (22): 95 g of dry cell material · mol of 3OC6HSL−1. However, V. paradoxus does not use the 4-carbon lactone ring as a growth nutrient. Instead, it releases HSL into the extracellular milieu. Thus, it utilizes only 6 of the available 10 carbons. Taking into account this incomplete oxidation of 30C6HSL by Variovorax, we found that each strain achieved similar, normalized yields of 15 to 16 g of dry cell material · mol of carbon utilized−1.
We found that Arthrobacter strain VAI-A utilized HSL quite rapidly as an N source. Other than that V. paradoxus VAI-C can assimilate lactone nitrogen very slowly, little is known about the biodegradation of the nonstandard amino acid homoserine lactone. We do not know the mechanism by which HSL is degraded by any microbe. Two possible routes for HSL degradation by bacteria have been proposed previously (22). One involves the activity of an HSL lactonase that would yield homoserine (which could be further degraded by established pathways). Such an activity has been shown to be present in mammalian sera, where an HSL lactonase is involved in HSL and homocysteine thiolactone (HCTL) detoxification (13). However, the mammalian enzyme has no obvious homologues that can be presently identified in any Bacteria or Archaea. Alternatively, an enzyme with an α,γ-lyase activity might, in principle, catalyze the concurrent deamination and ring cleavage of HSL, yielding α-ketobutyrate and ammonium.
HSL and homoserine have been reported to be inhibitors of bacterial and eukaryotic cell growth and health (13, 45). With the aim of optimizing the cultivation conditions for strain VAI-A, we examined the effect of HSL and homoserine concentration on growth kinetics. Homoserine had no deleterious impact on the growth of the isolate, but HSL was mildly to extremely inhibitory in concentrations above ca. 1 mM (Fig. 6). When provided as the sole, limiting N nutrient at concentrations below 1 mM, small amounts of HSL also negatively influenced the growth rate (Fig. 5). Thus, ca. 1 mM appears to be the optimal concentration for in vitro studies on the utilization of this N substrate.
While its environmental fate has been poorly studied, HSL (as well as its sulfur-containing analogue, HCTL) is likely to be frequently encountered in nature, and not just at the sites of acyl-HSL decomposition. Both of these lactones are known to be the products of the housekeeping metabolism of all biota, as they are generated during amino-acyl tRNA editing events (14, 15) as well as during S-adenosyl methionine degradation by several bacterial species (37). Additionally, intracellularly generated HSL and HCTL are considered to be employed as mediators of starvation sensing by several bacteria species (9, 12). Because of its rapid growth rate and excellent growth yield on this substrate, Arthrobacter strain VAI-A is a good candidate for use in further investigations of how prokaryotes utilize HSL.
We had originally anticipated that the cocultivation of V. paradoxus VAI-C and Arthrobacter strain VAI-A in 30C6HSL-containing media might result in the latter being outcompeted, inasmuch as strain VAI-A is not able to use this substrate. Yet the performance of V. paradoxus VAI-C and Arthrobacter strain VAI-A growing together on 3OC6HSL was superior to that seen when either of the two was grown alone (Fig. 7). The growth yield of the coculture was higher than the cumulative yield of the two strains under otherwise identical conditions. Moreover, during the utilization of 30C6HSL as the sole C and N source, the growth rates of each strain in the coculture exceeded those of the pure cultures under the same conditions. We do not yet know the physiological and molecular details underpinning this nutritional symbiosis. Although we have been unable to demonstrate that strain VAI-A is capable of using HSL as an energy source, we postulate that the energy nutrient serving as the tie that binds this consortium is ultimately derived from HSL-carbon but is not HSL itself. V. paradoxus VAI-C is known to release HSL as a product of an acyl-HSL acylase-mediated reaction (20). Studies have shown that even when this bacterium utilizes HSL as an N source, the lactone ring is not tapped as a growth nutrient. Thus, the carbon and energy of HSL, embodied in the form of an unidentified intermediate, likely become available for utilization by strain VAI-A. The possibility that carbon and energy derived from the HSL lactone ring might track to cells of Arthrobacter strain VAI-A growing in consortia can be examined by combining the techniques of acyl-HSL lactone ring radiolabeling and whole-cell microautoradiography (22, 23). However, the exact structure of the nutrient passaged between the two strains may prove to be very challenging to identify. This has certainly proven to be the case during studies on consortia engaging in interspecies electron transfer or in anaerobic methane oxidation, wherein H2, formate, methanol, and acetate have alternatively been proposed as the energy-rich, free intermediate passaged from one species to another (28, 39, 40).
That Arthrobacter strain VAI-A can degrade HSL and 3OC6-homoserine by itself and can engage in a nutritional symbiosis with another species that degrades acyl-HSLs suggests that it may be well adapted to fill an oligotrophic niche found in close proximity to or even within quorum-sensing microbial communities. Oxohexanoyl-HSL is a quorum signal known to be produced by a variety of marine and terrestrial quorum-sensing species, i.e., Vibrio fischeri, Pantoea stewartii, Pseudomonas syringae pv. tabaci, and diverse Erwinia species (38). At circumneutral and higher environmental pHs, acyl-HSLs produced by quorum-sensing strains rapidly decompose to the corresponding acyl-homoserine, which now can be considered to be subject to a direct degradation by bacteria similar to strain VAI-A. However, at lower pH values (e.g., <6, which is not uncommonly encountered in soils and other environments), the chemical half-life of acyl-HSL quorum signals increases to weeks or even months. Under such conditions, microbial physiotypes represented by Arthrobacter strain VAI-A appear to be well poised to coordinate their activities with species capable of biochemically degrading acyl-HSLs. Here, we have shown that strain VAI-A can grow synergistically with V. paradoxus, which exhibits an HSL-releasing, acyl-HSL acylase activity. We speculate that because strain VAI-A can utilize 3OC6-homoserine, it might also engage in nutritional interactions with acyl-homoserine-generating biota, e.g., Bacillus cereus, Agrobacterium tumefaciens, and other species expressing acyl-HSL lactonases (Fig. 1). Microbial consortia now appear likely to play a role in quorum signal turnover and mineralization. This begins to address why neither acyl-HSLs nor their nitrogenous degradation products are known to accumulate in the environment.
Acknowledgments
This research was supported by an infrastructural grant from the National Science Foundation (DBI-0107908) and research grants from the Department of Agriculture (CSREES 2001-01242) and the Schlumberger Foundation.
We thank N. Dalleska and R. Becker for technical discussions and for help in performing LC-MS analyses and D. Newman for helpful comments.
REFERENCES
- 1.Beck von Bodman, S., D. R. Majerczak, and D. L. Coplin. 1998. A negative regulator mediates quorum-sensing control of exopolysaccharide production in Pantoea stewartii subsp. stewartii. Proc. Natl. Acad. Sci. USA 95:7687-7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowman, J. P., S. A. McCammon, M. V. Brown, D. S. Nichols, and T. A. McMeekin. 1997. Diversity and association of psychrophilic bacteria in Antarctic sea ice. Appl. Environ. Microbiol. 63:3068-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charlton, T. S., R. de Nys, A. Netting, N. Kumar, M. Hentzer, M. Givskov, and S. Kjelleberg. 2000. A novel and sensitive method for the quantification of N-3-oxoacyl homoserine lactones using gas chromatography-mass spectrometry: application to a model bacterial biofilm. Environ. Microbiol. 2:530-541. [DOI] [PubMed] [Google Scholar]
- 4.Dong, Y. H., A. R. Gusti, Q. Zhang, J. L. Xu, and L. H. Zhang. 2002. Identification of quorum-quenching N-acyl homoserine lactonases from Bacillus species. Appl. Environ. Microbiol. 68:1754-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong, Y. H., L. H. Wang, J. L. Xu, H. B. Zhang, X. F. Zhang, and L. H. Zhang. 2001. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 411:813-817. [DOI] [PubMed] [Google Scholar]
- 6.Dong, Y. H., J. L. Xu, X. Z. Li, and L. H. Zhang. 2000. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. USA 97:3526-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eberhard, A., A. L. Burlingame, C. Eberhard, G. L. Kenyon, K. H. Nealson, and N. J. Oppenheimer. 1981. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry 20:2444-2449. [DOI] [PubMed] [Google Scholar]
- 8.Fuqua, C., M. R. Parsek, and E. P. Greenberg. 2001. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 35:439-468. [DOI] [PubMed] [Google Scholar]
- 9.Goodrich-Blair, H., and R. Kolter. 2000. Homocysteine thiolactone is a positive effector of σS levels in Escherichia coli. FEMS Microbiol. Lett. 185:117-121. [DOI] [PubMed] [Google Scholar]
- 10.Hanzelka, B., M. R. Parsek, D. L. Val, P. V. Dunlap, J. J. E. Cronan, and E. P. Greenberg. 1999. Acylhomoserine lactone synthase activity of the Vibrio fischeri AinS protein. J. Bacteriol. 181:5766-5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hess, W. R. 1997. Localization of an open reading frame with homology to human aspartoacylase upstream from psbA in the prokaryote Prochlorococcus marinus CCMP 1375. DNA Sequence 7:301-306. [DOI] [PubMed] [Google Scholar]
- 12.Huisman, G. W., and R. Kolter. 1994. Sensing starvation: a homoserine lactone-dependent signaling pathway in Escherichia coli. Science 265:537-539. [DOI] [PubMed] [Google Scholar]
- 13.Jakubowski, H. 2000. Calcium-dependent human serum homocysteine thiolactone hydrolase. A protective mechanism against protein N-homocysteinylation. J. Biol. Chem. 275:3957-3962. [DOI] [PubMed] [Google Scholar]
- 14.Jakubowski, H. 2000. Homocysteine thiolactone: metabolic origin and protein homocysteinylation in humans. J. Nutr. 130:377S-381S. [DOI] [PubMed]
- 15.Jakubowski, H. 1995. Proofreading in vivo. Editing of homocysteine by aminoacyl-tRNA synthetases in Escherichia coli. J. Biol. Chem. 270:17672-17673. [PubMed] [Google Scholar]
- 16.Kaplan, H. B., and E. P. Greenberg. 1985. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J. Bacteriol. 163:1210-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi, M., M. Shinohara, C. Sakoh, M. Kataoka, and S. Shimizu. 1998. Lactone-ring-cleaving enzyme: genetic analysis, novel RNA editing, and evolutionary implications. Proc. Natl. Acad. Sci. USA 95:12787-12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koch, C., F. A. Rainey, and E. Stackebrandt. 1994. 16S rDNA studies on members of Arthrobacter and Micrococcus: an aid for their taxonomic restructuring. FEMS Microbiol. Lett. 123:167-172. [Google Scholar]
- 19.Koch, C., P. Schumann, and E. Stackebrandt. 1995. Reclassification of Micrococcus agilis (Ali-Cohen 1889) to the genus Arthrobacter as Arthrobacter agilis comb. nov. and emendation of the genus Arthrobacter. Int. J. Syst. Bacteriol. 45:837-839. [DOI] [PubMed] [Google Scholar]
- 20.Leadbetter, J. R. 2001. News and views: plant microbiology—quieting the raucous crowd. Nature 411:748-749. [DOI] [PubMed] [Google Scholar]
- 21.Leadbetter, J. R., and J. A. Breznak. 1996. Physiological ecology of Methanobrevibacter cuticularis sp. nov. and Methanobrevibacter curvatus sp. nov., isolated from the hindgut of the termite Reticulitermes flavipes. Appl. Environ. Microbiol. 62:3620-3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leadbetter, J. R., and E. P. Greenberg. 2000. Metabolism of acyl-homoserine lactone quorum-sensing signals by Variovorax paradoxus. J. Bacteriol. 182:6921-6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee, N., P. H. Nielsen, K. H. Andreasen, S. Juretschko, J. L. Nielsen, K. H. Schleifer, and M. Wagner. 1999. Combination of fluorescent in situ hybridization and microautoradiography—a new tool for structure-function analyses in microbial ecology. Appl. Environ. Microbiol. 65:1289-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, S. J., S. Y. Park, J. J. Lee, D. Y. Yum, B. T. Koo, and J. K. Lee. 2002. Genes encoding the N-acyl homoserine lactone-degrading enzyme are widespread in many subspecies of Bacillus thuringiensis. Appl. Environ. Microbiol. 68:3919-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makarova, K. S., and N. V. Grishin. 1999. The Zn-peptidase superfamily: functional convergence after evolutionary divergence. J. Mol. Biol. 292:11-17. [DOI] [PubMed] [Google Scholar]
- 26.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 27.Moré, M. I., D. Finger, J. L. Stryker, C. Fuqua, A. Eberhard, and S. C. Winans. 1996. Enzymatic synthesis of a quorum-sensing autoinducer though the use of defined substrates. Science 272:1655-1658. [DOI] [PubMed] [Google Scholar]
- 28.Nauhaus, K., A. Boetius, M. Kruger, and F. Widdel. 2002. In vitro demonstration of anaerobic oxidation of methane coupled to sulphate reduction in sediment from a marine gas hydrate area. Environ. Microbiol. 4:296-305. [DOI] [PubMed] [Google Scholar]
- 29.Parsek, M. R., D. L. Val, B. L. Hanzelka, J. E. Cronan, and E. P. Greenberg. 1999. Acyl homoserine-lactone quorum-sensing signal generation. Proc. Natl. Acad. Sci. USA 96:4360-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearson, J. P., L. Passador, B. H. Iglewski, and E. P. Greenberg. 1995. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:1490-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poindexter, J. 1981. Oligotrophy: fast and famine existence. Adv. Microbiol. Ecol. 5:63-89. [Google Scholar]
- 32.Puskas, A., E. P. Greenberg, S. Kaplan, and A. L. Schaefer. 1997. A quorum-sensing system in the free-living photosynthetic bacterium Rhodobacter sphaeroides. J. Bacteriol. 179:7530-7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reimmann, C., N. Ginet, L. Michel, C. Keel, P. Michaux, V. Krishnapillai, M. Zala, K. Heurlier, K. Triandafillu, H. Harms, G. Defago, and D. Haas. 2002. Genetically programmed autoinducer destruction reduces virulence gene expression and swarming motility in Pseudomonas aeruginosa PAO1. Microbiology 148:923-932. [DOI] [PubMed] [Google Scholar]
- 34.Schaefer, A. L., B. L. Hanzelka, M. R. Parsek, and E. P. Greenberg. 2000. Detection, purification and structural elucidation of acylhomoserine lactone inducer of Vibrio fischeri luminescence and other related molecules. Methods Enzymol. 305:288-301. [DOI] [PubMed] [Google Scholar]
- 35.Schaefer, A. L., D. L. Val, B. L. Hanzelka, J. E. Cronan, Jr., and E. P. Greenberg. 1996. Generation of cell-to-cell signals in quorum sensing: acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protein. Proc. Natl. Acad. Sci. USA 93:9505-9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt, H. A., K. Strimmer, M. Vingron, and A. von Haeseler. 2002. TREE-PUZZLE: maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics 18:502-504. [DOI] [PubMed] [Google Scholar]
- 37.Shapiro, S., and A. Mather. 1958. The enzymatic decomposition of S-adenosyl-l-methionine. J. Biol. Chem. 233:631-633. [PubMed] [Google Scholar]
- 38.Shaw, P. D., G. Ping, S. L. Daly, C. Cha, J. J. E. Cronan, K. L. Rinehart, and S. K. Farrand. 1997. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc. Natl. Acad. Sci. USA 94:6036-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorensen, K. B., K. Finster, and N. B. Ramsing. 2001. Thermodynamic and kinetic requirements in anaerobic methane oxidizing consortia exclude hydrogen, acetate, and methanol as possible electron shuttles. Microb. Ecol. 42:1-10. [DOI] [PubMed] [Google Scholar]
- 40.Stams, A. J. 1994. Metabolic interactions between anaerobic bacteria in methanogenic environments. Antonie Leeuwenhoek 66:271-294. [DOI] [PubMed] [Google Scholar]
- 41.Strimmer, K., and A. von Haeseler. 1996. Quartet puzzling: a quartet maximum likelihood method for reconstructing tree topologies. Mol. Biol. Evol. 13:964-969. [Google Scholar]
- 42.Voelkert, E., and D. R. Grant. 1970. Determination of homoserine as the lactone. Anal. Biochem. 34:131-137. [DOI] [PubMed] [Google Scholar]
- 43.Wauters, G., J. Charlier, M. Janssens, and M. Delmee. 2000. Identification of Arthrobacter oxydans, Arthrobacter luteolus sp. nov., and Arthrobacter albus sp. nov., isolated from human clinical specimens. J. Clin. Microbiol. 38:2412-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whiteley, M., K. M. Lee, and E. P. Greenberg. 1999. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 96:13904-13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zakataeva, N. P., V. V. Aleshin, I. L. Tokmakova, P. V. Troshin, and V. A. Livshits. 1999. The novel transmembrane Escherichia coli proteins involved in the amino acid efflux. FEBS Lett. 452:228-232. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, H. B., L. H. Wang, and L. H. Zhang. 2002. Genetic control of quorum-sensing signal turnover in Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. USA 99:4638-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]