Abstract
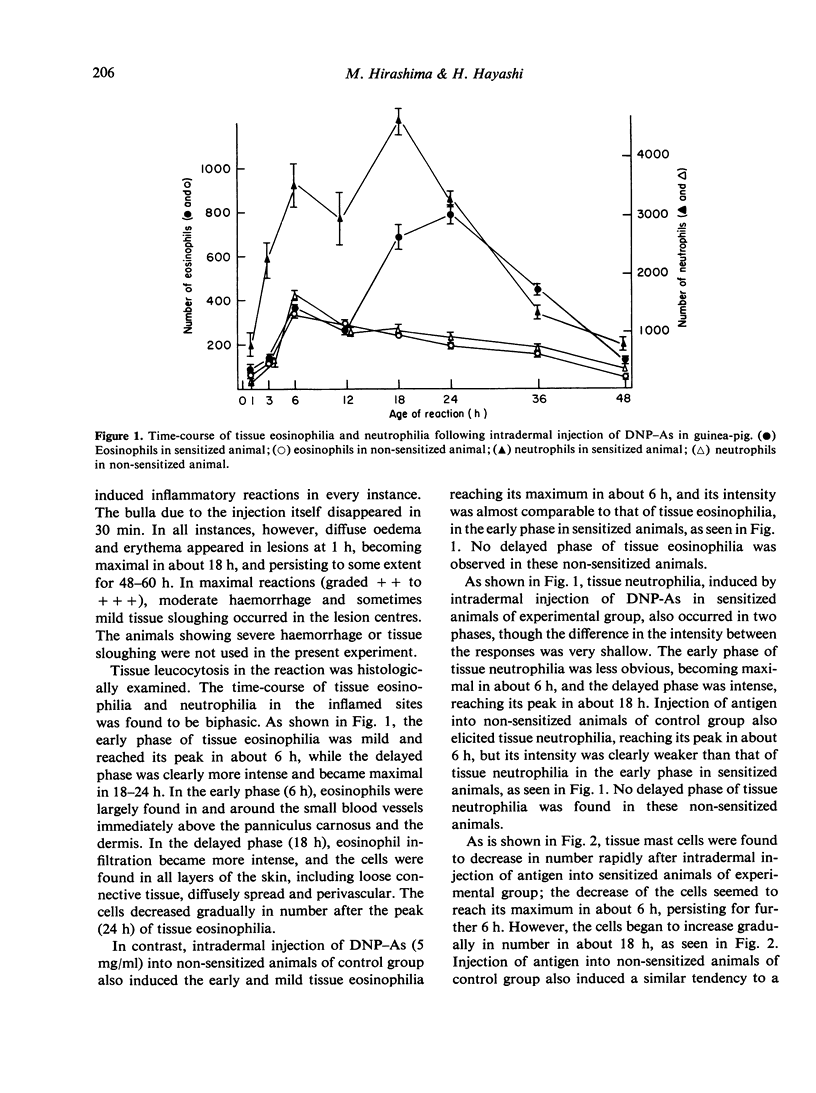
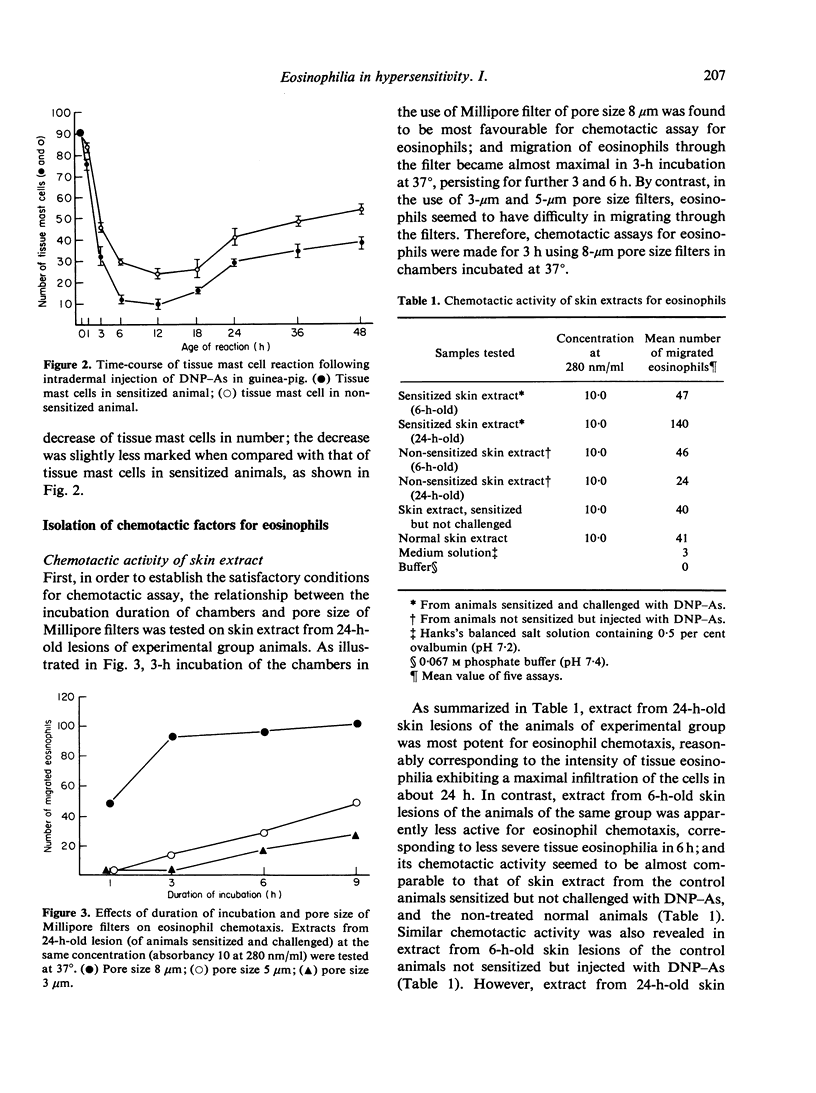
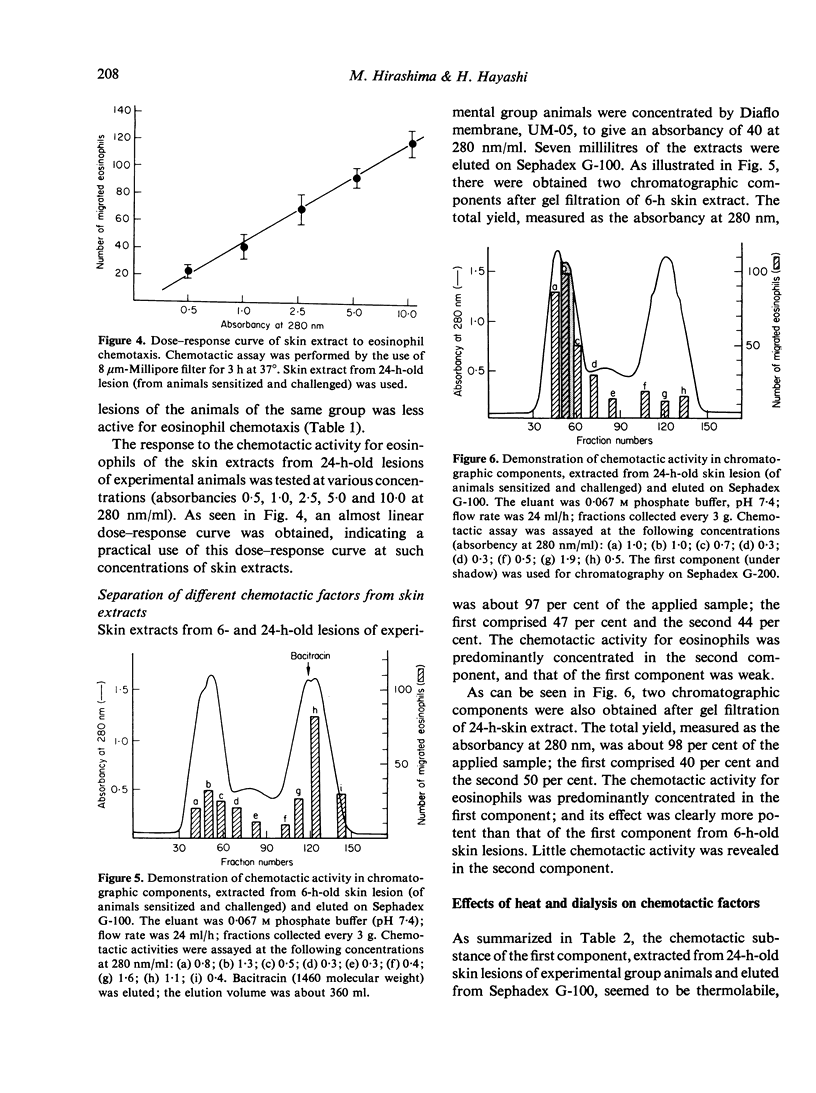
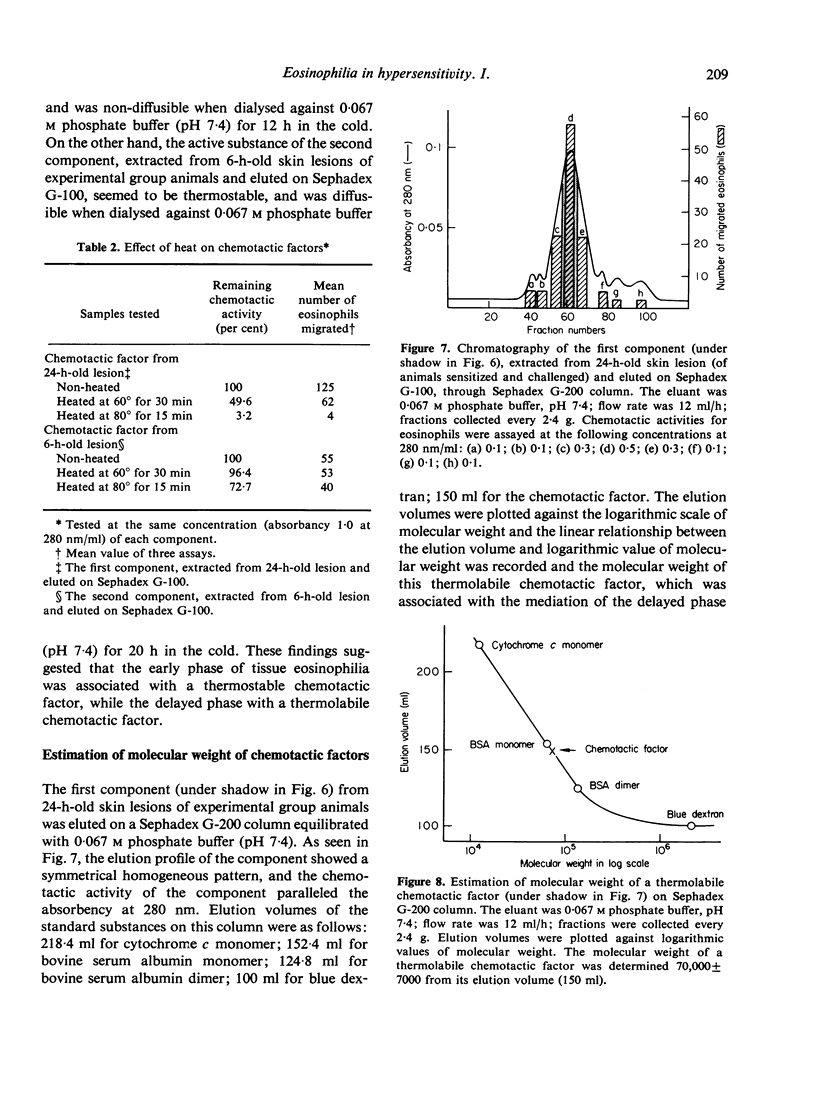
In an active cutaneous anaphylaxis induced by DNP-ascaris extract in guinea-pig, tissue eosinophilia manifested two phases; the early and mild phase became maximal in about 6 h, while the delayed and intense phase in 18-24 h. Skin extracts from the lesions exhibited chemotactic activities for eosinophils, respectively comparable to the intensity of tissue eosinophilia in each phase; and two different chemotactic factors for eosinophils of skin extracts were separated by gel filtration on Sephadex G-100. The mediation of the early phase seemed to be associated with a thermostable factor with amolefular weight of less than 1400; this factor seemed to be related to mast cell degranulation. The mediation of the delayed phase appeared to be associated with a thermolabile factor with a molecular weight of about 70,000, probably independent of mast cell degranulation; the factor was considered to be more significant than the thermostable factor, because the delayed tissue eosinophilia was more intense than the early tissue eosinophilia.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYDEN S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962 Mar 1;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson C., Morseth D. J., Soulsby J. L. Immunoglobulin E-type antibodies induced by Ascaris suum infections in guinea pigs. J Immunol. 1971 Jan;106(1):128–133. [PubMed] [Google Scholar]
- Hayashi H. The intracellular neutral SH-dependent protease associated with inflammatory reactions. Int Rev Cytol. 1975;40:101–151. [PubMed] [Google Scholar]
- Higuchi Y., Honda M., Hayashi H. Production of chemotactic factor for lymphocytes by neutral SH-dependent protease of rabbit PMN leukocytes from immunoglobulins, especially IgM. Cell Immunol. 1975 Jan;15(1):100–108. doi: 10.1016/0008-8749(75)90168-9. [DOI] [PubMed] [Google Scholar]
- Kay A. B., Stechschulte D. J., Austen K. F. An eosinophil leukocyte chemotactic factor of anaphylaxis. J Exp Med. 1971 Mar 1;133(3):602–619. doi: 10.1084/jem.133.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay A. B. Studies on eosinophil leucocyte migration. I. Eosinophil and neutrophil accumulation following antigen-antibody reactions in guinea-pig skin. Clin Exp Immunol. 1970 Jan;6(1):75–86. [PMC free article] [PubMed] [Google Scholar]
- Kay A. B. Studies on eosinophil leucocyte migration. II. Factors specifically chemotactic for eosinophils and neutrophils generated from guinea-pig serum by antigen-antibody complexes. Clin Exp Immunol. 1970 Nov;7(5):723–737. [PMC free article] [PubMed] [Google Scholar]
- Kouno T. The role of neutral SH-dependent protease of PMN leukocyte lysosome in inflammation. Kumamoto Med J. 1971 Dec 31;24(3):135–150. [PubMed] [Google Scholar]
- LITT M. EOSINOPHILS AND ANTIGEN-ANTIBODY REACTIONS. Ann N Y Acad Sci. 1964 Aug 27;116:964–985. doi: 10.1111/j.1749-6632.1964.tb52562.x. [DOI] [PubMed] [Google Scholar]
- LITT M. Studies in experimental eosinophilia. I. Repeated quantitation of peritoneal eosinophilia in guinea pigs by a method of peritoneal lavage. Blood. 1960 Sep;16:1318–1329. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAYASHI H., MIYOSHI H., NITTA R., UDAKA K. Proteolytic mechanism in recurrence of Arthus-type inflammation by thiol compounds. Br J Exp Pathol. 1962 Oct;43:564–573. [PMC free article] [PubMed] [Google Scholar]
- Margni R. A., Hajos S. E. Guinea-pig reaginic antibody. II. Physicochemical and biological properties. Immunology. 1973 Aug;25(2):333–342. [PMC free article] [PubMed] [Google Scholar]
- Nishiura M., Yamamoto S., Hayashi H. The natural mediator for PMN emigration in inflammation. VI. Production of leucoegresin by inflammatory SH-dependent protease in active Arthus reactions in complement-depleted rabbits. Immunology. 1974 Dec;27(6):1023–1031. [PMC free article] [PubMed] [Google Scholar]
- OVARY Z., BIER O. G. Quantitative studies on passive cutaneous anaphylaxis in the guinea pig and its relationship to the Arthus phenomenon. J Immunol. 1953 Jul;71(1):6–11. [PubMed] [Google Scholar]
- Ogata T. The role of inflammatory chemotactic factor (leukoegresin) and permeability factor (vasoexin) in acute inflammation: an electron microscopic observation of biologic action of these natural mediators. Kumamoto Med J. 1971 Dec 31;24(3):103–123. [PubMed] [Google Scholar]
- PORATH J., FLODIN P. Gel filtration: a method for desalting and group separation. Nature. 1959 Jun 13;183(4676):1657–1659. doi: 10.1038/1831657a0. [DOI] [PubMed] [Google Scholar]
- Parish W. E., Coombs R. R. Peripheral blood eosinophilia in guinea-pigs following implantation of anaphylactic guinea-pig and human lung. Br J Haematol. 1968 Apr;14(4):425–445. doi: 10.1111/j.1365-2141.1968.tb06994.x. [DOI] [PubMed] [Google Scholar]
- Parish W. E. Investigations on eosinophilia. The influence of histamine, antigen-antibody complexes containing gamma-1 or gamma-2 globulins, foreign bodies (phagocytosis) and disrupted mast cells. Br J Dermatol. 1970 Jan;82(1):42–64. doi: 10.1111/j.1365-2133.1970.tb02193.x. [DOI] [PubMed] [Google Scholar]
- SAMTER M., KOFOED M. A., PIEPER W. A factor in lungs of anaphylactically shocked guinea pigs which can induce eosinophilia in normal animals. Blood. 1953 Dec;8(12):1078–1090. [PubMed] [Google Scholar]
- Strejan G., Campbell D. H. Hypersensitivity to Ascaris antigens. I. Skin-sensitizing activity of serum fractions from guinea pigs sensitized to crude extracts. J Immunol. 1967 May;98(5):893–900. [PubMed] [Google Scholar]
- Tada T., Okumura K. Regulation of homocytotropic antibody formation in the rat. I. Feed-back regulation by passively administered antibody. J Immunol. 1971 Apr;106(4):1002–1011. [PubMed] [Google Scholar]
- Yamamoto S., Nishiura M., Matsumura K. In vitro production of a chemotactic factor by inflammatory SH-dependent protease from rabbit and human immunoglobulin G subclasses. Tohoku J Exp Med. 1974 Sep;114(1):49–54. doi: 10.1620/tjem.114.49. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Yoshinaga M., Hayashi H. The natural mediator for PMN emigration in inflammation. II. Common antigenicity of leucoegresin with immunoglobulin G. Immunology. 1971 May;20(5):803–808. [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga M., Yamamoto S., Maeda S., Hayashi H. The natural mediator for PMN emigration in inflammation. 3. In vitro production of a chemotactic factor by inflammatory SH-dependent protease from serum immunoglobulin G. Immunology. 1971 May;20(5):809–815. [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga M., Yoshida K., Tashiro A., Hayashi H. The natural mediator for PMN emigration in inflammation. I. Purification and characterization of leucoegresin from Arthus skin site. Immunology. 1971 Aug;21(2):281–298. [PMC free article] [PubMed] [Google Scholar]


