Abstract
Human cytomegalovirus (HCMV) masterfully evades adaptive and innate immune responses, allowing infection to be maintained and periodically reactivated for the life of the host. Here we show that cells also possess an intrinsic immune defense against HCMV that is disarmed by the virus. In HCMV-infected cells, the promyelocytic leukemia nuclear body (PML-NB) protein Daxx silences viral immediate-early gene expression through the action of a histone deacetylase. However, this antiviral tactic is efficiently neutralized by the viral pp71 protein, which is incorporated into virions, delivered to cells upon infection, and mediates the proteasomal degradation of Daxx. This work demonstrates the mechanism through which pp71 activates viral immediate-early gene expression in HCMV-infected cells. Furthermore, it provides insight into how a PML-NB protein institutes an intrinsic immune defense against a DNA virus and how HCMV pp71 inactivates this defense.
The adaptive, innate, and intrinsic branches of the human immune system share the same goal of detecting and neutralizing non-self pathogens such as viruses. Adaptive (e.g., antibody and T cell) and innate (e.g., NK cell and interferon) responses require virus-induced signaling cascades for their initiation and can take from hours to days to weeks before becoming active. Intrinsic immune defenses (3) are mediated by constitutively expressed proteins, are active even before the pathogen is present and represent the very first means of protection against infecting viruses.
Successful viruses such as human cytomegalovirus (HCMV) inactivate, modulate, or evade all aspects of immune surveillance. HCMV causes severe disease in immunocompromised or immunosuppressed patients, is the leading viral cause of birth defects, and likely impacts the etiology and/or progression of certain cardiovascular diseases and some cancers (12, 49, 61, 62). After primary infection, persistent and latent infections are established, maintained, and periodically reactivated for the life of the host. Adaptive and innate immune responses wage a war with HCMV and control viral pathogenesis but fail to clear the infection because of multiple viral measures that circumvent these arms of the immune system (2, 21, 50). Here we describe a newly identified intrinsic immune defense against HCMV instituted by the cellular Daxx protein and the mechanism through which the viral pp71 protein neutralizes it.
Daxx localizes to promyelocytic leukemia nuclear bodies (31) (PML-NBs; also called PODs, for PML oncogenic domains; or ND10, for nuclear domain 10), associates with DNA-binding transcription factors (16, 30, 40), recruits histone deacetyleses (HDACs) to targeted promoters to repress transcription (29, 39), and, in HCMV-infected cells, interacts with the viral pp71 protein (7, 28, 32). pp71 is incorporated into the tegument layer of the HCMV virion (54), is essential for efficient viral replication at low multiplicities of infection (MOIs) (4), stimulates cell cycle progression (33-35), and activates transcription from several viral and cellular promoters (37, 41, 57), including the HCMV major immediate-early promoter (MIEP).
The MIEP directs the synthesis of the immediate-early 1 (IE1) and IE2 proteins (49). IE1 is essential for efficient viral replication at low multiplicities of infection (23), and IE2 is absolutely required for viral replication (42). Failure to express IE1 and IE2 halts the lytic replication cycle and may promote the establishment of a latent infection. In reporter assays, pp71 transactivation of the MIEP depends upon its ability to bind Daxx (28). A recombinant HCMV in which the wild-type allele of pp71 was replaced with a Daxx-binding mutant shows the same phenotype as the pp71-null mutant, namely the inability to replicate after low-multiplicity infection and a decrease in the expression of IE genes (7).
Thus, it is clear that the pp71-Daxx interaction facilitates IE gene expression and lytic viral replication; however, the molecular mechanism through which this occurs is not understood. While others have proposed that pp71 and Daxx cooperate to activate the MIEP (28), three observations prompted us to examine if Daxx silences HCMV gene expression and if this repression is relieved by the pp71-mediated degradation of Daxx. First, Daxx is known to repress transcription through the action of HDACs (29, 39), and inhibitors of HDACs activate HCMV gene expression (46, 51, 52). Second, pp71 induces the degradation of the other transcriptional repressors to which it binds (33, 35). Third, Daxx localizes to PML-NBs, and accumulating evidence implicates the proteins that localize to PML-NBs or the structures themselves in cellular antiviral defenses and innate immunity, including their transcriptional induction by interferon, the ability of overexpressed PML to inhibit the replication of some viruses, and the disruption of these structures upon viral infection (10, 19, 25, 38, 44, 45, 59, 60).
Here we provide evidence that the molecular mechanism through which pp71 activates the HCMV MIEP is by inducing Daxx degradation. We show that proteasome function (i.e., protein degradation) is required for HCMV IE gene expression at the very start of low-multiplicity infections in fully permissive fibroblasts and that Daxx is the one and only protein that needs to be degraded to permit viral IE gene expression. Furthermore, we demonstrate that pp71 (but not IE1) is necessary for Daxx degradation in HCMV-infected cells and also that pp71 is sufficient for Daxx degradation in the absence of every other HCMV protein. Finally, we show that HCMV IE gene expression can be rescued in the presence of Daxx by inhibiting HDACs. Our data allow us to build a model in which Daxx silences the viral MIEP by recruiting an HDAC and in which pp71 reverses this silencing by degrading Daxx. Based on previously described cellular antiviral defenses (3, 24), we characterize Daxx as a mediator of intrinsic immunity against HCMV that is neutralized by pp71.
MATERIALS AND METHODS
Cells and viruses.
Human foreskin fibroblasts (HFs) were cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with 10% (vol/vol) fetal bovine serum (Gemini), 100 U/ml penicillin, and 100 μg/ml streptomycin plus 0.292 mg/ml glutamine (Gibco), in a 5% CO2 atmosphere at 37°C. The wild-type virus strains employed were AD169 (HCMV) and KOS (herpes simplex virus type 1 [HSV-1]). The HCMV pp71-null (ADsubUL82) (4) and IE1-null (CR208) (23) viruses have been described previously. For UV inactivation, virus stocks were placed on ice in a UV Stratalinker 2400 (Stratagene) and exposed to a 254-nm light source at 0.12 J/cm2 for 3 min. Crude viral stocks represent extracellular and intracellular particles, and purified stocks are crude stocks pelleted through a cushion (20% [wt/vol] d-sorbitol, 50 mM Tris, pH 7.2, 1 mM MgCl2) and resuspended in DMEM; both will contain many more defective particles (that are able to deliver tegument proteins such as pp71) than infectious virions (26, 64). Banded virus stock (provided by K. Boehme and T. Compton) contains infectious virions separated from noninfectious particles by gradient centrifugation. For infection, the medium was removed from confluent cells which were then incubated with virus at 4°C for 60 min. After a shift to 37°C for 5 min, the viral inoculum was removed and the old medium was returned to the cells. A recombinant adenovirus (rAD) based on pADIC that expresses the pp71 mutant (Did 2-3) unable to bind Daxx (7, 28) was generated as described previously (33). Other rADs have been described previously (33). Transductions were performed at the indicated particle/cell ratio (48).
Inhibitors and antibodies.
Lactacystin (20 μM) or E64 (50 μM) (Calbiochem) dissolved in dimethyl sulfoxide (DMSO) were added at the time of infection with HCMV and 6 h after transduction with rADs. Cycloheximide (100 μg/ml in water) (Sigma) was added 1 h before infection, and trichostatin A (TSA [100 ng/ml in DMSO]) (Upstate Biotechnology) was added 18 h before infection. Antibodies to the following proteins were from commercial sources: Daxx (M-112 from Santa Cruz and D7810 from Sigma), hemagglutinin (HA; Ha.11; Covance), tubulin (DM 1A; Sigma), E2F-1 (KH95; Santa Cruz), PML (H-238; Santa Cruz), pp65 (1025; Rumbaugh-Goodwin Institute), ICP0 (1112; Rumbaugh-Goodwin Institute), Sp100 (AB1380; Chemicon), and Skp-1 (clone 52; Transduction Laboratories). Antibodies against pp71 (2H10-9 and IE-233), IE1 (1B12), and pp28 (CMV 157) have been previously described (33, 55, 66). Secondary horseradish peroxidase-conjugated goat anti-mouse and goat anti-rabbit antibodies were from Chemicon. Secondary antibodies for indirect immunofluorescence conjugated with Alexa Fluor 488 (A-11029 and A-11034) or Alexa Fluor 546 (A-11030) were from Molecular Probes.
Western blots and immunofluorescence.
Cells were lysed in radioimmunoprecipitation assay buffer as described previously (33). Equal amounts of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immobilized on Optitran membranes (Schleicher & Schuell). Blots were blocked in 5% nonfat dry milk dissolved in TBST (10 mM Tris [pH 8.0], 150 mM NaCl, 0.05% Tween 20). Antibody incubations were in 1% milk-TBST, and the blots were developed with the ECL enhanced chemiluminescence system (Amersham). For indirect immunofluorescence, fibroblasts were grown on glass coverslips to confluence and infected as described. Coverslips were washed twice with phosphate-buffered saline (PBS; Gibco) and fixed with 4% paraformaldehyde in PBS. Cells were blocked for 25 min and incubated with primary and subsequently secondary antibodies for 1 h each at room temperature in PBST (PBS plus 0.1% Triton X-100 and 0.05% Tween 20) plus 5% goat serum and 0.5% bovine serum albumin and subsequently washed three times for 5 min with PBST after each incubation. After washing with distilled water twice, nuclei were counterstained with Hoechst 33342 for 10 min, washed twice more with distilled water, and mounted with Fluoromount-G (Southern Biotech). Images were taken using a Zeiss microscope and camera, model Axiovert 200 M.
RNA interference.
Synthetic, annealed 21-base oligonucleotides (small interfering RNA [siRNA]) were purchased from Dharmacon. The Daxx sequence (hDx2) has been published (47); the Skp1 sense sequence was 5′-ACACCAUGCCUUCAAUUAAdTdT-3′. HFs were transfected to a final concentration of 25 nM with siRNA using the TransIT-TKO reagent (Mirus) following the manufacturer's protocol. The next day, the medium was removed and the cells were transfected with the siRNA again. The next day, the medium was replaced, and 24 h later, the cells were infected with HCMV at an MOI of 5. A high multiplicity of infection is required because the transfection reagent appears to inhibit the entry of HCMV (data not shown).
RESULTS
A virion protein rapidly induces the proteasomal degradation of Daxx in HCMV-infected cells.
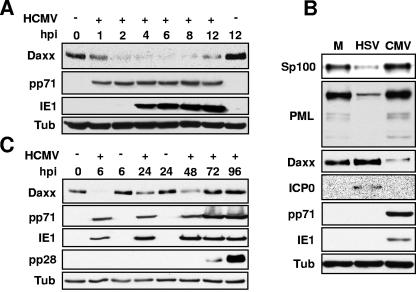
We reasoned that if Daxx were to repress the MIEP and if pp71 were to relieve this repression by degrading Daxx, then the levels of Daxx should drop after HCMV infection. Experimentally, we found that Daxx levels are dramatically reduced as early as 2 h after infection, before detectable levels of viral IE genes are expressed (Fig. 1A). The levels of two other PML-NB proteins, PML and Sp100, are not altered in HCMV-infected cells, but were decreased, as expected, in HSV-1-infected cells (9, 18) (Fig. 1B). Daxx levels return to those observed in mock-infected cells at later times during HCMV infection (Fig. 1C), likely indicating that HCMV may only need to neutralize the antiviral functions of Daxx at the earliest stages of the infectious cycle.
FIG. 1.
Daxx is degraded early during HCMV infection. (A) HFs were uninfected (−) or infected (+) with HCMV at an MOI of 3. Lysates were harvested at the indicated hours postinfection (hpi) and analyzed by Western blotting. Tubulin (Tub) was analyzed as an internal loading control. (B) HFs were either mock infected (M) or infected with HSV-1 or HCMV at an MOI of 3. Lysates harvested at 4 hpi were analyzed by Western blotting. (C) HFs were treated as in panel A. pp28 is an HCMV protein that serves as a marker for the late stages of viral infection.
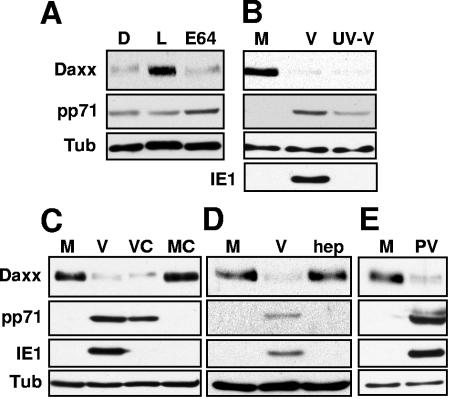
The reduction in the level of Daxx is due to degradation by the proteasome since Daxx is stabilized in the presence of the proteasome inhibitor lactacystin, but not by the calpain inhibitor E64 or the solvent in which both inhibitors are dissolved, DMSO (Fig. 2A). Daxx degradation prior to viral IE gene expression (Fig. 1A) implicates a component of the virion as a mediator of Daxx degradation. We confirmed this prediction by observing Daxx degradation in cells infected with UV-inactivated HCMV virions (Fig. 2B). Exposure of HCMV virions to UV light damages the viral genome and prevents viral gene expression, but UV-inactivated virions still enter cells and induce Daxx degradation (Fig. 2B). Likewise, pretreatment of cells with the protein synthesis inhibitor cycloheximide does not inhibit HCMV-induced Daxx degradation (Fig. 2C), indicating that neither viral nor cellular gene expression is required for Daxx degradation in HCMV-infected cells. It is important to note that in mock-infected cells treated with cycloheximide, Daxx levels do not detectably change, indicating that in the absence of HCMV infection, Daxx is a stable protein (Fig. 2C).
FIG. 2.
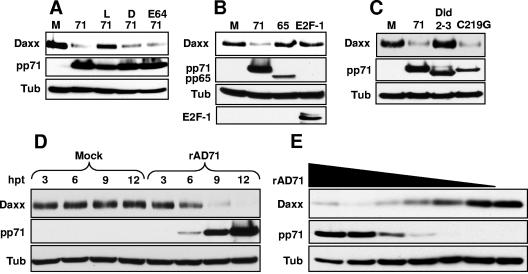
Daxx is degraded by a tegument protein in a proteasome-dependent manner. (A) HFs infected with HCMV at an MOI of 3 were treated with DMSO (D), lactacystin (L), or E64. Lysates were harvested 6 h postinfection (6 hpi) and analyzed by Western blotting. Tub, tubulin. (B) HFs were mock infected, infected with HCMV (V), or infected with HCMV particles exposed to UV light (UV-V) at an MOI of 3. Lysates were harvested at 6 hpi and analyzed by Western blotting. (C) Untreated HFs or HFs preincubated with cycloheximide (C) were mock infected or infected with HCMV at an MOI of 3. Lysates were harvested at 6 hpi and analyzed by Western blotting. (D) HFs were mock infected, infected with HCMV, or infected with HCMV treated with heparin (hep) at an MOI of 0.1. Lystates were harvested at 6 hpi and analyzed by Western blotting. (E) HFs were mock infected or infected with purified HCMV particles (PV) at an MOI of 3. Lysates were harvested at 6 hpi and analyzed by Western blotting.
Because HCMV-infected cells secrete many signaling molecules, including cytokines, chemokines, and interferons, we wanted to rule out the possibility that a nonvirion component of our viral stocks was responsible for inducing Daxx degradation. Pretreatment of crude viral stocks with soluble heparin inhibits viral entry (13) and prevents Daxx degradation (Fig. 2D). In addition, purified HCMV particles were able to induce Daxx degradation upon infection (Fig. 2E). These two experiments demonstrate that viral entry, and not a soluble component of the viral stocks, is required for Daxx degradation.
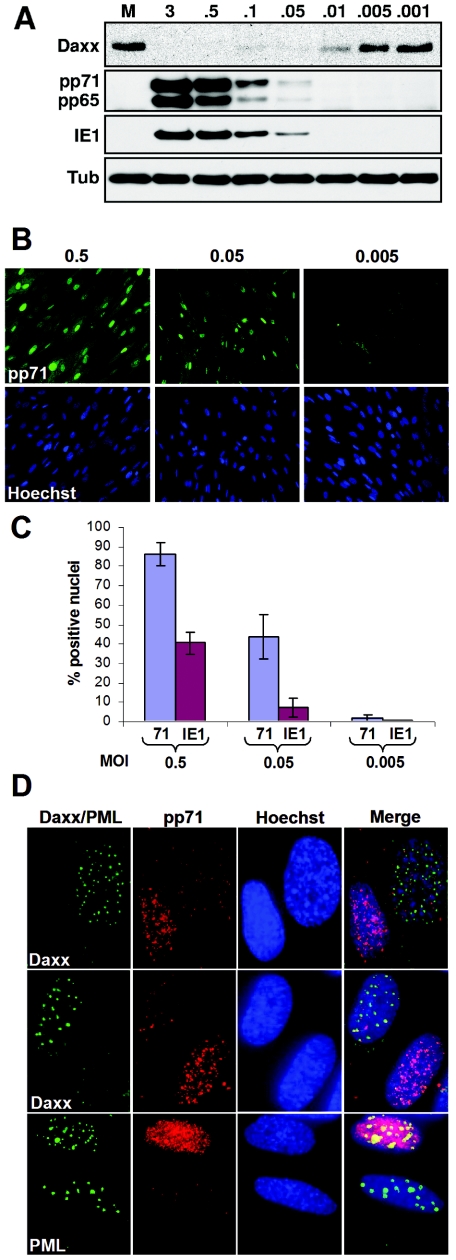
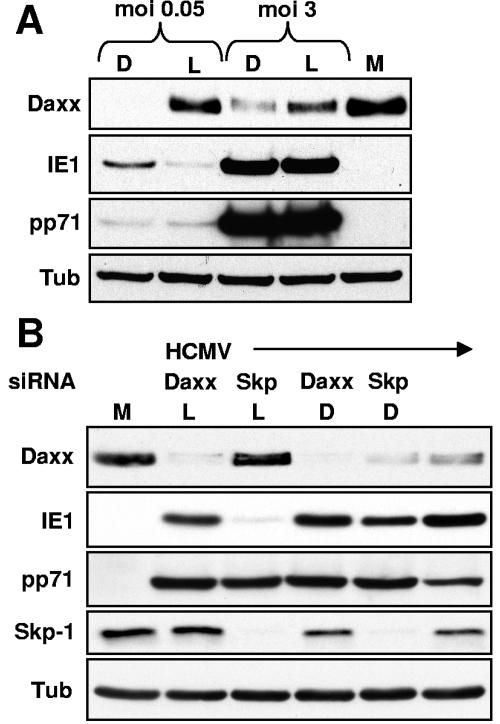
While heparin inhibited Daxx degradation after HCMV infection at an MOI of 0.1, infection at the same MOI in the absence of heparin resulted in the efficient degradation of Daxx (Fig. 2D). In fact, using serial dilutions of crude stocks, we found that, on a population basis, Daxx was efficiently degraded at MOIs as low as 0.05 (Fig. 3A), a multiplicity where only a small percentage of the cells would be productively infected. However, because HCMV stocks are known to contain many noninfectious particles (1 PFU of crude stock has been found to contain from 80 to 200 particles) (26, 64), each cell will be transduced with multiple particles even at this low MOI. In fact, others have shown that at an MOI of 0.1, the majority of cells stain positive for pp71 (32). To confirm this with our viral stock, we used indirect immunofluorescence to determine the percentage of cells that either received tegument proteins or were productively infected after infections at decreasing MOIs (Fig. 3B and 3C). At an MOI of 0.5, we detected pp71 in the majority of nuclei, but only about half of the cells expressed IE1. At an MOI of 0.05, we could detect pp71 in about half of the nuclei and around 10% of the cells were IE1 positive. At an MOI of 0.005, only rarely did we detect either pp71 delivery or IE1 expression (Fig. 3B and 3C). Thus, the high particle/PFU ratio of our crude viral stocks explains why the majority of Daxx expressed by a population of cells can be degraded at such low MOIs when assayed by Western blotting.
FIG. 3.
Low-multiplicity infection is sufficient to deliver tegument proteins and induce the degradation of Daxx. (A) HFs were mock infected (M) or infected with HCMV at the indicated MOIs. Lysates were harvested 6 h postinfection (hpi) and analyzed by Western blotting. Tub, tubulin. (B) HFs grown on coverslips were infected with HCMV at an MOI of 0.5, 0.05, or 0.005. Cells were fixed, and delivery of pp71 to the nucleus was determined by indirect immunofluorescence. The nuclei were counterstained with Hoechst. (C) The percentage of nuclei positive for either pp71 or IE1 was compared from HCMV-infected HFs at an MOI of 0.5, 0.05, or 0.005. (D) HFs were infected at an MOI of 0.5 with gradient-purified HCMV for 4 h in the presence of cycloheximide and analyzed by indirect immunofluorescence. Fixed cells were stained for pp71 and either Daxx or PML, and nuclei were counterstained with Hoechst. Two separate panels are shown for the pp71-Daxx costaining, and a single panel is shown for the pp71-PML costaining.
To determine if we could observe HCMV-mediated Daxx degradation by microscopy, we used indirect immunofluorescence to compare Daxx levels in adjacent cells that either did or did not receive HCMV tegument proteins, assayed here by delivery of pp71 to the nucleus. To accomplish this, we infected cells in the presence of cycloheximide (to prevent IE1 expression from disrupting PML-NBs) at a low MOI with infectious particles that were gradient purified. These stocks should contain very few defective particles, and thus the fraction of cells to which pp71 is delivered should more closely match the MOI. Using this approach, we were able to visualize adjacent cells that either did or did not stain positive for pp71 and found that cells which did not receive tegument-delivered pp71 clearly contain significantly more Daxx that the neighboring, infected cells (Fig. 3D). The level of PML was not different in cells that did or did not receive pp71 (Fig. 3D), which is consistent with the inability of HCMV to induce the degradation of PML (Fig. 1B). Therefore, we can observe Daxx degradation both on a population basis by Western blotting and at the level of an individual cell by indirect immunofluorescence. In total, our data demonstrate that a component of the virion is responsible for Daxx degradation in HCMV-infected cells.
pp71 is necessary and sufficient to induce proteasome-mediated Daxx degradation.
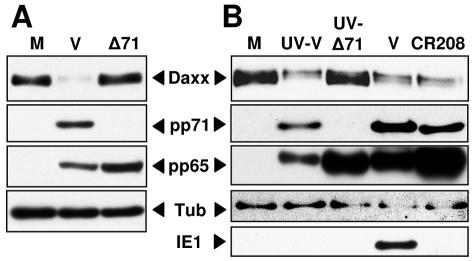
We suspected that pp71 was the virion protein responsible for Daxx degradation in HCMV-infected cells. We have previously shown that pp71 degrades the transcriptional repressors of the retinoblastoma family (Rb, p107, and p130) through a proteasome-dependent, ubiquitin-independent mechanism to stimulate cell cycle progression (33-35). Furthermore, others have shown that pp71 binds to Daxx in HCMV-infected cells (7, 28, 32) and that HCMV mutants that lack wild-type pp71 or that express only a pp71 mutant protein unable to bind Daxx do not efficiently express IE genes after infection at low multiplicities and fail to initiate a lytic replication cycle (4, 7). We used the pp71-null mutant HCMV to show that pp71 was required for Daxx degradation in HCMV-infected cells. While the pp71-null virus entered cells as assayed by delivery of another viral tegument protein, pp65, it failed to induce Daxx degradation (Fig. 4A).
FIG. 4.
pp71 is necessary for Daxx degradation in HCMV-infected cells. (A) HFs were mock infected (M), infected with HCMV (V), or infected with the pp71-null HCMV virus (Δ71) at an MOI of 1. Lysates were harvested at 6 h postinfection (hpi) and analyzed by Western blotting. Tub, tubulin. (B) HFs were mock infected or infected with UV-inactivated wild-type HCMV (UV-V), UV-inactivated pp71-null HCMV (UV-Δ71), wild-type HCMV, or an IE1-null HCMV (CR208) at an MOI of 1. Lysates were harvested at 6 hpi and analyzed by Western blotting.
The HCMV IE1 protein disrupts PML-NBs (36). Although our previous experiments show that viral IE gene synthesis in general is not necessary for Daxx degradation in HCMV-infected cells (Fig. 2), we wanted to confirm that IE1 in particular was not required for this process. We found that cells infected with an IE1-null virus (23) had lower levels of Daxx than mock-infected cells (Fig. 4B), indicating that IE1 is not required for Daxx degradation during HCMV infection. In this experiment, we use UV-inactivated pp71-null virus to again confirm that pp71 is necessary for Daxx degradation (Fig. 4B) and to show that UV treatment does not confer to virions the ability to induce Daxx degradation.
In addition, we show that pp71 is able to induce Daxx degradation in the absence of other HCMV proteins. Daxx is degraded in a proteasome-dependent manner in HCMV-permissive fibroblasts transduced with a defective rAD that expresses pp71 (Fig. 5A). We demonstrate specificity by showing that another viral tegument protein, pp65, and E2F-1, a cellular protein that stimulates cell cycle progression (15), fail to degrade Daxx when overexpressed in cells from rADs (Fig. 5B). In addition, the pp71 mutant (Did 2-3) that fails to bind Daxx (7, 28) fails to induce Daxx degradation, while the pp71 mutant (C219G) which fails to degrade Rb and p130 (33) still induces the degradation of Daxx (Fig. 5C). Furthermore, we show that rAD-expressed pp71, like HCMV tegument-delivered pp71, rapidly (Fig. 1A and 5D) and efficiently (Fig. 3A and 5E) induces Daxx degradation. In combination, these experiments show that pp71 is necessary and sufficient for the proteasome-mediated degradation of Daxx in HCMV-infected cells.
FIG. 5.
pp71 induces the proteasome-dependent degradation of Daxx in the absence of all other HCMV proteins. (A) HFs mock transduced (M) or transduced with rAD71 (lanes 71) at 10,000 particles per cell (ppc) were treated with lactacystin (L), DMSO (D), or E64 at 6 h posttransduction (hpt). Lysates were harvested at 18 hpt and analyzed by Western blotting. (B) HFs were mock transduced or transduced with recombinant adenoviruses that express pp71 (lane 71), pp65 (lane 65), or E2F-1 at 30,000 ppc. Lysates were harvested at 24 hpt and analyzed by Western blotting. (C) HFs were mock transduced or transduced with a recombinant adenovirus that expresses pp71 (lane 71) at 3,000 ppc, a mutant pp71 which fails to bind Daxx (Did 2-3) at 30,000 ppc, or a pp71 mutant that fails to degrade Rb and p130 (C219G) at 3,000 ppc. Lysates were harvested 24 hpt. (D) Lysates from HFs either mock transduced or transduced with rAD71 at 30,000 ppc were harvested at the indicated times (hpt) and analyzed by Western blotting. (E) HFs were transduced with rAD71 at decreasing ppc (from left to right: 30,000, 10,000, 3,000, 1,000, 300, 100, and 0 ppc), and lysates harvested at 24 hpt were analyzed by Western blotting.
Daxx represses HCMV immediate-early gene synthesis.
Finally, because Daxx can repress transcription (30, 39, 40) and because Daxx degradation precedes IE1 production in HCMV-infected cells (Fig. 1A), we asked if Daxx degradation was required for IE1 synthesis. We found that, at a high MOI, preventing Daxx degradation by treating infected cells with the proteasome inhibitor lactacystin had no effect on IE1 production. However, at a low MOI, IE1 production was dramatically reduced in the presence of lactacystin (Fig. 6A). This was not a result of decreased viral entry since equal amounts of the pp71 tegument protein were delivered to the infected cells. We suspect that this steady-state analysis detects a higher residual level of Daxx in cells infected with the higher MOI because a more robust interferon response at the higher MOI results in a higher rate of synthesis for Daxx, an interferon-stimulated gene.
FIG. 6.
Daxx silences the HCMV MIEP. (A) Mock-infected HFs (M) or HFs infected with HCMV at the indicated MOIs were treated with DMSO (D) or lactacystin (L). Lysates were harvested 6 h postinfection (hpi) and analyzed by Western blotting. Tub, tubulin. (B) HFs transfected with siRNA directed at either Daxx or Skp-1 and subsequently infected with HCMV were treated with either DMSO or lactacystin. Lysates were harvested 6 hpi and analyzed by Western blotting. Lysates from HCMV-infected (MOI of 0.1) and mock-infected cells not treated with siRNA were also analyzed.
Lactacystin will stabilize all of the proteins degraded by the proteasome in HCMV-infected cells. To specifically determine if Daxx degradation was required for IE1 production, we used RNA silencing to knock-down the levels of Daxx (47) prior to HCMV infection and lactacystin treatment. The Skp-1 protein, a component of cellular ubiquitin ligase complexes (14), was silenced as a negative control. We found that when Daxx was eliminated by siRNA treatment, IE1 was produced in HCMV-infected cells even when lactacystin was present (Fig. 6B). Decreasing the levels of Skp-1 by siRNA had no effect on Daxx degradation in the absence of lactacystin and did not rescue IE1 synthesis in the presence of lactacystin (Fig. 6B). This experiment demonstrates that, in the presence of Daxx, IE1 production is inhibited and that Daxx is the only protein that needs to be degraded by the proteasome to permit IE gene synthesis in HCMV-infected cells.
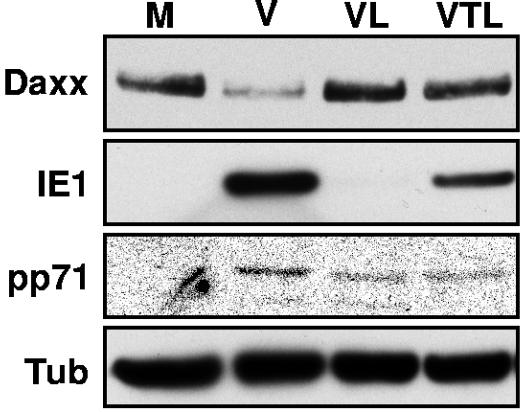
Daxx is known to repress transcription through association with HDACs (29, 39). We found that pretreatment of cells with the HDAC inhibitor TSA before HCMV infection, much like the treatment with siRNA of Daxx described above, allowed IE1 synthesis in the presence of lactacystin (Fig. 7) and increased IE1 production in infected cells not treated with the proteasome inhibitor (data not shown). These data are consistent with Daxx recruiting an HDAC to repress the HCMV MIEP at very early times after HCMV infection. The MIEP produces a transcript that is differentially spliced to produce messenger RNAs for both the IE1 and IE2 proteins (49). IE1 and IE2 initiate the lytic viral replication cycle; thus, preventing their synthesis by Daxx-mediated repression of the MIEP would halt HCMV replication. However, pp71 mediates Daxx degradation, thus allowing for the production of viral IE genes and the initiation of the lytic replication cycle.
FIG. 7.
Daxx represses HCMV immediate-early gene expression through the action of an HDAC. HFs pretreated with trichostatin A (T) were mock infected (M) or infected with HCMV (V) at an MOI of 0.05 and then treated with lactacystin (L). Lysates were harvested 6 h postinfection and analyzed by Western blotting. Tub, tubulin.
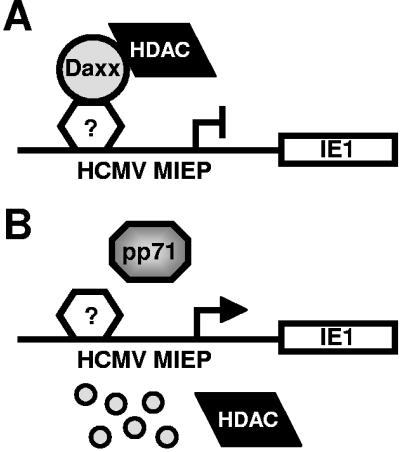
This series of experiments demonstrates that the PML-NB protein Daxx is degraded in HCMV-infected cells by the viral pp71 protein and that in the absence of Daxx degradation, HCMV IE gene expression is silenced. Because Daxx interacts with cellular transcription factors (30, 40) but has not been shown to bind DNA directly, we speculate that it is recruited to the MIEP by directly interacting with one or more of the many cellular transcription factors known to bind this promoter and that an HDAC bound by Daxx represses promoter activity (Fig. 8A). This repression is observed either when cells are infected with wild-type virus in the presence of the proteasome inhibitor lactacystin (Fig. 6A) or when cells are infected with an HCMV mutant that fails to express pp71 (4) or that express a pp71 mutant which fails to bind Daxx (7). However, when pp71 and the proteasome are functional, Daxx is degraded and viral IE genes are efficiently expressed (Fig. 8B).
FIG. 8.
Model for gene expression from the viral MIEP at the onset of lytic viral replication in HCMV-infected cells. (A) In HFs infected with the pp71-null virus or with wild-type virus in the presence of lactacystin, Daxx represses viral transcription through its interactions with an HDAC and an unknown transcription factor bound to the promoter. (B) During infection with HCMV, tegument-delivered pp71 enters the nucleus, degrades Daxx, and thus activates viral immediate-early gene expression.
DISCUSSION
Silencing of herpesviral genomes and cell-based viral immunity.
Our data demonstrate the molecular mechanism through which HCMV pp71 activates transcription from the viral MIEP during lytic infection of fully permissive fibroblasts. We show that by inducing the degradation of Daxx, pp71 relieves HDAC-mediated repression of the MIEP. Previous studies (28) that employed transient transfection reporter assays in semipermissive U373MG cells concluded that Daxx overexpression could enhance the ability of pp71 to activate the MIEP almost threefold. However, since results from reporter assays with the MIEP are known to not always mimic the regulation of the promoter in HCMV-infected cells (46), we are confident that our results indicate the true manner in which pp71 and Daxx regulate the MIEP during lytic infection of fully permissive cells.
For both HCMV and HSV-1, the acetylation status of histones associated with these herpesviral genomes controls viral gene expression (27, 51, 56, 58) in a manner likely identical to the way that the transcription of cellular genes is controlled by histone acetylation. Our new data indicate that preventing the silencing of their genomes by inducing the degradation of cellular repressors that localize to PML-NBs may be a conserved feature of both HCMV and HSV-1. It has been known for some time that HSV-1 ICP0 degrades PML-NB proteins (9, 18) and that the ability of ICP0 to activate transcription correlates with its ability to induce protein degradation (17), but the identity of the cellular protein(s) that needs to be degraded for ICP0-mediated transcriptional activation remains elusive. As shown here, we have not only correlated protein degradation with viral gene expression in HCMV-infected cells but gone on to identify the one and only protein that needs to be degraded by pp71 for the activation of HCMV IE gene synthesis as the cellular PML-NB protein Daxx.
Proteins that localize to PML-NBs can effect transcription (1, 6, 8, 29, 40, 65), are induced by interferon (25, 38), and have antiviral activities (10, 24, 60), and thus have been described as mediators of cellular innate immunity (24, 53). Because Daxx-mediated transcriptional repression of reporter gene expression from integrated avian sarcoma virus genomes has been described as a form of cellular antiviral immunity (24), we also describe the Daxx-mediated transcriptional repression of HCMV shown here as a form of “intrinsic” cellular immunity. The term “intrinsic” was originally used to describe immunity that was independent of cytokines and white blood cells (20) and refers to immune defenses that do not respond to viral infections like the innate and adaptive immune systems, but are always present in cells and ready to function even before the pathogen is encountered (3). Along with their constitutive presence and antiviral activities, other defining characteristics of intrinsic immune defenses are that they are saturable (titratable) and are subject to viral countermeasures (3, 22). Because Daxx is a constitutively expressed protein with an antiviral effect against HCMV that is saturable and is inactivated by the viral pp71 protein, we characterize Daxx-mediated silencing of HCMV IE gene expression as an example of cellular intrinsic immunity.
pp71 orchestrates the viral attack on PML-NB function during HCMV infection.
pp71 could be thought of as the master regulator of PML-NBs during HCMV infection. It induces Daxx degradation, the only PML-NB protein shown to have antiviral properties during HCMV infection, and it induces the expression of IE1, which eventually disrupts these structures (36), and thus presumably attenuates any putative antiviral properties of the proteins remaining at PML-NBs after pp71 degrades Daxx.
While previous studies identified the interaction between pp71 and Daxx, ours is the first to show that pp71 induces the proteasomal degradation of Daxx. Interestingly, all of the studies that have analyzed the subcellular localization of tegument-delivered pp71 during HCMV infection of permissive cells have used either Sp100 or PML (and not Daxx) to establish that pp71 localizes to PML-NBs (7, 28, 32, 43). In nonpermissive mouse cells infected with HCMV, pp71 and Daxx showed a weak colocalization (32), but only pp71-positive cells were imaged, so it is impossible to compare the levels of Daxx in the presence and absence of pp71 in this experiment. Another report visualized a pp71-green fluorescent protein fusion protein introduced into either permissive or nonpermissive cells by transfection, and observed that Daxx, a stable protein, was limiting for the recruitment of pp71 to PML-NBs, even at low levels of pp71 (28). While this could be interpreted as evidence of Daxx degradation, once again, only pp71-positive cells were imaged, and without a pp71-negative cell for comparison, specific conclusions cannot be drawn. Therefore, it is likely that previous studies failed to detect the pp71-mediated degradation of Daxx because of their experimental design. Both our Western blots and immunofluorescence studies employ negative controls that allow us to clearly demonstrate that Daxx is degraded in the presence of pp71.
Near complete Daxx degradation is observed at low MOIs because of the high particle/PFU ratio of the crude viral stocks used. Indeed, we have shown that almost half of the cells in a population stain positive for pp71 after infection at an MOI of 0.05, and the experimentally determined particle to PFU ratio (26, 64) would argue that this is an underestimate of the number of cells that were transduced with HCMV particles. It would be possible for pp71 to degrade the majority of Daxx within a cell, even at levels of pp71 undetectable above background by fluorescence microscopy, if each molecule of pp71 could act catalytically to degrade multiple molecules of Daxx.
Daxx reaccumulates at late times during HCMV infection (Fig. 1C), implying that the antiviral effect of this protein may only be observed prior to the expression of IE genes. In addition, because the absence of Daxx sensitizes cells to apoptosis (11), allowing Daxx to reaccumulate at late times may be another means by which apoptosis is inhibited in HCMV-infected cells. Daxx reaccumulates either because of an increased production resulting from the cellular interferon response, an inhibition of pp71-mediated degradation, or both. The inability of pp71 to bind Daxx cannot explain Daxx reaccumulation in HMCV-infected cells because pp71 still associates with Daxx at late times during infection (7). Interestingly, our preliminary experiments indicate that Daxx does not reaccumulate in cells transduced with an rAD that expresses pp71 (Saffert and Kalejta, unpublished observations). We are currently exploring the reasons for the difference in the steady-state levels of Daxx in rADpp71-transduced and HCMV-infected cells at late time points.
Silencing of the HCMV MIEP by Daxx.
For 25 years, “the CMV promoter” has been the prototype of highly active promoters, and numerous papers both analyzing its regulation and using it to express proteins have been published. Our data demonstrate how this strong, ubiquitously utilized promoter can be silenced during lytic infections by host factors inside living cells. We note that this silencing is only observed at low promoter concentrations. At the high promoter numbers likely obtained with transient transfection of expression plasmids driven by the MIEP, the Daxx intrinsic defense is probably saturated. However, long-term promoter silencing does plague gene therapy approaches that employ the HCMV MIEP (5). Thus, our data showing how this promoter is silenced may reveal ways to increase long-term expression from gene therapy vectors, both improving the efficacy of these approaches and decreasing side effects by allowing viral vectors to be used at lower titers to prevent strong adverse immune responses (63). In addition, regulation of the MIEP by Daxx and pp71 could be exploited both as a target for the treatment for HCMV disease and as a novel regulatable expression system.
In summary, our discoveries presented here demonstrate the mechanism through which pp71 activates the viral MIEP at the very start of the lytic replication cycle in HCMV-infected cells, define another approach utilized by HCMV to escape immune regulation, show how physiologic levels of a PML-NB protein function as an antiviral, and represent the initial example of a cellular intrinsic immune defense against a DNA virus.
Acknowledgments
We thank Phil Balandyk for technical assistance; Michelle Bonar and Brian Olson for preliminary experiments; Wade Bresnahan, Tom Shenk, Ed Mocarski, Richard Greaves, Teresa Compton, and Rebecca Montgomery for reagents; Bill Sugden for the use of his microscope; and Teresa Compton and the members of her laboratory for helpful discussions.
Work in the Kalejta lab is supported by a Scientist Development Grant from the American Heart Association (0430186N) and by start-up funds provided by the University of Wisconsin—Madison that included institutional grants from the American Cancer Society and the Howard Hughes Medical Institute.
REFERENCES
- 1.Alcalay, M., L. Tomassoni, E. Colombo, S. Stoldt, F. Grignani, M. Fagioli, L. Szekely, K. Helin, and P. G. Pelicci. 1998. The promyelocytic leukemia gene product (PML) forms stable complexes with the retinoblastoma protein. Mol. Cell. Biol. 18:1084-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnon, T. I., H. Achdout, O. Levi, G. Markel, N. Saleh, G. Katz, R. Gazit, T. Gonen-Gross, J. Hanna, E. Nahari, A. Porgador, A. Honigman, B. Plachter, D. Mevorach, D. G. Wolf, and O. Mandelboim. 2005. Inhibition of the NKp30 activating receptor by pp65 of human cytomegalovirus. Nat. Immunol. 6:515-523. [DOI] [PubMed] [Google Scholar]
- 3.Bieniasz, P. D. 2004. Intrinsic immunity: a front-line defense against viral attack. Nat. Immunol. 5:1109-1115. [DOI] [PubMed] [Google Scholar]
- 4.Bresnahan, W. A., and T. E. Shenk. 2000. UL82 virion protein activates expression of immediate early viral genes in human cytomegalovirus-infected cells. Proc. Natl. Acad. Sci. USA 97:14506-14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks, A. R., R. N. Harkins, P. Wang, H. S. Qian, P. Liu, and G. M. Rubanyi. 2004. Transcriptional silencing is associated with extensive methylation of the CMV promoter following adenoviral gene delivery to muscle. J. Gene Med. 6:395-404. [DOI] [PubMed] [Google Scholar]
- 6.Cairo, S., F. De Falco, M. Pizzo, P. Salomoni, P. P. Pandolfi, and G. Meroni. 2005. PML interacts with Myc, and Myc target gene expression is altered in PML-null fibroblasts. Oncogene 24:2195-2203. [DOI] [PubMed] [Google Scholar]
- 7.Cantrell, S. R., and W. A. Bresnahan. 2005. Interaction between the human cytomegalovirus UL82 gene product (pp71) and hDaxx regulates immediate-early gene expression and viral replication. J. Virol. 79:7792-7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, C. C., D. Y. Lin, H. I. Fang, R. H. Chen, and H. M. Shih. 2005. Daxx mediates the small ubiquitin-like modifier-dependent transcriptional repression of Smad4. J. Biol. Chem. 280:10164-10173. [DOI] [PubMed] [Google Scholar]
- 9.Chelbi-Alix, M. K., and H. de The. 1999. Herpes virus induced proteasome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene 18:935-941. [DOI] [PubMed] [Google Scholar]
- 10.Chelbi-Alix, M. K., F. Quignon, L. Pelicano, M. H. M. Koken, and H. de Thë. 1998. Resistance to virus infection conferred by the interferon-induced promyelocytic leukemia protein. J. Virol. 72:1043-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, L.-Y., and J. D. Chen. 2003. Daxx silencing sensitizes cells to multiple apoptotic pathways. Mol. Cell. Biol. 23:7108-7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cinatl, J., J. U. Vogel, R. Kotchetkov, and H. W. Doerr. 2004. Oncomodulatory signals by regulatory proteins encoded by human cytomegalovirus: a novel role for viral infection in tumor progression. FEMS Microbiol. Rev. 28:59-77. [DOI] [PubMed] [Google Scholar]
- 13.Compton, T., D. M. Nowlin, and N. R. Cooper. 1993. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology 193:834-841. [DOI] [PubMed] [Google Scholar]
- 14.Craig, K. L., and M. Tyers. 1999. The F-box: a new motif for ubiquitin dependent proteolysis in cell cycle regulation and signal transduction. Prog. Biophys. Mol. Biol. 72:299-328. [DOI] [PubMed] [Google Scholar]
- 15.DeGregori, J., G. Leone, A. Miron, L. Jakoi, and J. R. Nevins. 1997. Distinct roles for E2F proteins in cell growth control and apoptosis. Proc. Natl. Acad. Sci. USA 94:7245-7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emelyanov, A. V., C. R. Kovac, M. A. Sepulveda, and B. K. Birshtein. 2002. The interaction of Pax5 (BSAP) with Daxx can result in transcriptional activation in B cells. J. Biol. Chem. 277:11156-11164. [DOI] [PubMed] [Google Scholar]
- 17.Everett, R., P. O'Hare, D. O'Rourke, P. Barlow, and A. Orr. 1995. Point mutations in the herpes simplex virus type 1 Vmw110 RING finger helix affect activation of gene expression, viral growth, and interaction with PML-containing nuclear structures. J. Virol. 69:7339-7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Everett, R. D., P. Freemont, H. Saitoh, M. Dasso, A. Orr, M. Kathoria, and J. Parkinson. 1998. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J. Virol. 72:6581-6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Everett, R. D., and G. G. Maul. 1994. HSV-1 IE protein Vmw110 causes redistribution of PML. EMBO J. 13:5062-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flint, S. J., L. W. Enquist, V. R. Racaniello, and A. M. Skalka. 2004. Principles of virology: molecular biology, pathogenesis, and control of animal viruses, 2nd ed., p. 532-536. ASM Press, Washington, D.C.
- 21.Gandhi, M. K., and R. Khanna. 2004. Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect. Dis. 4:725-738. [DOI] [PubMed] [Google Scholar]
- 22.Goff, S. P. 2004. Retrovirus restriction factors. Mol. Cell 16:849-859. [DOI] [PubMed] [Google Scholar]
- 23.Greaves, R. F., and E. S. Mocarski. 1998. Defective growth correlates with reduced accumulation of a viral DNA replication protein after low-multiplicity infection by a human cytomegalovirus ie1 mutant. J. Virol. 72:366-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greger, J. G., R. A. Katz, A. M. Ishov, G. G. Maul, and A. M. Skalka. 2005. The cellular protein Daxx interacts with avian sarcoma virus integrase and viral DNA to repress viral transcription. J. Virol. 79:4610-4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guldner, H. H., C. Szostecki, T. Grotzinger, and H. Will. 1992. IFN enhance expression of Sp100, an autoantigen in primary biliary cirrhosis. J. Immunol. 149:4067-4073. [PubMed] [Google Scholar]
- 26.Heider, J. A., W. A. Bresnahan, and T. E. Shenk. 2002. Construction of a rationally designed human cytomegalovirus variant encoding a temperature-sensitive immediate-early 2 protein. Proc. Natl. Acad. Sci. USA 99:3141-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hobbs, W. E., II, and N. A. DeLuca. 1999. Perturbation of cell cycle progression and cellular gene expression as a function of herpes simplex virus ICP0. J. Virol. 73:8245-8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofmann, H., H. Sindre, and T. Stamminger. 2002. Functional interaction between the pp71 protein of human cytomegalovirus and the PML-interacting protein human Daxx. J. Virol. 76:5769-5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollenbach, A. D., C. J. McPherson, E. J. Mientjes, R. Iyengar, and G. Grosveld. 2002. Daxx and histone deacetylase II associate with chromatin through an interaction with core histones and the chromatin-associated protein Dek. J. Cell Sci. 115:3319-3330. [DOI] [PubMed] [Google Scholar]
- 30.Hollenbach, A. D., J. E. Sublett, C. J. McPherson, and G. Grosveld. 1999. The Pax3-FKHR oncoprotein is unresponsive to the Pax3-associated repressor hDaxx. EMBO J. 18:3702-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishov, A. M., A. G. Sotnikov, D. Negorev, O. V. Vladimirova, N. Neff, T. Kamitani, E. T. Yeh, J. F. Strauss III, and G. G. Maul. 1999. PML is critical for ND10 formation and recruits the PML-interacting protein daxx to this nuclear structure when modified by SUMO-1. J. Cell Biol. 147:221-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishov, A. M., O. V. Vladimirova, and G. G. Maul. 2002. Daxx-mediated accumulation of human cytomegalovirus tegument protein pp71 at ND10 facilitates initiation of viral infection at these nuclear domains. J. Virol. 76:7705-7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalejta, R. F., J. T. Bechtel, and T. Shenk. 2003. Human cytomegalovirus pp71 stimulates cell cycle progression by inducing the proteasome-dependent degradation of the retinoblastoma family of tumor suppressors. Mol. Cell. Biol. 23:1885-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalejta, R. F., and T. Shenk. 2003. The human cytomegalovirus UL82 gene product (pp71) accelerates progression through the G1 phase of the cell cycle. J. Virol. 77:3451-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalejta, R. F., and T. Shenk. 2003. Proteasome-dependent, ubiquitin-independent degradation of the Rb family of tumor suppressors by the human cytomegalovirus pp71 protein. Proc. Natl. Acad. Sci. USA 100:3263-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korioth, F., G. G. Maul, B. Plachter, T. Stamminger, and J. Frey. 1996. The nuclear domain 10 (ND10) is disrupted by the human cytomegalovirus gene product IE1. Exp. Cell Res. 229:155-158. [DOI] [PubMed] [Google Scholar]
- 37.Kronschnabl, M., and T. Stamminger. 2003. Synergistic induction of intercellular adhesion molecule-1 by the human cytomegalovirus transactivators IE2p86 and pp71 is mediated via an Sp1-binding site. J. Gen. Virol. 84:61-73. [DOI] [PubMed] [Google Scholar]
- 38.Lavau, C., A. Marchio, M. Fagioli, J. Jansen, B. Falini, P. Lebon, F. Grosveld, P. P. Pandolfi, P. G. Pelicci, and A. Dejean. 1995. The acute promyelocytic leukaemia-associated PML gene is induced by interferon. Oncogene 11:871-876. [PubMed] [Google Scholar]
- 39.Li, H., C. Leo, J. Zhu, X. Wu, J. O'Neil, E.-J. Park, and J. D. Chen. 2000. Sequestration and inhibition of Daxx-mediated transcriptional repression by PML. Mol. Cell. Biol. 20:1784-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li, R., H. Pei, D. K. Watson, and T. S. Papas. 2000. EAP1/Daxx interacts with ETS1 and represses transcriptional activation of ETS1 target genes. Oncogene 19:745-753. [DOI] [PubMed] [Google Scholar]
- 41.Liu, B., and M. F. Stinski. 1992. Human cytomegalovirus contains a tegument protein that enhances transcription from promoters with upstream ATF and AP-1 cis-acting elements. J. Virol. 66:4434-4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marchini, A., H. Liu, and H. Zhu. 2001. Human cytomegalovirus with IE-2 (UL122) deleted fails to express early lytic genes. J. Virol. 75:1870-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marshall, K. R., K. V. Rowley, A. Rinaldi, I. P. Nicholson, A. M. Ishov, G. G. Maul, and C. M. Preston. 2002. Activity and intracellular localization of the human cytomegalovirus protein pp71. J. Gen. Virol. 83:1601-1612. [DOI] [PubMed] [Google Scholar]
- 44.Maul, G. G. 1998. Nuclear domain 10, the site of DNA virus transcription and replication. Bioessays 20:660-667. [DOI] [PubMed] [Google Scholar]
- 45.Maul, G. G., E. Yu, A. M. Ishov, and A. L. Epstein. 1995. Nuclear domain 10 (ND10) associated proteins are also present in nuclear bodies and redistribute to hundreds of nuclear sites after stress. J. Cell Biochem. 59:498-513. [DOI] [PubMed] [Google Scholar]
- 46.Meier, J. L. 2001. Reactivation of the human cytomegalovirus major immediate-early regulatory region and viral replication in embryonal NTera2 cells: role of trichostatin A, retinoic acid, and deletion of the 21-base-pair repeats and modulator. J. Virol. 75:1581-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michaelson, J. S., and P. Leder. 2003. RNAi reveals anti-apoptotic and transcriptionally repressive activities of DAXX. J. Cell Sci. 116:345-352. [DOI] [PubMed] [Google Scholar]
- 48.Mittereder, N., K. L. March, and B. C. Trapnell. 1996. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J. Virol. 70:7498-7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mocarski, E. S., and C. T. Courcelle. 2001. p. 2629-2673. In D. M. Knipe and P. Howley (ed.), Fields virology. Lippincott, Philadelphia, Pa.
- 50.Mocarski, E. S., Jr. 2004. Immune escape and exploitation strategies of cytomegaloviruses: impact on and imitation of the major histocompatibility system. Cell Microbiol. 6:707-717. [DOI] [PubMed] [Google Scholar]
- 51.Murphy, J. C., W. Fischle, E. Verdin, and J. H. Sinclair. 2002. Control of cytomegalovirus lytic gene expression by histone acetylation. EMBO J. 21:1112-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nevels, M., C. Paulus, and T. Shenk. 2004. Human cytomegalovirus immediate-early 1 protein facilitates viral replication by antagonizing histone deacetylation. Proc. Natl. Acad. Sci. USA 101:17234-17239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nisole, S., J. P. Stoye, and A. Saib. 2005. TRIM family proteins: retroviral restriction and antiviral defence. Nat. Rev. Microbiol. 3:799-808. [DOI] [PubMed] [Google Scholar]
- 54.Nowak, B., A. Gmeiner, P. Sarnow, A. J. Levine, and B. Fleckenstein. 1984. Physical mapping of human cytomegalovirus genes: identification of DNA sequences coding for a virion phosphoprotein of 71 kDa and a viral 65-kDa polypeptide. Virology 134:91-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nowak, B., C. Sullivan, P. Sarnow, R. Thomas, F. Bricout, J. C. Nicolas, B. Fleckenstein, and A. J. Levine. 1984. Characterization of monoclonal antibodies and polyclonal immune sera directed against human cytomegalovirus virion proteins. Virology 132:325-338. [DOI] [PubMed] [Google Scholar]
- 56.Poon, A. P. W., Y. Liang, and B. Roizman. 2003. Herpes simplex virus 1 gene expression is accelerated by inhibitors of histone deacetylases in rabbit skin cells infected with a mutant carrying a cDNA copy of the infected-cell protein no. 0. J. Virol. 77:12671-12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Preston, C. M., and M. J. Nicholl. 2005. Human cytomegalovirus tegument protein pp71 directs long-term gene expression from quiescent herpes simplex virus genomes. J. Virol. 79:525-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reeves, M. B., P. A. MacAry, P. J. Lehner, J. G. Sissons, and J. H. Sinclair. 2005. Latency, chromatin remodeling, and reactivation of human cytomegalovirus in the dendritic cells of healthy carriers. Proc. Natl. Acad. Sci. USA 102:4140-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Regad, T., and M. K. Chelbi-Alix. 2001. Role and fate of PML nuclear bodies in response to interferon and viral infections. Oncogene 20:7274-7286. [DOI] [PubMed] [Google Scholar]
- 60.Regad, T., A. Saib, V. Lallemand-Breitenbach, P. P. Pandolfi, H. de The, and M. K. Chelbi-Alix. 2001. PML mediates the interferon-induced antiviral state against a complex retrovirus via its association with the viral transactivator. EMBO J. 20:3495-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reinhardt, B., R. Minisini, and T. Mertens. 2002. Cytomegalovirus is a risk factor in atherogenesis. Herpes 9:21-23. [PubMed] [Google Scholar]
- 62.Revello, M. G., and G. Gerna. 2002. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin. Microbiol. Rev. 15:680-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ritter, T., M. Lehmann, and H. D. Volk. 2002. Improvements in gene therapy: averting the immune response to adenoviral vectors. Bio Drugs 16:3-10. [DOI] [PubMed] [Google Scholar]
- 64.Stinski, M. F., E. S. Mocarski, and D. R. Thomsen. 1979. DNA of human cytomegalovirus: size heterogeneity and defectiveness resulting from serial undiluted passage. J. Virol. 31:231-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhong, S., P. Salomoni, and P. P. Pandolfi. 2000. The transcriptional role of PML and the nuclear body. Nat. Cell Biol. 2:E85-E90. [DOI] [PubMed] [Google Scholar]
- 66.Zhu, H., Y. Shen, and T. Shenk. 1995. Human cytomegalovirus IE1 and IE2 proteins block apoptosis. J. Virol. 69:7960-7970. [DOI] [PMC free article] [PubMed] [Google Scholar]