Abstract
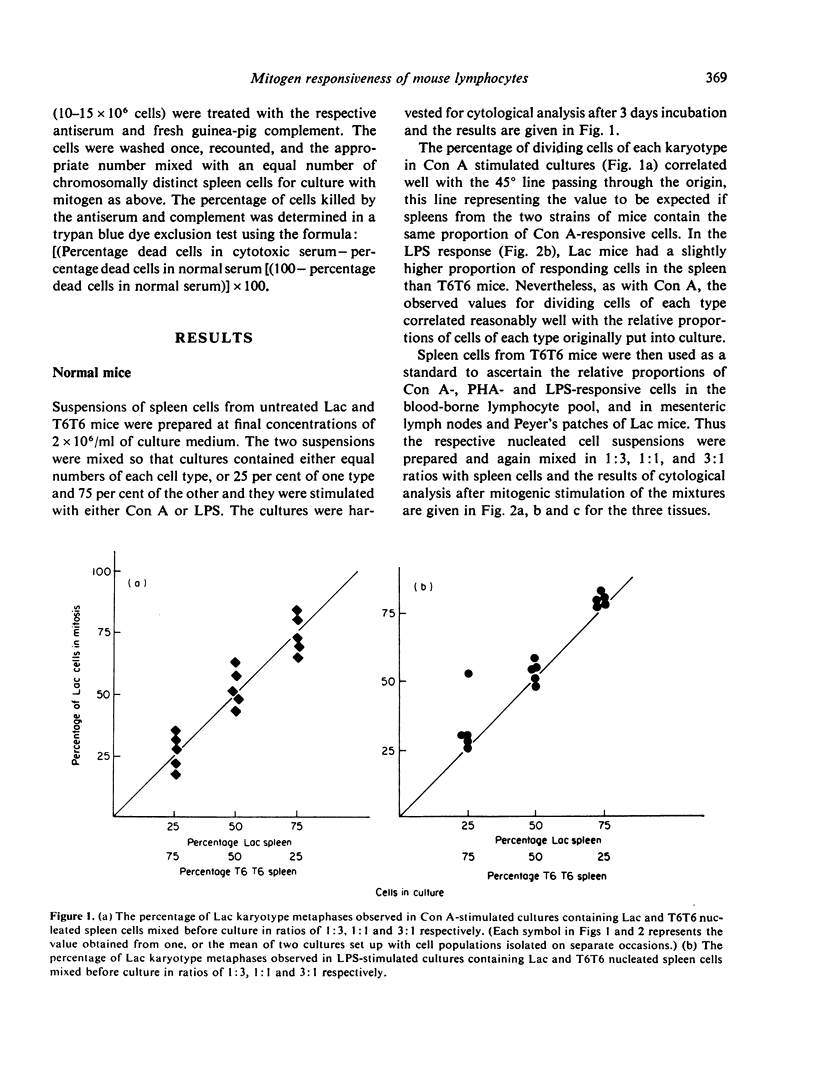
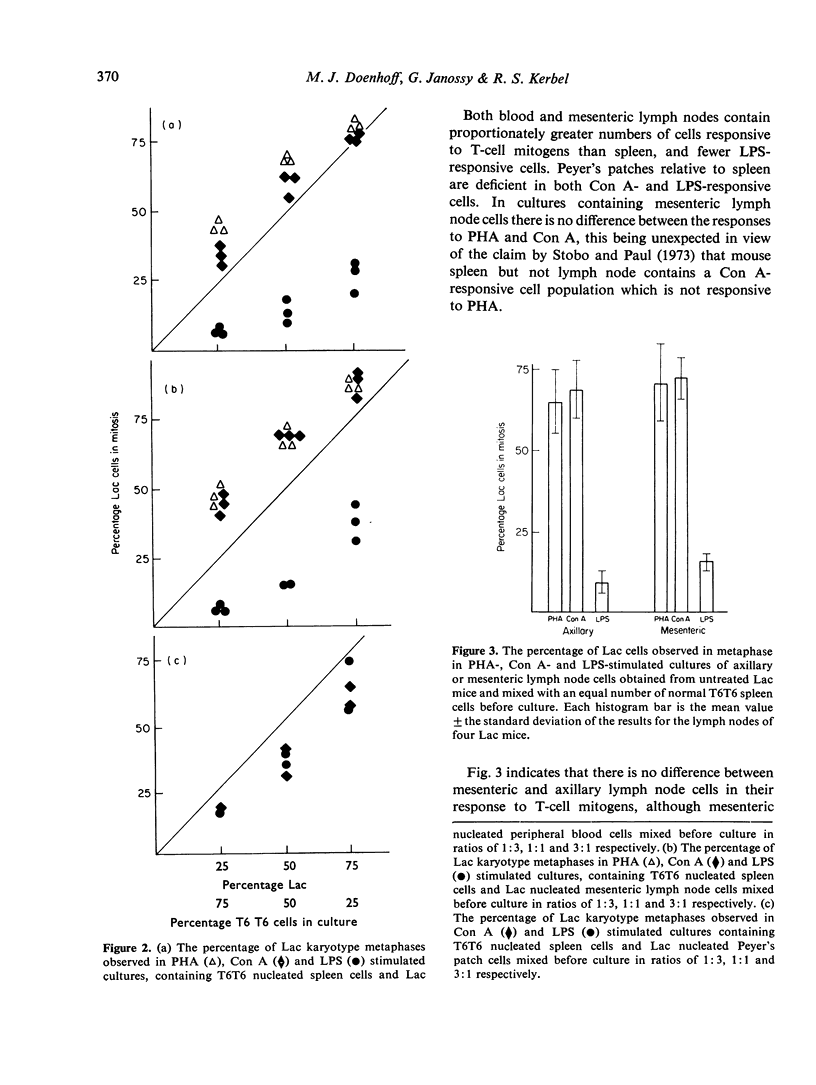
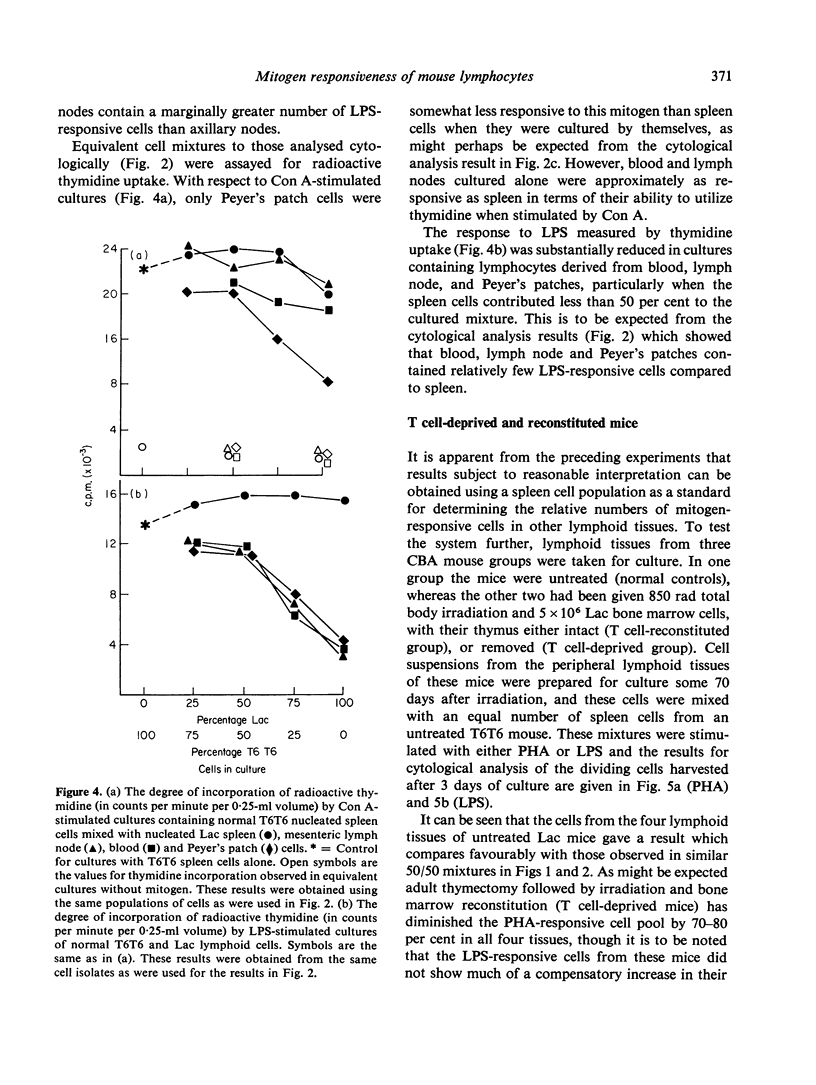
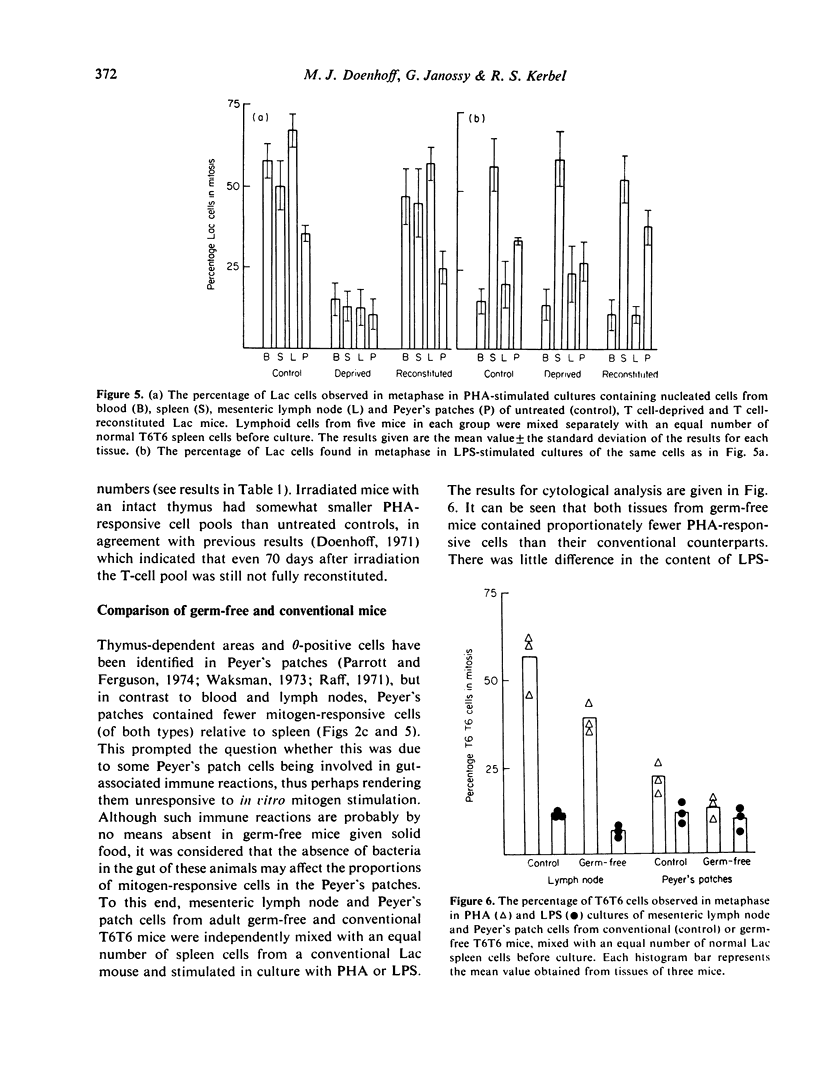
The relative number of cells capable of responding to Con A, PHA and LPS in the spleen, blood, lymph node and Peyer's patches of CBA mice has been quantified by means of a cytological analysis technique. No difference has been found between Con A- and PHA-responsive cells in spleen and lymph node. The lymphoid tissues of T cell-deprived mice have a reduced content of PHA responsive cells, but LPS responsiveness is within normal limits. Pretreatment of peripheral lymphocyte populations with high concentrations of anti-O antiserum and complement abolishes the response of the treated cells to PHA, but not to LPS, whereas similar treatment with a cytotoxic anti-immunoglobulin serum, which has no effect on PHA-responsive cells, only partially reduces the response to LPS. The results for mitogen responsiveness are discussed with reference to other methods of quantifying T and B cells using cell-surface markers.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davies A. J., Leuchars E., Wallis V., Koller P. C. The mitotic response of thymus-derived cells to antigenic stimulus. Transplantation. 1966 Jul;4(4):438–451. doi: 10.1097/00007890-196607000-00008. [DOI] [PubMed] [Google Scholar]
- Doenhoff M. J., Davies A. J. Reconstitution of the T-cell pool after irradiation of mice. Cell Immunol. 1971 Feb;2(1):82–90. doi: 10.1016/0008-8749(71)90027-x. [DOI] [PubMed] [Google Scholar]
- Doenhoff M. J., Janossy G., Greaves M. F., Gomer K. J., Snajdr J. Lymphocyte activation. VI. A re-evaluation of factors affecting the selectivity of polyclonal mitogens for mouse T and B cells. Clin Exp Immunol. 1974 Jul;17(3):475–490. [PMC free article] [PubMed] [Google Scholar]
- Doenhoff M. J. The effect of x-rays on circulating lymphocytes of mice quantitated by PHA-responsiveness. Clin Exp Immunol. 1971 Apr;8(4):603–615. [PMC free article] [PubMed] [Google Scholar]
- Forman J., Möller G. The effector cell in antibody-induced cell mediated immunity. Transplant Rev. 1973;17(0):108–149. doi: 10.1111/j.1600-065x.1973.tb00125.x. [DOI] [PubMed] [Google Scholar]
- Gery I., Krüger J., Spiesel S. Z. Stimulation of B-lymphocytes by endotoxin. Reactions of thymus-deprived mice and karyotypic analysis of dividing cells in mice bearing T 6 T 6 thymus grafts. J Immunol. 1972 Apr;108(4):1088–1091. [PubMed] [Google Scholar]
- Gorczynski R. M., Rittenberg M. B. Stimulation of early protein synthesis as an assay of immune reactivity: analysis of the cells responding to mitogens and alloantigens. J Immunol. 1974 Jan;112(1):47–55. [PubMed] [Google Scholar]
- Greaves M., Janossy G., Doenhoff M. Selective triggering of human T and B lymphocytes in vitro by polyclonal mitogens. J Exp Med. 1974 Jul 1;140(1):1–18. doi: 10.1084/jem.140.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M., Janossy G. Elicitation of selective T and B lymphocyte responses by cell surface binding ligands. Transplant Rev. 1972;11:87–130. doi: 10.1111/j.1600-065x.1972.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F., Doenhoff M. J., Snajdr J. Lymphocyte activation. V. Quantitation of the proliferative responses to mitogens using defined T and B cell populations. Clin Exp Immunol. 1973 Aug;14(4):581–596. [PMC free article] [PubMed] [Google Scholar]
- Kagnoff M. F., Billings P., Cohn M. Functional characteristics of Peyer's patch lymphoid cells. II. Lipopolysaccharide is thymus dependent. J Exp Med. 1974 Feb 1;139(2):407–413. doi: 10.1084/jem.139.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel R. S., Doenhoff M. J. Resistance of mitotic B lymphocytes to cytotoxic effects of anti-Ig serum. Nature. 1974 Jul 26;250(464):342–344. doi: 10.1038/250342a0. [DOI] [PubMed] [Google Scholar]
- Kerbel R. S., Elliott E. V., Wallis V. J. Assessment of mitotic thymus-derived lymphocytes by their sensitivity to the cytotoxic effects of anti-theta serum. Cell Immunol. 1974 Mar 30;11(1-3):146–161. doi: 10.1016/0008-8749(74)90015-x. [DOI] [PubMed] [Google Scholar]
- Parrott D. M., Ferguson A. Selective migration of lymphocytes within the mouse small intestine. Immunology. 1974 Mar;26(3):571–588. [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Sternberg M., Taylor R. B. Immunoglobulin determinants on the surface of mouse lymphoid cells. Nature. 1970 Feb 7;225(5232):553–554. doi: 10.1038/225553a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplant Rev. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Two distinct populations of peripheral lymphocytes in mice distinguishable by immunofluorescence. Immunology. 1970 Oct;19(4):637–650. [PMC free article] [PubMed] [Google Scholar]
- Scher I., Strong D. M., Ahmed A., Knudsen R. C., Sell K. W. Specific murine B-cell activation by synthetic single-and double-stranded polynucleotides. J Exp Med. 1973 Dec 1;138(6):1545–1563. doi: 10.1084/jem.138.6.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobo J. D., Paul W. E. Functional heterogeneity of murine lymphoid cells. 3. Differential responsiveness of T cells to phytohemagglutinin and concanavalin A as a probe for T cell subsets. J Immunol. 1973 Feb;110(2):362–375. [PubMed] [Google Scholar]
- Stobo J. D., Rosenthal A. S., Paul W. E. Functional heterogeneity of murine lymphoid cells. I. Responsiveness to and surface binding of concanavalin A and phytohemagglutinin. J Immunol. 1972 Jan;108(1):1–17. [PubMed] [Google Scholar]
- Stobo J. D., Rosenthal A. S., Paul W. E. Functional heterogeneity of murine lymphoid cells. V. Lymphocytes lacking detectable surface theta or immunoglobulin determinants. J Exp Med. 1973 Jul 1;138(1):71–88. doi: 10.1084/jem.138.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waksman B. H. The homing pattern of thymus-derived lymphocytes in calf and neonatal mouse Peyer's patches. J Immunol. 1973 Sep;111(3):878–884. [PubMed] [Google Scholar]
- de Petris S., Raff M. C. Distribution of immunoglobulin on the surface of mouse lymphoid cells as determined by immunoferritin electron microscopy. Antibody-induced, temperature-dependent redistribution and its implications for membrane structure. Eur J Immunol. 1972 Dec;2(6):523–535. doi: 10.1002/eji.1830020611. [DOI] [PubMed] [Google Scholar]


