Abstract
The product of the herpes simplex virus 1 (HSV-1) US3 gene is a multifunctional serine-threonine protein kinase that can block apoptosis induced by proapoptotic cellular proteins, exogenous agents, or replication-defective viruses. Earlier studies showed that the US3 kinase activates and functionally overlaps cellular protein kinase A (PKA). In this study we examined the status of phosphatidylinositol 3-kinase [PI(3)K] and of its effector, protein kinase B/Akt (PKB/Akt), a component of a major pathway of mammalian antiapoptotic signaling systems. We report the following. (i) Infection of target cells with HSV-1 induces transient phosphorylation of serine 473 of PKB/Akt early in infection, with a mechanism that is dependent on PI(3)K. Inhibition of PI(3)K induced apoptosis in mock-infected or ΔUS3 mutant-virus-infected but not in wild-type-virus-infected cells and reduced the accumulation of specific viral gene products, including the US3 protein kinase, but had a marginal effect on virus yields. (ii) At later times after infection, the total amounts of PKB/Akt decreased and phosphorylated PKB/Akt forms disappeared in a US3-dependent and protein phosphatase 2A-independent manner. (iii) Activation of PKA by forskolin did not mediate significant dephosphorylation of PKB/Akt. Our results are consistent with the model that PKB/Akt is activated early in infection and acts to block apoptosis in infected cells prior to the accumulation of US3 protein kinase and that it persists and continues to function as an antiapoptotic protein in the absence of US3 but becomes redundant or even inimical once US3 protein kinase accumulates in effective amounts.
Earlier studies from this and other laboratories have shown that cells infected with wild-type herpes simplex virus 1 (HSV-1) are protected against apoptosis induced by a number of exogenous agents as well as by replication-incompetent mutants lacking the regulatory genes α4 and/or α27 (13, 14, 18, 22, 24). The antiapoptotic functions identified to date map to glycoprotein D (48), glycoprotein J (17, 18, 48), the protein kinase encoded by the US3 gene (24, 25, 37), and the viral ribonucleotide reductase (33). The US3 protein kinase, the focus of this and several preceding reports (4, 5, 8, 9, 15, 17, 25, 29, 30, 32, 34, 37, 39), is a multifunctional protein. The three major functions associated with this kinase are the disruption of nuclear lamina to enable egress of capsids from nuclei (39), phosphorylation of histone-deacetylating enzymes 1 and 2 (35), and blocking apoptosis induced by viral gene products or exogenous agents (5, 8, 9, 17, 25, 29, 30, 32). It is noteworthy that the transcriptional unit encoding the US3 protein kinase contains in its domain a shorter transcript encoding a protein lacking the 76 amino-terminal residues of the US3 protein. The truncated protein, designated US3.5, is catalytically active and mediates the phosphorylation of histone-deacetylating enzymes 1 and 2 but is unable to block apoptosis induced by viral gene products or exogenous agents (34). The US3 protein kinase appears to be the major antiapoptotic protein encoded by the virus, inasmuch as it protects cells from apoptosis induced by the proapoptotic cellular proteins BAD (5, 9, 28, 32), BID (8, 32) and BAX (32), exogenous agents such as sorbitol (15), and viruses lacking key regulatory genes (e.g., ΔICP4 mutants) (25, 30). An important clue to the potential targets of the US3 protein kinase emerged from the observation that the motif of the preferred phosphorylation sites of the US3 protein kinase is similar to that of cyclic AMP-dependent protein kinase (protein kinase A [PKA]) (19, 40). Indeed, activation of PKA by forskolin blocked apoptosis induced by BAD or infection with the ΔICP4 mutant virus. Furthermore, the US3 protein kinase could phosphorylate both PKA targets in vitro and a regulatory subunit of PKA in infected cells. These studies led to the conclusion that the US3 protein kinase both activates and mimics protein kinase A and that the antiapoptotic effects of the US3 kinase reflect the functions of PKA, US3, or both enzymes (4). It is also noteworthy that ΔUS3 mutants induce apoptosis weakly and in only a few cell lines, suggesting that HSV also triggers additional pathways of resistance to apoptosis.
Protein kinase A is a prototype member of the AGC (PKA/protein kinase G/protein kinase C) protein kinase extended family, which comprises over 80 members. The kinases belonging to this group share similarities in the sequences of their catalytic domains, but not their flanking domains, and in their mechanisms of activation; many are often deregulated in diseases, including cancer (28). In particular, one member of this family, protein kinase B (PKB)/Akt, has a well-documented role in promoting cell survival and blocking apoptosis (2, 11, 21, 41, 43). Akt is activated in response to insulin and growth signals through the phosphatidylinositol 3-kinase [PI(3)K] signaling pathway (3). Activation requires the phosphorylation of serine residue at position 473 and of the threonine residue at position 308. PKA/Akt is activated by phosphorylation of residue 473, but the activity is significantly higher when both residues are phosphorylated (3). Furthermore, an important downstream regulator of Akt, protein phosphatase 2A (PP2A), can be activated by PKA-mediated phosphorylation (16).
The purpose of the study presented in this report was to investigate the effect of HSV-1 replication on PI(3)K signaling and Akt phosphorylation. The key finding is that serine 473 of PKB/Akt is phosphorylated in a PI(3)K-dependent fashion early in infection. Inhibition of PI(3)K results in a decrease in the accumulation of several viral proteins but only marginal decrease in viral yields. At intermediate and late stages of infection the phosphorylated species of PKB/Akt disappear concurrently with a decrease in the amount of total PKB/Akt protein. The PKB/Akt negative regulator protein phosphatase 2A did not appear to be activated or involved in the observed PKB/Akt dephosphorylation. In cells infected with a ΔUS3 mutant, PKB/Akt remained phosphorylated for a much longer time interval—an observation that suggests the possibility that PKB/Akt is phosphorylated in infected cells by activation of the PI(3)K signaling pathway and that this pathway protects ΔUS3 mutant-infected cells from apoptosis. Finally, we report that that in wild-type- or ΔUS3 mutant-infected cells treated with forskolin at concentrations sufficient to block apoptosis, the phosphorylation of PKB/Akt was largely unchanged.
MATERIALS AND METHODS
Cells and viruses.
SK-N-SH and HEp2 cells were obtained from the American Type Culture Collection and propagated in Dulbecco's modified Eagle’s medium (DMEM) supplemented with 10% (SK-N-SH) or 5% (HEp2) newborn calf serum (NBCS). HSV-1(F) is the prototype HSV-1 strain used in the laboratory. The recombinant virus R7041, lacking the US3 gene (ΔUS3), is described in reference 37. The HSV-1(KOS) d120 mutant, a kind gift from N. DeLuca (University of Pittsburgh, Pittsburgh, Pa.), lacks both copies of the α4 gene (ΔICP4) and was grown in a Vero-derived cell line (E5) expressing the α4 gene (12). In all experiments in which cells were infected with virus, cells grown in 25-cm2 flasks were either mock infected or exposed to approximately 10 PFU of virus per cell for 1 h and 30 min at 37°C in medium 199V (mixture 199 [Sigma] supplemented with 1% calf serum) and then maintained in DMEM supplemented with the proper amount of serum as described above.
Antibodies and reagents.
Rabbit polyclonal antibody against total Akt, phospho-Akt (Ser473-P), phospho-Akt (Thr308-P) (Cell Signaling Technologies, Beverly, MA) were all used at a dilution of 1:1,000. Rabbit polyclonal antibody against poly(ADP-ribosyl) polymerase (PARP) (Santa Cruz Biotechnology) was used at a dilution of 1:700. Mouse monoclonal antibody against β-actin (Sigma) was used at a dilution of 1:1,000. Monoclonal antibodies to ICP0 and glycoprotein D were purchased from the Goodwin Institute (Plantation, Fla.). Monoclonal antibody against ICP4 was described in reference 1. Rabbit polyclonal antibody against viral thymidine kinase (TK), a product of the UL23 ORF (M. Sarmiento and B. Roizman, unpublished data), was used at a dilution of 1:500.
Epidermal growth factor (EGF), the phosphatidylinositol 3-kinase inhibitor LY294002, the PP2A inhibitor okadaic acid, and the adenylate cyclase activator forskolin were obtained from Sigma. The proteasome inhibitors MG132 and lactacystin were from Calbiochem.
Immunoblot assays.
Cells were harvested at various times after infection, rinsed three times with phosphate-buffered saline (PBS), and solubilized in radioimmunoprecipitation assay buffer in the presence of phosphatase inhibitors (10 mM NaF, 10 mM β-glycerophosphate, 0.1 mM sodium vanadate) and protease inhibitors (Complete; Roche). Lysed cells were stored on ice for 10 min before centrifugation at 14,000 rpm for 10 min. The protein concentration of the supernatant fluids was determined with the aid of a Bio-Rad protein assay. Protein samples denatured in disruption buffer (50 mM Tris [pH 7.0], 2.75% sucrose, 5% 2-β-mercaptoethanol, 2% sodium dodecyl sulfate) were heated at 95°C for 5 min, electrophoretically separated in denaturing polyacrylamide gels, electrically transferred to a nitrocellulose sheet, blocked, and reacted with primary antibody followed by appropriate secondary antibody conjugated to alkaline phosphatase (Bio-Rad). Protein bands were visualized with 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium (Denville Scientific, Metuchen, NJ).
PP2A activity assay.
The activity of cellular protein phosphatase 2A was assayed with a PP2A immunoprecipitation phosphatase assay kit (Upstate) with minor modifications. Briefly, cells were harvested at various times after infection, rinsed and solubilized in TBS (150 mM NaCl, 3 mM KCl, 20 mM Tris-base), and disrupted with 50 strokes of a Dounce homogenizer. Cellular debris was precipitated by low-speed centrifugation, and the catalytic subunit of PP2A was immune precipitated from the supernatants. Protein phosphatase 2A activity was then assayed by incubating the immune-precipitated protein with the synthetic phosphopeptide K-R-pT-I-R-R, as per the manufacturer's instruction (Upstate).
DEVDase activity assay.
Caspase 3 activity was assayed by using a tetrapeptide conjugated to phenylnitraniline (DEVD-pNA) (Biomol). Cells were harvested at various times after infection, rinsed three times with PBS, resuspended in 75 μl of lysis solution A {0.1% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate, 50 mM HEPES (pH 7.4)-1 mM dithiothreitol-0.1 mM EDTA}, held on ice for 10 min, and centrifuged at 14,000 rpm for 10 min at 4°C in an Eppendorf 5415C centrifuge. Equal amounts of protein in supernatant fluids were tested for DEVDase activity according to the manufacturer's instructions (Biomol).
RESULTS
PKB/Akt is transiently phosphorylated in HSV-1-infected cells.
The objective of this series of experiments was to determine whether PKB/Akt is activated by phosphorylation in cells infected with HSV-1. HEp-2 cells were either mock infected or infected with wild-type HSV-1(F) or ΔUS3 or ΔICP4 mutant viruses. At times indicated in Fig. 1, replicate infected cultures were harvested, and the cells were solubilized and subjected to electrophoresis on a denaturing gel. The separated proteins were reacted with antibodies that recognize total PKB/Akt protein or specifically the phosphorylated form of the regulatory threonine 308 or serine 473. As shown in Fig. 1, PKB/Akt phosphorylated on serine 473 accumulated in lysates of cells harvested 3.5 h after infection with either wild-type or mutant HSV (lanes 6 to 8). Phosphorylated PKB/Akt persisted in cells infected with the ΔUS3 mutant virus but disappeared in wild-type-virus-infected cells in a manner that correlated with maximal accumulation of the protein kinase (lane 10). This pattern was reproduced in cells maintained for longer intervals after infection (lanes 13 to 25). It should be noted that at no time were we able to detect the presence in mock-infected or infected cells of PKB/Akt phosphorylated at threonine 308 (Fig. 1 and data not shown). An identical pattern of phosphorylated PKB/Akt was observed in SK-N-SH cells at late time points (data not shown). These results indicate that PKB/Akt is phosphorylated early in infection with HSV-1 and that the phosphorylated species disappear concomitantly with the appearance and accumulation of the US3 protein kinase.
FIG. 1.
Photographs of electrophoretically separated cell lysates reacted with antibody against total PKB/Akt, phosphorylated PKB/Akt serine 473 or threonine 308, US3, or β-actin. HEp-2 cells were mock infected or exposed to 10 PFU of HSV-1(F), ΔUS3, or ΔICP4 per cell and maintained in DMEM supplemented with 5% NBCS. The cells were harvested at the time points shown, solubilized, and normalized for protein content. Equal amounts of protein lysates (120 μg/lane) were subjected to electrophoresis on a denaturing gel, transferred to a nitrocellulose sheet, and reacted with antibodies as indicated.
The amount of total PKB/Akt decreases in infected cells by a proteasome-independent mechanism.
In the results presented above, as infection progressed, the amounts of total PKB/Akt decreased compared to those in mock-infected cells. This decrease was US3 protein kinase independent, inasmuch as it was also observed in ΔUS3- and ΔICP4 mutant-virus-infected cells. Two series of experiments were done to test the hypothesis that HSV-1 infection induces proteasomal degradation of PKB/Akt. In the first, replicate cultures of HEp-2 cells were either mock infected or exposed to HSV-1(F) or ΔUS3 mutant virus for 1 h, maintained for 6.5 h in DMEM supplemented with 5% NBCS, and then mock treated or incubated for 2.5 h in medium containing MG132 (50 μM). In the second series, the cells were mock infected or exposed to HSV-1(F), ΔUS3, or ΔICP4 for 1 h, maintained for 5 h as described above, and then incubated in medium containing lactacystin (10 μM) for an additional 2.5 h. After the treatments, cells were harvested, solubilized, subjected to electrophoresis on denaturing gels, and reacted with antibody against total PKB/Akt, or against β-actin as a loading control. As shown in Fig. 2, neither MG132 nor lactacystin altered the pattern of accumulation of PKB/Akt in infected cells. We conclude from these results that the amount of total PKB/Akt decreases during infection by a mechanism other than proteasome-dependent degradation.
FIG. 2.
Proteasomal inhibitors do not affect the accumulation of total PKB/Akt in infected HEp2 cells. (A) Cells were mock infected or exposed to 10 PFU of HSV-1(F) or ΔUS3 mutant virus per cell, maintained for 6.5 h in DMEM supplemented with 5% NBCS, and treated with 50 μM MG132 for 2.5 h. (B) HEp-2 cells were mock infected or exposed to 10 PFU of HSV-1(F) or ΔUS3 or ΔICP4 mutant virus per cell, maintained for 5 h in DMEM supplemented with 5% NBCS, and treated with 10 μM lactacystin for 2.5 h. The cells were then harvested and solubilized. Equal amounts of protein lysates (120 μg/lane) were subjected to electrophoresis and subsequent immunoblotting with the indicated antibodies.
HSV infection induces signaling through PI(3)K.
The next series of experiments was designed to determine the role of the PI(3)K signaling pathway in the phosphorylation of PKB/Akt during HSV-1 infection. In order to test whether the PI(3)K pathway was intact in HEp2 cells, replicate cultures were either mock infected or exposed to HSV-1(F) or ΔUS3 mutant virus, maintained for 7 h, and incubated for 20 min in medium containing epidermal growth factor (EGF) (100 ng/ml). EGF is a mitogenic polypeptide used for activation of PKB/Akt. The cells were then harvested, solubilized, subjected to electrophoresis on a denaturing gel, and reacted with antibody against total PKB/Akt or PKB/Akt 473-P. As shown in Fig. 3A, EGF treatment resulted in phosphorylation of PKB/Akt in uninfected cells; furthermore, EGF treatment also reversed the loss of PKB/Akt phosphorylation observed at the late stages of HSV-1(F) infection. As an additional control, we investigated whether PKB/Akt phosphorylation in infected cells was dependent on PI(3)K activation. Replicate cultures of HEp2 cells were either mock infected or infected with wild-type virus for 1 h and incubated for an additional 2 h in medium containing the PI(3)K inhibitor LY294002 (75 μM) (7, 44-46). Cells were then exposed to EGF (100 ng/ml) for additional 30 min, harvested, solubilized, and subjected to electrophoresis and immunoblotting as described above. As shown in Fig. 3B, PI(3)K inhibition prevented the phosphorylation of PKB/Akt induced either by EGF treatment, by early events of HSV-1(F) infection, or by both. These results suggest that the PI(3)K pathway is intact in HEp2 cells and that phosphorylation of PKB/Akt in infected cells is dependent on PI(3)K activity. We next assessed the effect of PI(3)K inhibition on PKB/Akt phosphorylation and accumulation of viral proteins in wild-type- and mutant-virus-infected HEp2 cells. Replicate cultures were either mock infected or exposed to HSV-1(F), ΔUS3, or ΔICP4 viruses for 1 h. The cells were then maintained for an additional 2.5 h in the presence or absence of the PI(3)K inhibitor LY294002 (75 μM) and then harvested, solubilized, subjected to electrophoresis on a denaturing gel, and reacted with antibody against total PKB/Akt or PKB/Akt 473-P and against viral proteins of different kinetic classes, i.e., α (ICP0 and ICP4), β (TK and US3), and γ (glycoprotein D [gD]). As expected, treatment with LY294002 precluded the phosphorylation of PKB/Akt in infected cells (shown in Fig. 3C). In addition, while the inhibitor had no effect on the accumulation of ICP0 or ICP4, it decreased the accumulation of viral TK and of the US3 protein kinase. The accumulation of gD was largely unchanged in wild-type-virus- or ΔICP4 mutant-infected cells but reduced in ΔUS3 mutant-virus-infected cells. Consistent with these results, the yield of virus from HEp-2 cells exposed to 10 PFU of HSV-1(F) and to the drug was only fivefold lower than that from untreated infected cells (∼4.8 × 107 PFU compared to ∼2.4 × 108 PFU). We conclude that, at least in cultured cells, while PI(3)K is activated it may not play a major role in the replication of wild-type virus.
FIG. 3.
Role of PI(3)K pathway in PKB/Akt phosphorylation and herpesvirus gene expression. (A) Replicate cultures of HEp-2 cells were mock infected or exposed to 10 PFU of HSV-1(F) or ΔUS3 mutant virus per cell; after 7 h, cells were exposed to EGF (100 ng/ml) for 20 min, harvested, solubilized, and normalized for protein content. Equal amounts of protein lysates (120 μg/lane) were subjected to electrophoresis on a denaturing gel, transferred to a nitrocellulose sheet, and reacted with the indicated antibodies. (B) HEp-2 cells were mock infected or incubated with HSV-1(F) for 1 h and then exposed to the PI(3)K inhibitor LY294002 (75 μM) for 2 h. The cells were then treated with EGF (100 ng/ml) for an additional 30 min, harvested, and solubilized. Equal amounts of protein lysates (120 μg/lane) were subjected to electrophoresis and immunoblotting with the indicated antibodies. (C) Replicate cultures of HEp-2 cells were mock infected or exposed to 10 PFU of HSV-1(F) or ΔUS3 or ΔICP4 mutant virus per cell and then exposed to LY294002 (75 μM) solubilized in dimethyl sulfoxide or to an equivalent amount of dimethyl sulfoxide added to the medium. The cells were harvested at 3.5 h after the infection and processed as described above. While 120 μg/lane of protein lysates was used for immunoblotting with antibodies against total and phosphorylated PKB/Akt, 60 μg/lane was used for immunoblotting with antibodies against viral proteins.
Viral protein kinase US3 blocks apoptosis induced by PI(3)K inhibition.
The objective of the next series of experiments was to determine the effect of prolonged inhibition of the PI(3)K signaling pathway. Replicate cultures of HEp-2 cells were exposed to wild-type or mutant virus for 1 h as described above, maintained for 5 h in DMEM supplemented with 5% NBCS, and then mock treated or exposed to LY294002 (75 μM). The cells were harvested 18 h after the exposure to virus, harvested, solubilized, subjected to electrophoresis on a denaturing gel, transferred to a nitrocellulose sheet, and reacted with antibody to PARP. In parallel, lysates of the infected cells were tested for DEVDase activity. The results were as follows. Inhibition of PI(3)K resulted in the induction of programmed cell death, as measured by both PARP cleavage (Fig. 4A) and caspase 3 activity assay (Fig. 4B), in mock-, ΔUS3- and ΔICP4-infected cells. In particular, cells infected with the ΔUS3 mutant virus and maintained in the presence of LY294002 were committed to apoptosis in a manner comparable to that of untreated cells infected with ΔICP4 mutant virus (compare lanes 6 and 7). In contrast to these results, there was no evidence of apoptosis in wild-type-virus-infected cells exposed to the LY294002 inhibitor (lane 4). Finally, PKB/Akt was phosphorylated in mock-treated, ΔUS3-infected cells but not in LY294002-treated infected cells (data not shown).
FIG. 4.
Effect of PI(3)K inhibition on infected-cell viability. Replicate cultures of HEp-2 cells were mock infected or exposed to 10 PFU of HSV-1(F) or ΔUS3 or ΔICP4 mutant virus per cell, maintained for 5 h in DMEM supplemented with 5% NBCS, and then exposed to the PI(3)K inhibitor LY294002 (75 μM) or medium containing dimethyl sulfoxide as described above. The cells were harvested at 18 h after infection, solubilized, and processed as described above for immunoblotting with antibody directed against PARP (A) or tested for DEVDase activity (B). The results for the DEVDase activity assays are indicated as increase (-fold) above activity of untreated, mock-infected cells.
We conclude that an early event in HSV-1 infection triggers signaling through the PI(3)K pathway, resulting in activation of PKB/Akt. Expression of the US3 protein kinase both mediates loss of phosphorylated PKB/Akt and blocks apoptosis induced by viral gene products.
Effect of okadaic acid treatment on the status of PKB/Akt in HSV-1-infected HEp-2 cells.
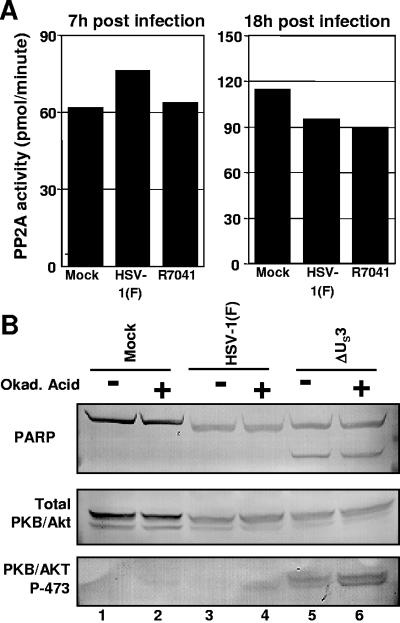
Members of the family of protein phosphatases 2A (PP2A) can be activated by PKA to regulate PKB/Akt (reviewed in reference 16). Since the US3 protein kinase can functionally overlap protein kinase A, we investigated whether activation of PP2A could account for the loss of phosphorylated PKB/Akt correlated with the accumulation of US3. Replicate cultures of HEp-2 cells were either mock infected or exposed to HSV-1(F) or the ΔUS3 mutant virus for 1 h. The cells were then maintained in DMEM supplemented with 5% NBCS, harvested at either 7 or 18 h after infection, and disrupted with a Dounce homogenizer. The catalytic subunit of protein phosphatase 2A was then immune precipitated from the cell lysates and assayed for activity with a synthetic substrate as described in Materials and Methods. As shown in Fig. 5A, no significant changes in total PP2A activity were detected in infected cells compared to mock-infected cells.
FIG. 5.
(A) Assays of PP2A activity in HSV-1(F)-infected cells. Replicate cultures of HEp-2 cells were either mock infected or exposed to HSV-1(F) or the ΔUS3 mutant virus. At the indicated time points, cells were harvested and lysed with a Dounce homogenizer. The PP2A was precipitated from the lysates with the antibody to the catalytic subunit of PP2A. The immune-precipitated PP2A was then assayed for phosphatase activity, measured as release of phosphate groups in the supernatant fluid from a synthetic PP2A substrate as described in Materials and Methods. (B) Effect of okadaic acid treatment on the accumulation of phospho-Akt in HSV-1-infected cells. Replicate cultures of HEp-2 cells were either mock infected or exposed to 10 PFU of HSV-1(F) or ΔUS3 mutant virus per cell for 1 h and maintained in DMEM supplemented with 5% NBCS for 11 h. At that time the cells were mock treated or exposed to okadaic acid (50 ng/ml) for an additional 7 h. The cells were then harvested and solubilized, and the lysates were normalized for protein content: equal amounts of protein lysates were subjected to electrophoresis on a denaturing gel and reacted with antibody against either PARP, total PKB/Akt, or PKB/Akt P-473.
These results, however, did not exclude an alternative scenario in which US3 redirects part of the total PP2A pool to dephosphorylate PKB/Akt, without altering its total activity. In order to test this additional hypothesis, we analyzed okadaic acid-treated and untreated infected cells for the status of phosphorylated PKB/Akt and the induction of apoptotic events. HEp-2 cells were mock infected or infected with HSV-1(F) or ΔUS3 for 1 h. Cells were then maintained in DMEM supplemented with 5% NBCS for 11 h and subsequently exposed to okadaic acid (50 ng/ml) for seven additional hours. The cells were then harvested, solubilized, subjected to electrophoresis on a denaturing gel, transferred to nitrocellulose paper, and incubated with antibodies against PARP or either total PKB/Akt or PKB/Akt 473-P. The results (shown in Fig. 5B) were as follows. (i) Okadaic acid did not cause increased cleavage of PARP. (ii) Okadaic acid treatment resulted in detection of a faint band reactive with antibody against PKB/Akt 473-P in the lysates of both mock- and HSV-1(F)-infected cells (lanes 2 and 4). However, the intensity of such bands was much lower than the intensity of the band corresponding to PKB/Akt 473-P from the lysates of untreated ΔUS3-infected cells (lane 5). Moreover, exposure of ΔUS3-infected cells to okadaic acid also increased the intensity of the Akt-473-P band (compare lanes 5 and 6). Taken together, these results do not support the hypothesis that HSV-1 regulates PKB/Akt phosphorylation by activating PP2A.
Phosphorylation of PKB/Akt in cells exposed to forskolin.
The results presented above suggested that PKA-regulated PP2A was not involved in the observed dephosphorylation of PKB/Akt. The question remained, however, whether the disappearance of phosphorylated PKB/Akt observed at intermediate and late stages of infection was mediated by the US3 protein kinase-dependent activation of the PKA pathway. The experiments described in this section were designed to resolve this question. Specifically, earlier we showed that activation of PKA by forskolin complemented ΔUS3 mutants in blocking apoptosis induced by proapoptotic agents or by the ΔICP4 mutant (4). The objective of the experiment reported in this section was to determine whether PKB/Akt is dephosphorylated as a consequence of activation of PKA by forskolin in the absence of the US3 protein kinase. In this series of experiments, HEp-2 cells were mock infected or infected with HSV-1(F) or with ΔUS3 or ΔICP4 mutants for 1 h. The cells were then maintained in DMEM supplemented with forskolin (100 μg/ml) for 1, 2.5, or 3.5 additional hours. The cells were then harvested, solubilized, subjected to electrophoresis on a denaturing gel, transferred to a nitrocellulose sheet, and incubated with antibodies against total PKB/Akt or PKB/Akt 473-P (Fig. 6, lanes 1 to 16). In addition, HEp-2 cells were infected as described above, exposed to forskolin at 3 h after infection, and harvested 18 h after infection (Fig. 6, lanes 17 to 24). The results were as follows. (i) As expected, forskolin treatment blocked ΔICP4-induced PARP cleavage (lanes 23 and 24). (ii) With the exception of cells harvested at 2 h after infection, the pattern of Akt phosphorylation in forskolin-treated cells could not be differentiated from that in untreated cells. At 2 h after infection, the level of phosphorylated Akt in treated cells was reduced with respect to that of untreated cells. A slight difference was also observed in ΔUS3 virus-infected cells at 18 h after infection. The data suggest that the antiapoptotic function of PKA does not involve the phosphorylation of PKB/Akt.
FIG. 6.
Effect of forskolin treatment on the accumulation of phospho-Akt. For lanes 1 to 16, HEp-2 cells were either mock infected or exposed to 10 PFU of HSV-1(F) or ΔUS3 per cell for 1 h. The cells were then exposed to medium containing forskolin (100 μg/ml) initially dissolved in ethanol or medium containing an equivalent amount of ethanol for 2.5 h. For lanes 17 to 24, the cells were maintained in DMEM supplemented with 5% NBCS for 3 h, then treated with forskolin as described above, and harvested at 17 h postinfection. The cells were solubilized, and equal amounts of protein lysates were subjected to electrophoresis on a denaturing gel, and reacted with antibody against PARP or against either total PKB/Akt or PKB/Akt P-473.
DISCUSSION
The salient features of the results reported in this article are as follows.
(i) PKB/Akt was phosphorylated early after infection of cells with either wild-type or mutant viruses. The extent of phosphorylation and the duration of accumulation of the phosphorylated PKB/Akt were not uniform.
The phosphorylated PKB/Akt disappeared at intermediate and late times after infection with wild-type virus but persisted in cells infected with the ΔUS3 mutant virus. In cells infected with the ΔICP4 mutant, the amounts of phosphorylated PKB/Akt were lower than in cells infected with either the wild type or the ΔUS3 mutant, and the phosphorylated species was not detected late in infection. In uninfected cells, PKB/Akt has been reported to be dephosphorylated by activated cellular protein phosphatase 2A. However, the disappearance of phosphorylated PKB/Akt we observed does not appear to be due to PP2A, inasmuch as the cellular phosphatase was not activated by HSV and, additionally, inhibition of PP2A by okadaic acid had no effect on the accumulation of phosphorylated PKB/Akt.
The conclusion to be derived from these results is that PKB/Akt phosphorylation was dependent primarily on post-α gene expression and did not require the US3 protein kinase. On the other hand, the results suggest that the US3 protein kinase mediates the disappearance of phosphorylated species of PKB/Akt by a mechanism other than dephosphorylation by PP2A.
Our results also indicate that the total PKB/Akt decreased during HSV infection in a manner that is independent of proteasomal degradation. Moreover, phosphorylation did not seem to affect the turnover of Akt, since PKB/Akt decreased at comparable rates at late time points in cells infected with either wild-type, ΔUS3, or ΔICP4 virus.
(ii) The PI(3)K signaling pathway was activated in cells infected with wild-type or mutant viruses, and activation of this pathway was associated with phosphorylation of PKB/Akt. Treatment with an inhibitor of the PI(3)K signaling pathway blocked the phosphorylation of PKB/Akt and reduced the accumulation of specific viral gene products but had only a marginal negative (fivefold) effect on the yield of wild-type virus. Furthermore, sustained inhibition of the PI(3)K signaling pathway resulted in apoptosis that is readily apparent in mock-infected cells and cells infected with ΔUS3 or ΔICP4 mutants but not in cells infected with the wild-type virus [HSV-1(F)]. The evidence that inhibition of PI(3)K induced apoptosis in ΔUS3 mutant-infected cells but not in wild-type-virus-infected cells raises the possibility that activated PKB/Akt can protect ΔUS3-infected cells from apoptosis. In wild-type-virus-infected cells, however, protection from apoptosis is achieved by pathways that do not involve activation of PKB/Akt.
(iii) Lastly, we showed that concentrations of forskolin sufficient to block apoptosis decreased the early accumulation of phosphorylated PKB/Akt and may have retarded the degradation of the kinase in mock-infected cells but not in cells infected with wild-type virus (Fig. 6, compare lanes 17 and 18 and lanes 19 and 20). The results suggest, but do not prove, that the loss of phosphorylated PKB/Akt mediated by US3 is unrelated to the antiapoptotic effects mediated by the activation of PKA by forskolin.
Activation of PI(3)K signaling is required for infectivity of human herpesvirus 8 (31). Activation of PKB/Akt in cells infected with human cytomegalovirus (20), Epstein-Barr virus (36), hepatitis C virus (26, 42), simian virus 40 (6, 47), dengue and Japanese encephalitis viruses (23), adenovirus type 19 (38), and severe acute respiratory syndrome coronavirus (27) has been reported.
A well-established feature of HSV is that it uses multiple pathways to achieve its objectives to suppress host responses to infection. The relevant example is that HSV contains five genes whose products block cell death (US3, gD, gJ, UL36, and γ134.5) (10, 17, 25, 33, 48). On the cellular side, activation of PKB/Akt is a major pathway for blocking apoptosis in uninfected cells. Given the obvious intent to suppress cellular suicidal impulses in every way possible, why are the phosphorylated forms of PKB/Akt removed from the milieu of infected cells? One possible explanation is that PKB/Akt serves an antiapoptotic function early in infection, prior to the accumulation of the US3 protein kinase. Late in infection, its presence is no longer necessary and may even be inimical to viral replication. The observation that phosphorylated PKB/Akt persists in ΔUS3 mutant-virus-infected cells and the observation that ΔUS3 mutant viruses induce apoptosis in only a few of the many cell lines tested are consistent with the hypothesis that PKB/Akt serves as an antiapoptotic protein on an as-needed basis.
Acknowledgments
This study was aided by National Cancer Institute grants CA115662, CA83939, CA71933, CA78766, and CA88860.
REFERENCES
- 1.Ackermann, M., D. K. Braun, L. Pereira, and B. Roizman. 1984. Characterization of herpes simplex virus 1 alpha proteins 0, 4, and 27 with monoclonal antibodies. J. Virol. 52:108-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed, N. N., H. Leighton Grimes, A. Bellacosa, T. O. Chan, and P. N. Tsichilis. 1997. Transduction of interleukin-2 antiapoptotic and proliferative signals via Akt protein kinase. Proc. Natl. Acad. Sci. USA 94:3627-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alessi, D. R., M. Andjelkovic, B. Caudwell, P. Cron, N. Morrice, P. Cohen, and B. A. Hemmings. 1996. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15:6541-6551. [PMC free article] [PubMed] [Google Scholar]
- 4.Benetti, L., and B. Roizman. 2004. Herpes simplex virus protein kinase US3 activates and functionally overlaps protein kinase A to block apoptosis. Proc. Natl. Acad. Sci. USA 101:9411-9416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benetti, L., J. Munger, and B. Roizman. 2003. The herpes simplex virus 1 US3 protein kinase blocks caspase dependent double cleavage and activation of the proapoptotic protein BAD. J. Virol. 77:6567-6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cacciotti, P., D. Barbone, C. Porta, D. A. Altomare, J. R. Testa, L. Mutti, and G. Gaudino. 2005. SV40-dependent AKT activity drives mesothelial cell transformation after asbestos exposure. Cancer Res. 15:5256-5262. [DOI] [PubMed] [Google Scholar]
- 7.Cardone, M. H., N. Roy, H. R. Stennicke, G. S. Salvesen, T. F. Franke, E. Stanbridge, E. Frisch, and J. C. Reed. 1998. Regulation of cell death protease caspase 9 by phosphorylation. Science 282:1318-1321. [DOI] [PubMed] [Google Scholar]
- 8.Cartier, A., E. Broberg, T. Komai, M. Henriksson, and M. G. Masucci. 2003. The herpes simplex virus-1 Us3 protein kinase blocks CD8T cell lysis by preventing the cleavage of Bid by granzyme B. Cell Death Differ. 10:1320-1328. [DOI] [PubMed] [Google Scholar]
- 9.Cartier, A., T. Komai, and M. G. Masucci. 2003. The Us3 protein kinase of herpes simplex virus 1 blocks apoptosis and induces phosphorylation of the Bcl-2 family member Bad. Exp. Cell Res. 291:242-250. [DOI] [PubMed] [Google Scholar]
- 10.Chou, J., and B. Roizman. 1992. The γ134.5 gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programmed cell death in neuronal cells. Proc. Natl. Acad. Sci. USA 89:3266-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 12.DeLuca, N. A., M. McCarth, and P. A. Schaffer. 1985. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J. Virol. 56:558-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galvan, V., and B. Roizman. 1998. Herpes simplex virus type 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc. Natl. Acad. Sci. USA 95:3931-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galvan, V., R. Brandimarti, and B. Roizman. 1999. Herpes simplex virus 1 blocks caspase-3-independent and caspase-dependent pathways to cell death. J. Virol. 73:3219-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hata, S., A. H. Koyama, H. Shiota, A. Adachi, F. Goshima, and Y. Nishiyama. 1999. Antiapoptotic activity of herpes simplex virus type 2: the role of US3 protein kinase gene. Microbes Infect. 1:601-607. [DOI] [PubMed] [Google Scholar]
- 16.Janssens, V., and J. Goris. 2001. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signaling. Biochem. J. 353:417-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jerome, K. R., R. Fox, Z. Chen, A. E. Sears, and L. Corey. 1999. Herpes simplex virus inhibits apoptosis through the action of two genes, Us5 and Us3. J. Virol. 73:8950-8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jerome, K. R., Z. Chen, R. Lang, M. R. Torres, J. Hofmeister, S. Smith, R. Fox, C. J. Froelich, and L. Corey. 2001. HSV and glycoprotein J inhibit caspase activation and apoptosis induced by granzyme B or Fas. J. Immunol. 167:3928-3935. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, D. A., P. Akamine, E. Radzio-Anzelm, M. Madhusudan, and S. S. Taylor. 2001. Dynamics of cAMP-dependent protein kinase. Chem. Rev. 101:2243-2270. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, R. A., X. Wang, X. L. Ma, S. M. Huong, and E. S. Huang. 2001. Human cytomegalovirus up-regulates the phosphatidylinositol 3-kinase (PI3-K) pathway: inhibition of PI3-K activity inhibits viral replication and virus-induced signaling. J. Virol. 75:6022-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy, S. G., A. J. Wagner, S. D. Conzen, J. Jordan, A. Bellacosa, P. N. Tsichilis, and N. Hay. 1997. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 11:701-713. [DOI] [PubMed] [Google Scholar]
- 22.Koyama, A. H., and Y. Miwa. 1997. Suppression of apoptotic DNA fragmentation in herpes simplex virus type 1-infected cells. J. Virol. 71:2567-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee, C. J., C. L. Liao, and Y. L. Lin. 2005. Flavivirus activates phosphatidylinositol 3-kinase signaling to block caspase-dependent apoptotic cell death at the early stage of virus infection. J. Virol. 79:8388-8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leopardi, R., and B. Roizman. 1996. The herpes simplex major regulatory protein ICP4 blocks apoptosis induced by the virus or hyperthermia. Proc. Natl. Acad. Sci. USA 93:9583-9587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leopardi, R., C. Van Sant, and B. Roizman. 1997. The herpes simplex virus 1 protein kinase Us3 is required for protection from apoptosis induced by the virus. Proc. Natl. Acad. Sci. USA 94:7891-7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mannova, P., and L. Beretta. 2005. Activation of the N-Ras-PI3K-Akt-mTOR pathway by hepatitis C virus: control of cell survival and viral replication. J. Virol. 79:8742-8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizutani, T., S. Fukushi, M. Saijo, I. Kurane, and S. Morikawa. 2005. JNK and PI3K/Akt signaling pathways are required for establishing persistent SARS-CoV infection in Vero E6 cells. Biochim. Biophys. Acta 1741:4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mora, A., D. Komander, D. M. Van Aalten, and D. R. Alessi. 2004. PDK1, the master regulator of AGC kinase signal transduction. Semin. Cell Dev. Biol. 15:161-170. [DOI] [PubMed] [Google Scholar]
- 29.Munger, J., and B. Roizman. 2001. The US3 protein kinase of herpes simplex virus 1 mediates the posttranslational modification of BAD and prevents BAD-induced programmed cell death in the absence of other viral proteins. Proc. Natl. Acad. Sci. USA 98:10410-10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munger, J., A. V. Chee, and B. Roizman. 2001. The US3 protein kinase blocks apoptosis induced by the d120 mutant of herpes simplex virus 1 at a premitochondrial stage. J. Virol. 75:5491-5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naranatt, P. P., S. M. Akula, C. A. Zien, H. H. Krishnan, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus induces the phosphatidylinositol 3-kinase-PKCζ-MEK-ERK signaling pathway in target cells early during infection: implications for infectivity. J. Virol. 77:1524-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogg, P. D., P. J. McDonell, B. J. Ryckman, C. M. Knudson, and R. J. Roller. 2004. The HSV-1 US3 protein kinase is sufficient to block apoptosis induced by overexpression of a variety of Bcl-2 family members. Virology 319:212-224. [DOI] [PubMed] [Google Scholar]
- 33.Perkins, D., E. F. R. Pereira, M. Gober, P. J. Yarowsky, and L. Aurelian. 2002. The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) blocks apoptosis in hippocampal neurons, involving activation of the MEK/MAPK survival pathway. J. Virol. 76:1435-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poon, A. P. W., and B. Roizman. 2005. Herpes simplex virus 1 ICP22 regulates the accumulation of a shorter mRNA and of a truncated US3 protein kinase that exhibits altered functions. J. Virol. 79:8470-8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poon, A. P. W., Y. Liang, and B. Roizman. 2003. Herpes simplex virus 1 gene expression is accelerated by inhibitors of histone deacetylases in rabbit skin cells infected with a mutant carrying a cDNA copy of the infected-cell protein no. 0. J. Virol. 77:12671-12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Portis, T., and R. Longnecker. 2004. Epstein-Barr virus (EBV) LMP2A mediates B-lymphocyte survival through constitutive activation of the Rasa/PI3K/Akt pathway. Oncogene 23:8619-8628. [DOI] [PubMed] [Google Scholar]
- 37.Purves, F. C., R. M. Longnecker, D. P. Leader, and B. Roizman. 1987. Herpes simplex virus 1 protein kinase is encoded by open reading frame US3 which is not essential for virus growth in cell culture. J. Virol. 61:2896-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajala, M. S., R. V. S. Rajala, R. A. Astley, A. L. Butt, and J. Chodosh. 2005. Corneal cell survival in adenovirus type 19 infection requires phosphoinositide 3-kinase/Akt activation. J. Virol. 79:12332-12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reynolds, A. E., E. G. Wills, R. J. Roller, B. J. Ryckman, and J. D. Baines. 2002. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J. Virol. 76:8939-8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shabb, J. B. 2001. Physiological substrates of cAMP-dependent protein kinase. Chem. Rev. 101:2381-2411. [DOI] [PubMed] [Google Scholar]
- 41.Song, G., G. Ouyang, and S. Bao. 2005. The activation of Akt/PKB signaling pathway and cell survival. J. Cell Mol. Med. 9:59-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Street, A., A. Macdonald, C. McCormick, and M. Harris. 2005. Hepatitis C virus NS5A-mediated activation of phosphoinositide 3-kinase results in stabilization of cellular beta-catenin and stimulation of beta-catenin-responsive transcription. J. Virol. 79:5006-5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Testa, J. R., and A. Bellacosa. 2001. AKT plays a central role in tumorigenesis. Proc. Natl. Acad. Sci. USA 98:10983-10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vlahos, C. J., W. F. Matter, R. F. Brown, A. E. Traynor-Kaplan, P. G. Heyworth, E. R. Prossnitz, R. D. Ye, P. Marder, J. A. Schelm, and K. J. Rothfuss. 1995. Investigation of neutrophil signal transduction using a specific inhibitor of phosphatidylinositol 3-kinase. J. Immunol. 154:2413-2422. [PubMed] [Google Scholar]
- 45.Vlahos, C. J., W. F. Matter, K. Y. Hui, and R. F. Brown. 1994. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY 294002). J. Biol. Chem. 269:5241-5248. [PubMed] [Google Scholar]
- 46.Yano, H., T. Agatsuma, S. Nakanishi. Y. Saitoh, Y. Fukui, Y. Nonomura, and Y. Matsuda. 1995. Biochemical and pharmacological studies with KT7692 and LY294002 on the role of phosphatidylinositol 3-kinase in FcɛRI-mediated signal transduction. Biochem. J. 312:145-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu, Y., and J. C. Alwine. 2002. Human cytomegalovirus major immediate early proteins and simian virus 40 large T antigen can inhibit apoptosis through activation of the phosphatidylinositide 3′-OH pathway and the cellular kinase Akt. J. Virol. 76:3731-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou, G., V. Galvan, G. Campadelli-Fiume, and B. Roizman. 2000. Glycoprotein D or J delivered in trans blocks apoptosis in SK-N-SH cells induced by a herpes simplex virus 1 mutant lacking intact genes expressing both glycoproteins. J. Virol. 74:11782-11791. [DOI] [PMC free article] [PubMed] [Google Scholar]