Abstract
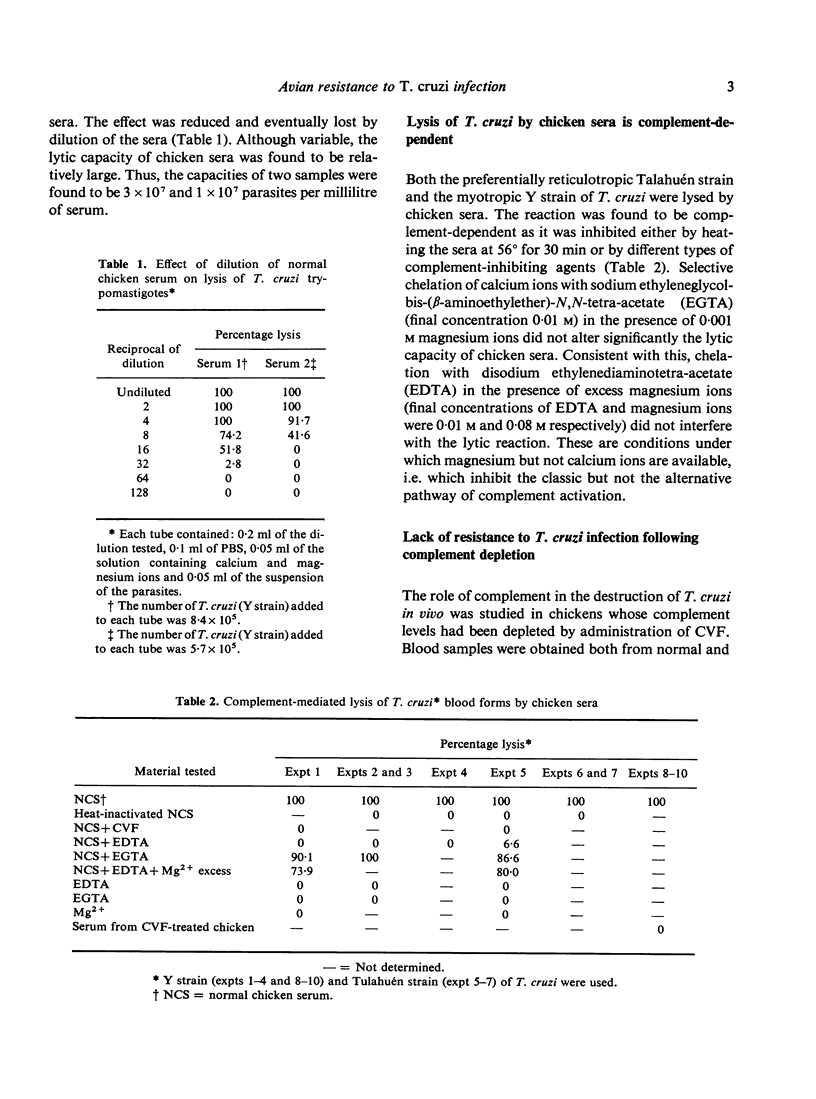
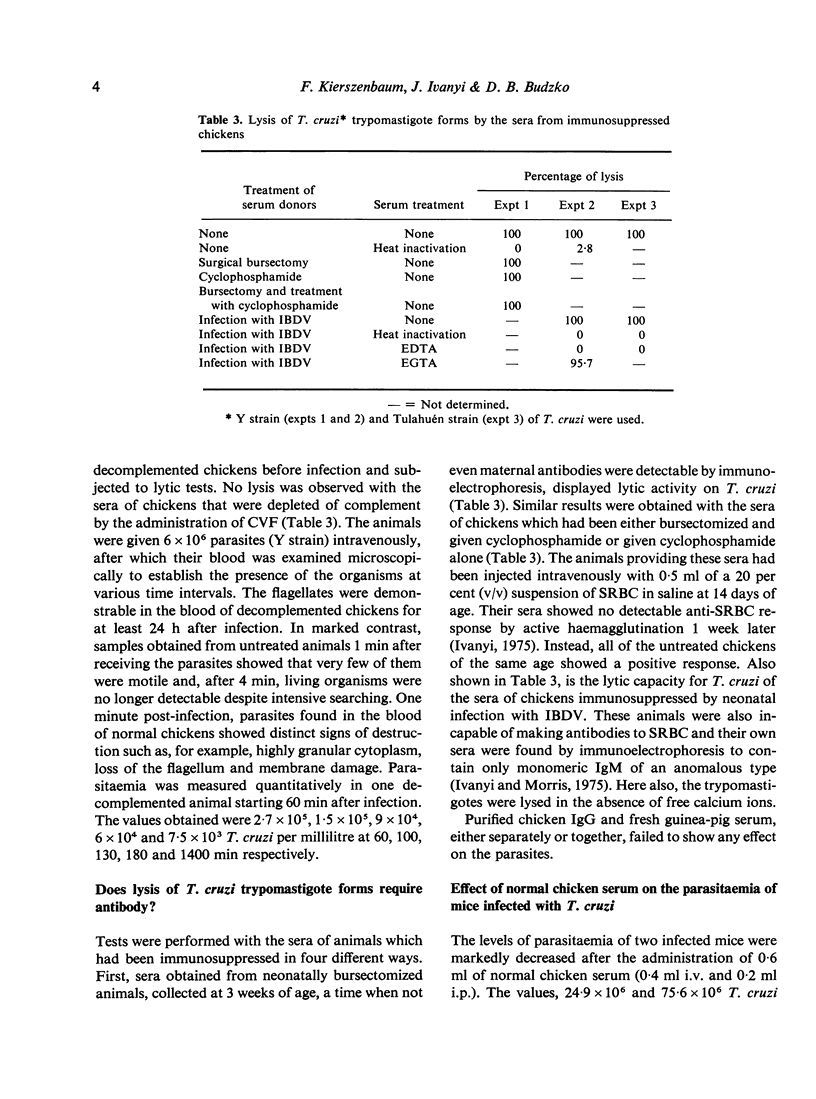
The natural resistance of chickens to Trypanosoma curzi infection and the capacity of their sera to lyse blood (trypomastigote) forms of the parasite in vitro were found to be complement-dependent phenomena. Parasites given intravenously to decomplemented chickens were detectable in their bloodstream for at least 24 h post-infection, whereas in untreated animals they became undetectable after 1 min (and destroyed flagellates were observed). One millilitre of serum had the capacity to lyse as many as 10-30 X 10(6) organisms. The lytic activity of serum in vitro was not impaired in chickens that had been immunosuppressed by four different procedures and was present in the absence of antibodies. In vitro lysis of T. cruzi by either normal or antibody-free chicken sera occurred in the absence of calcium ions but required magnesium ions, indicating that complement was activated via the alternative pathway. Administration of normal chicken serum to mice infected with T. cruzi provoked a marked decrease in their parasitaemias.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Budzko D. B., Pizzimenti M. C., Kierszenbaum F. Effects of complement depletion in experimental chagas disease: immune lysis of virulent blood forms of Trypanosoma cruzi. Infect Immun. 1975 Jan;11(1):86–91. doi: 10.1128/iai.11.1.86-91.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielsen A. E., Pickering R. J., Good R. A. Haemolysis in chicken serum. I. The ionic environment. Immunology. 1973 Aug;25(2):167–177. [PMC free article] [PubMed] [Google Scholar]
- Gabrielsen A. E., Pickering R. J., Linna T. J., Good R. A. Haemolysis in chicken serum. II. Ontogenetic development. Immunology. 1973 Aug;25(2):179–184. [PMC free article] [PubMed] [Google Scholar]
- Ivanyi J. Immunodeficiency in the chicken. II. Production of monomeric IgM following testosterone treatment or infection with Gumboro disease. Immunology. 1975 Jun;28(6):1015–1021. [PMC free article] [PubMed] [Google Scholar]
- Iványi J., Valentová V., Cerný J. The dose of antigen required for the suppression of the IgM and IgG antibody response in chickens. I. The kinetics and characterization of serum antibodies. Folia Biol (Praha) 1966;12(3):157–167. [PubMed] [Google Scholar]
- KORINEK J., PALUSKA E. Immunochemical studies of rivanol-soluble serum proteins. Folia Biol (Praha) 1961;7:185–192. [PubMed] [Google Scholar]
- Kierszenbaum F., Saavedra L. E. The effects of bacterial endotoxin on the infection of mice with Trypanosoma cruzi. J Protozool. 1972 Nov;19(4):655–657. doi: 10.1111/j.1550-7408.1972.tb03552.x. [DOI] [PubMed] [Google Scholar]
- McGHEE R. B. The effect of a malaria infection on the titer of complement and its components in ducks. J Immunol. 1952 Apr;68(4):421–427. [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Fjellström K. E. Isolation of the anticomplementary protein from cobra venom and its mode of action on C3. J Immunol. 1971 Dec;107(6):1666–1672. [PubMed] [Google Scholar]
- RUBIO M. Actividad lítica de sueros normales sobre formas de cultivo y sanguíneas de Trypanosoma cruzi. Bol Chil Parasitol. 1956 Oct-Dec;11(4):62–69. [PubMed] [Google Scholar]
- Stolfi R. L., Fugmann R. A., Jensen J. J., Sigel M. M. A C1-fixation method for the measurement of chicken anti-viral antibody. Immunology. 1971 Mar;20(3):299–306. [PMC free article] [PubMed] [Google Scholar]
- WARREN L. G. Biochemical studies on chicken macrophages infected in vitro with Trypanosoma cruzi. Exp Parasitol. 1958 Jan;7(1):82–91. doi: 10.1016/0014-4894(58)90007-9. [DOI] [PubMed] [Google Scholar]


