Abstract
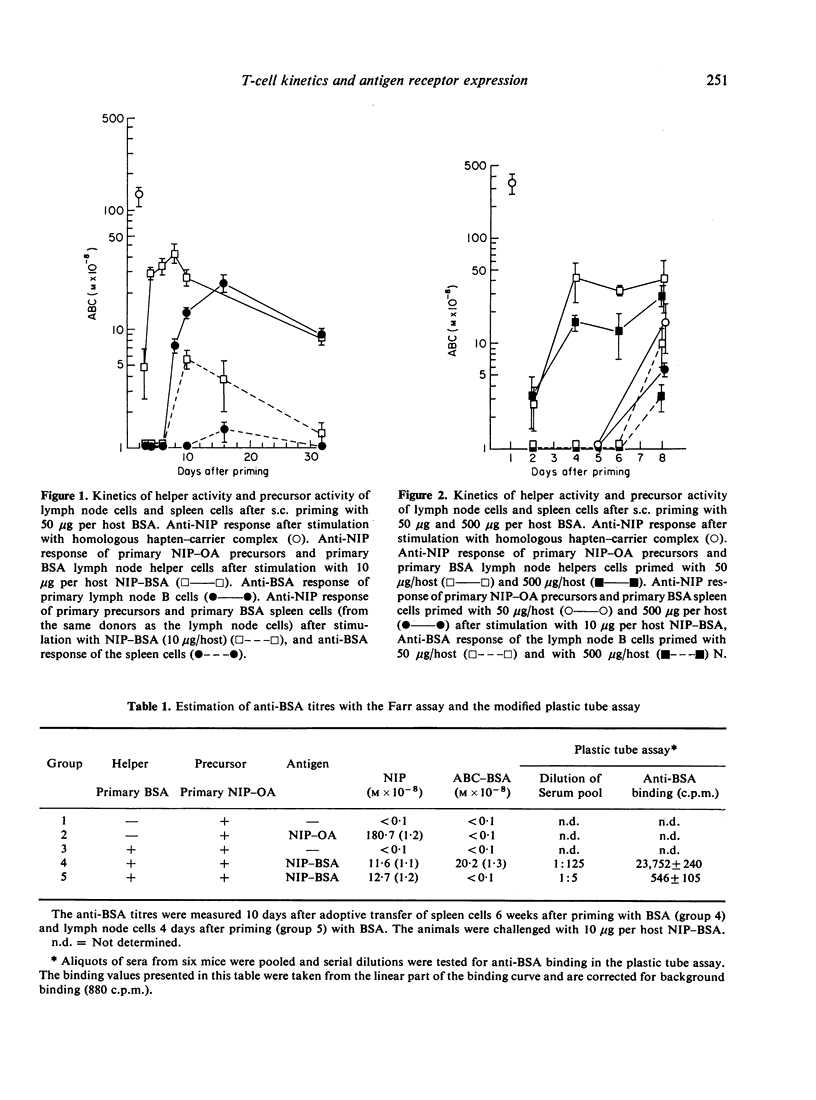
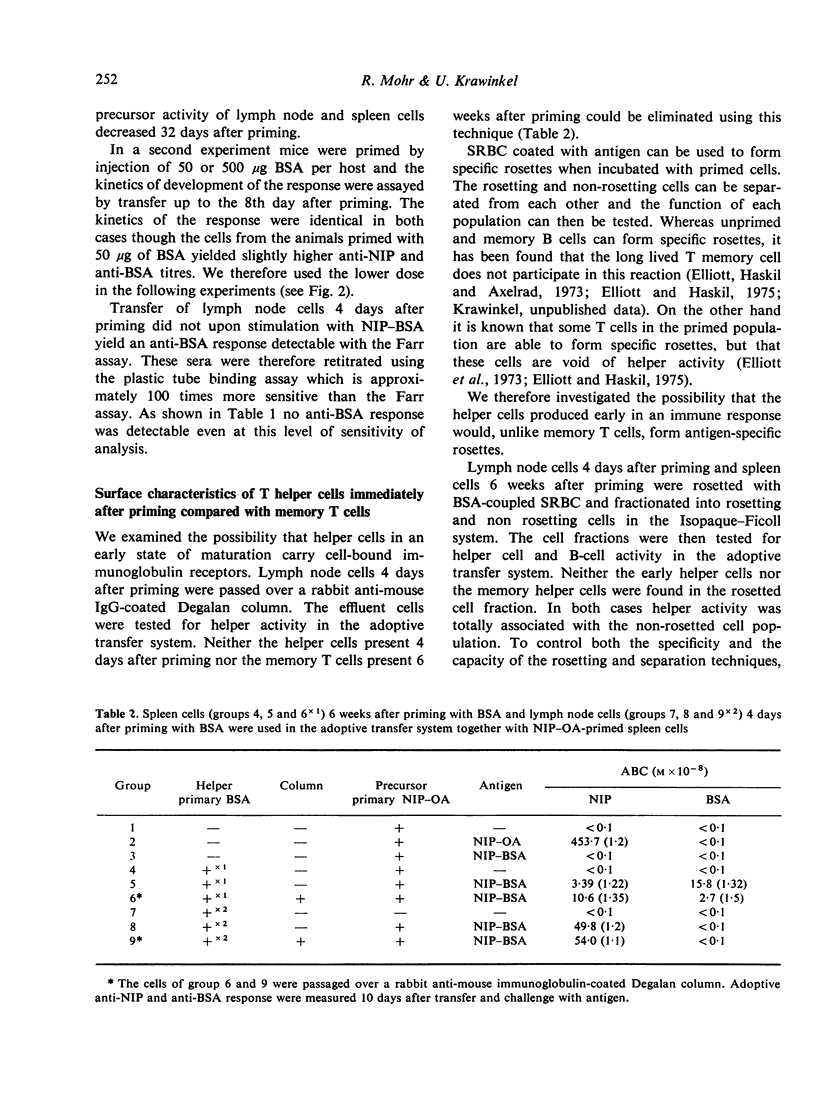
The subcutaneous application of soluble antigen resulted in generation of helper T lymphocytes in the draining lymph nodes, tested 4 days after priming in the adoptive transfer system. Four days later a specific helper T-cell population was detected in the spleen. B-cell activity could be demonstrated 4-5 days after helper T-cell activity in both organs. We investigated the early helper T-cell population and memory T cells with respect to differences in the expression of antigen receptors. No such differences could be detected, either by anti-immunoglobulin-coated Degalan columns or by an antigen-specific rosetting method.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashman R. F., Raff M. C. Direct demonstration of theta-positive antigen-binding cells, with antigen-induced movement of thymus-dependent cell receptors. J Exp Med. 1973 Jan 1;137(1):69–84. doi: 10.1084/jem.137.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach J. F., Dardenne M. Antigen recognition by T lymphocytes. Thymus and marrow dependence of spontaneous rosette forming cells in the mouse. Cell Immunol. 1972 Jan;3(1):1–10. doi: 10.1016/0008-8749(72)90220-1. [DOI] [PubMed] [Google Scholar]
- Basten A., Miller J. F., Warner N. L., Pye J. Specific inactivation of thymus-derived (T) and non-thymus-derived (B) lymphocytes by 125I-labelled antigen. Nat New Biol. 1971 May 26;231(21):104–106. doi: 10.1038/newbio231104a0. [DOI] [PubMed] [Google Scholar]
- Black S. J., Inchley C. J. Characteristics of immunological memory in mice. I. Separate early generation of cells mediating IgM and IgG memory to sheep erythrocytes. J Exp Med. 1974 Aug 1;140(2):333–348. doi: 10.1084/jem.140.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. J., Sercarz E. E. The asynchronous development of immunological memory in helper (T) and precursor (B) cell lines. Eur J Immunol. 1971 Dec;1(6):413–421. doi: 10.1002/eji.1830010602. [DOI] [PubMed] [Google Scholar]
- Elliott B. E., Haskill J. S., Axelrad M. A. Thymus-derived rosettes are not "helper" cells. J Exp Med. 1973 Nov 1;138(5):1133–1143. doi: 10.1084/jem.138.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott B. E., Haskill J. S. Rosette-forming ability of thymus-derived lymphocytes in humoral and cell-mediated immunity. II. Helper cell activity. J Exp Med. 1975 Mar 1;141(3):600–607. doi: 10.1084/jem.141.3.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANDL J. H., SIMMONS R. L. The agglutination and sensitization of red cells by metallic cations: interactions between multivalent metals and the red-cell membrane. Br J Haematol. 1957 Jan;3(1):19–38. doi: 10.1111/j.1365-2141.1957.tb05768.x. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Hoffmann M. Regulation of the immune response. 3. Kinetic differences between thymus- and bone marrow- derived lymphocytes in the proliferative response to heterologous erythrocytes. J Exp Med. 1973 Jun 1;137(6):1325–1337. doi: 10.1084/jem.137.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettman J., Dutton R. W. Radioresistance of the enhancing effect of cells from carrier-immunized mice in an in vitro primary immune response. Proc Natl Acad Sci U S A. 1971 Apr;68(4):699–703. doi: 10.1073/pnas.68.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell I., Munro A., Gurner B. W., Coombs R. R. Studies on actively allergized cells. I. The cyto-dynamics and morphology of rosete-forming lymph node cells in mice and inhibition of rosette-formation with antibody to mouse immunoglobulins. Int Arch Allergy Appl Immunol. 1969;35(3):209–227. [PubMed] [Google Scholar]
- Mitchison N. A. The carrier effect in the secondary response to hapten-protein conjugates. I. Measurement of the effect with transferred cells and objections to the local environment hypothesis. Eur J Immunol. 1971 Jan;1(1):10–17. doi: 10.1002/eji.1830010103. [DOI] [PubMed] [Google Scholar]
- NOSSAL G. J., ADA G. L., AUSTIN C. M. ANTIGENS IN IMMUNITY. II. IMMUNOGENIC PROPERTIES OF FLAGELLA, POLYMERIZED FLAGELLIN AND FLAGELLIN IN THE PRIMARY RESPONSE. Aust J Exp Biol Med Sci. 1964 Jun;42:283–294. [PubMed] [Google Scholar]
- Rajewsky K., Brenig C. Paralysis to serum albumins in T and B lymphocytes in mice. Dose dependence, specificity and kinetics of escape. Eur J Immunol. 1974 Feb;4(2):120–125. doi: 10.1002/eji.1830040211. [DOI] [PubMed] [Google Scholar]
- Rajewsky K., Mohr R. Specificity and heterogeneity of helper T cells in the response to serum albumins in mice. Eur J Immunol. 1974 Feb;4(2):111–119. doi: 10.1002/eji.1830040210. [DOI] [PubMed] [Google Scholar]
- Roelants G. E., Askonas B. A. Cell cooperation in antibody induction. The susceptibility of helper cells to specific lethal radioactive antigen. Eur J Immunol. 1971 Jun;1(3):151–157. doi: 10.1002/eji.1830010302. [DOI] [PubMed] [Google Scholar]
- Roelants G. E., Rydén A., Hägg L. B., Loor F. Active synthesis of immunoglobulin receptors for antigen by T lymphocytes. Nature. 1974 Jan 11;247(5436):106–108. doi: 10.1038/247106a0. [DOI] [PubMed] [Google Scholar]
- Roelants G., Rydén A. Dose dependence of antigen binding to B and T lymphocytes. Nature. 1974 Jan 11;247(5436):104–106. doi: 10.1038/247104a0. [DOI] [PubMed] [Google Scholar]
- Sprent J., Miller J. F., Mitchell G. F. Antigen-induced selective recruitment of circulating lymphocytes. Cell Immunol. 1971 Apr;2(2):171–181. doi: 10.1016/0008-8749(71)90036-0. [DOI] [PubMed] [Google Scholar]
- Wigzell H., Sundqvist K. G., Yoshida T. O. Separation of cells according to surface antigens by the use of antibody-coated columns. Fractionation of cells carrying immunoglobulins and blood group antigen. Scand J Immunol. 1972;1(1):75–87. doi: 10.1111/j.1365-3083.1972.tb03737.x. [DOI] [PubMed] [Google Scholar]


