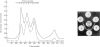Abstract
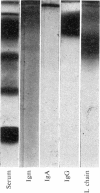
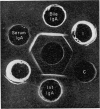
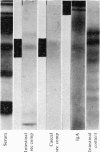
Immunochemical studies of the intestinal secretory immune system of the chicken have led to further characterization of IgA in bile, intestinal contents and serum. A component was detected in late Sephadex G-200 fractions of caecal and intestinal contents which showed partial identity with bile, intestinal and a high molecular weight fraction of serum IgA. This component showed similar sedimentation characteristics to bovine serum albumin in sucrose density gradients, a fast electrophoretic mobility on polyacrylamide gel and is a possible analogue of mammalian secretory component (SC). Fractionation of serum from birds affected with infectious synovitis revealed two moleculare classes of IgA. Comparative double diffusion studies produced a reaction of complete identity between bile IgA and high molecular weight serum IgA (15S) and partial identity with low molecular weight serum IgA (7S), suggesting a lack of an SC determinant on the latter. A spur of partial identity between 15S and 7S serum IgA was also observed. Although no direct structural homology with mammalian or human IgA could be demonstrated by immunological cross-reactivity, the similarities of molecular characteristics, particularly emphasized by the presence of a secretory component, favour a functional analogy between the secretory immune system of the fowl and mammalian species.
Full text
PDF


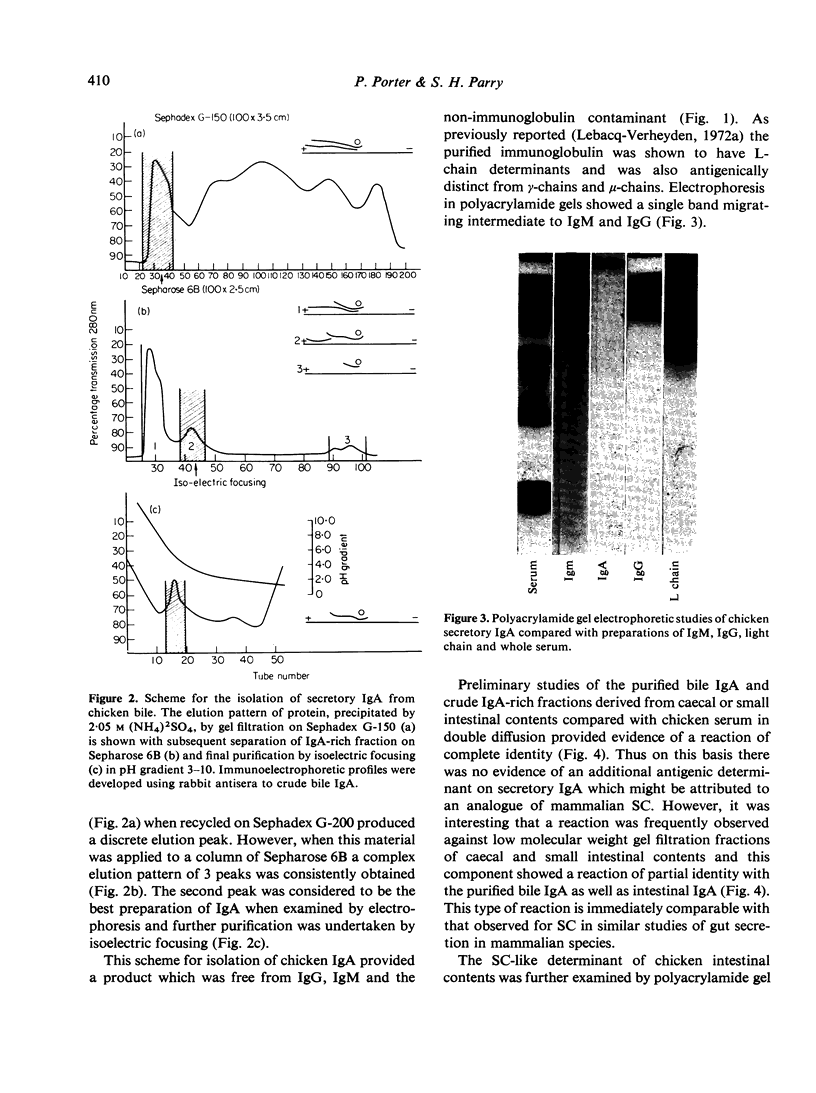
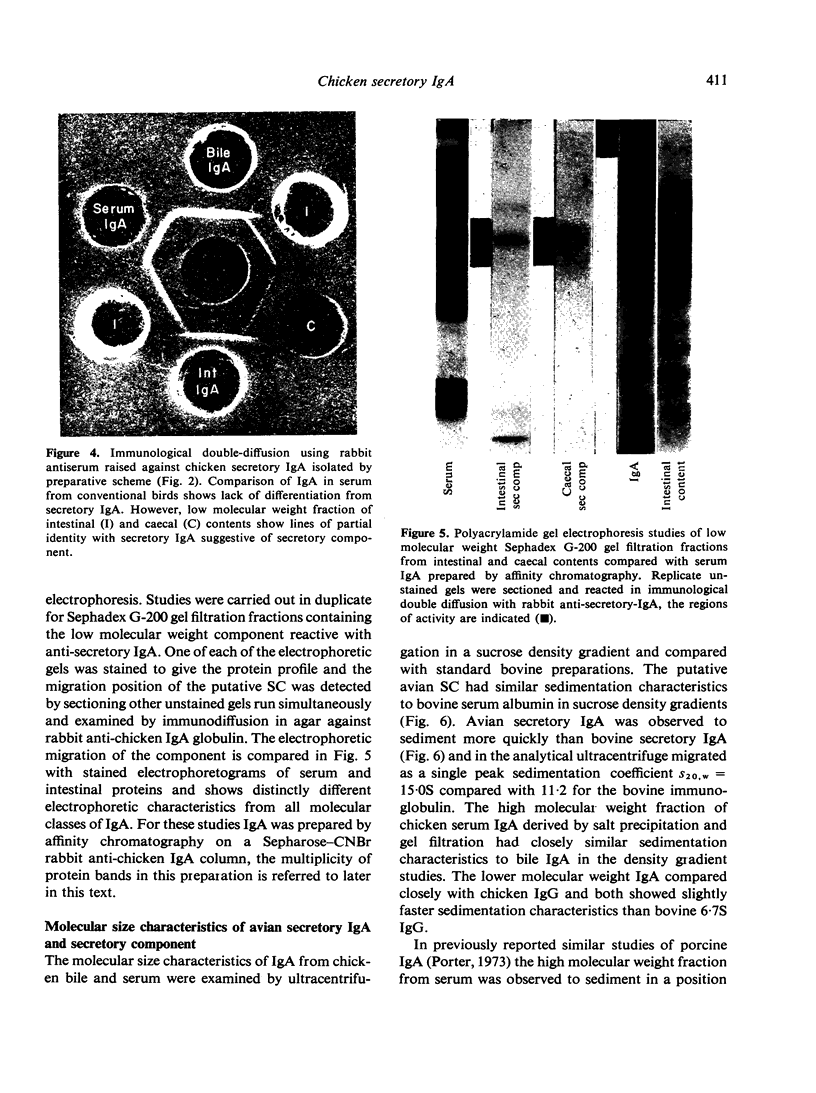
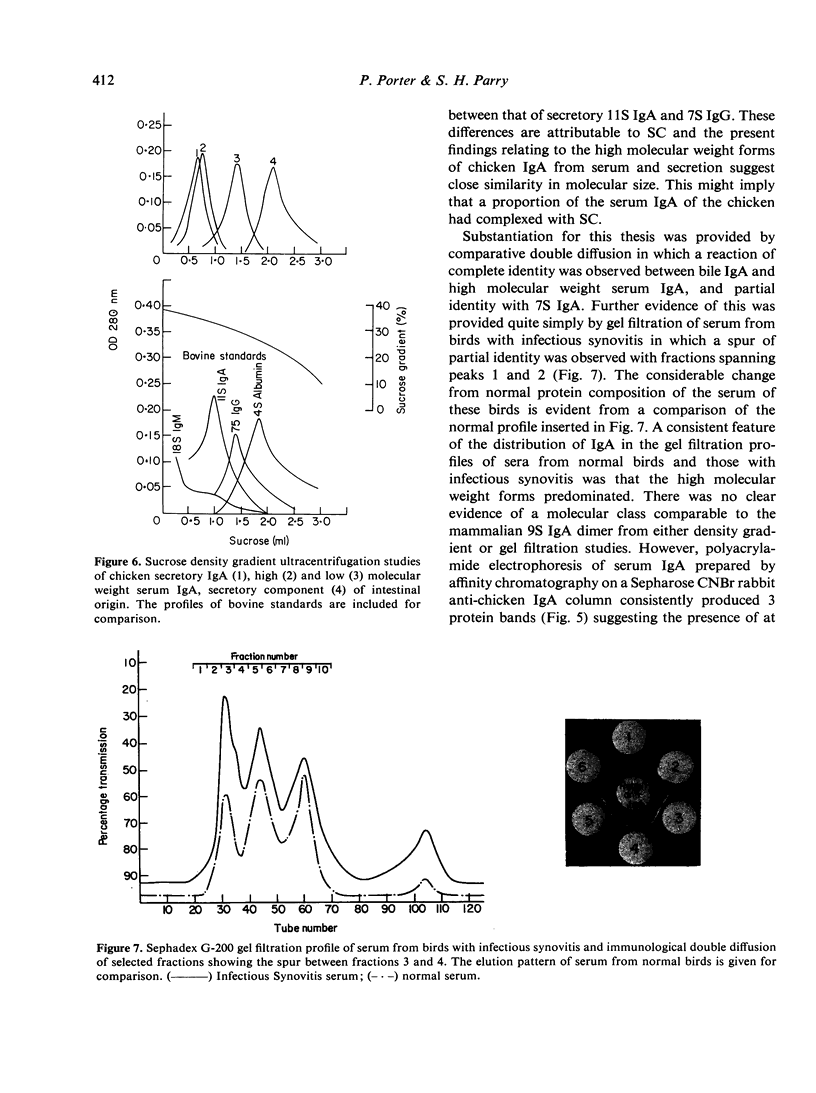



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bienenstock J. Immunoglobulins of the hamster. II. Characterization of the gamma A and other immunoglobulins in serum and secretions. J Immunol. 1970 May;104(5):1228–1235. [PubMed] [Google Scholar]
- Bienenstock J., Perey D. Y., Gauldie J., Underdown B. J. Chicken A: physicochemical and immunochemical characteristics. J Immunol. 1973 Feb;110(2):524–533. [PubMed] [Google Scholar]
- Bienenstock J., Perey D. Y., Gauldie J., Underdown B. J. Chicken immunoglobulin resembling A. J Immunol. 1972 Aug;109(2):403–406. [PubMed] [Google Scholar]
- Brandtzaeg P. Purification of J chain after mild reduction of human immunoglobulins. Scand J Immunol. 1975;4(4):309–320. doi: 10.1111/j.1365-3083.1975.tb02631.x. [DOI] [PubMed] [Google Scholar]
- Esteves M. B., Binaghi R. A. Antigenic similarities among mammalian immunoglobulins. Immunology. 1972 Aug;23(2):137–145. [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., Vaerman J. P., Bazin H., LeBacq-Verheyden A. M., Heremans J. F. Identification of J-chain in polymeric immunoglobulins from a variety of species by cross-reaction with rabbit antisera to human J-chain. J Immunol. 1973 Nov;111(5):1590–1594. [PubMed] [Google Scholar]
- Lebacq-Verheyden A. M., Vaerman J. P., Heremans J. F. A possible homologue of mammalian IgA in chicken serum and secretions. Immunology. 1972 Jan;22(1):165–175. [PMC free article] [PubMed] [Google Scholar]
- Lebacq-Verheyden A. M., Vaerman J. P., Heremans J. F. Immunohistologic distribution of the chicken immunoglobulins. J Immunol. 1972 Sep;109(3):652–654. [PubMed] [Google Scholar]
- Leslie G. A., Clem L. W. Phylogen of immunoglobulin structure and function. 3. Immunoglobulins of the chicken. J Exp Med. 1969 Dec 1;130(6):1337–1352. doi: 10.1084/jem.130.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie G. A., Martin L. N. Studies on the secretory immunologic system of fowl. 3. Serum and secretory IgA of the chicken. J Immunol. 1973 Jan;110(1):1–9. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Noll H. Characterization of macromolecules by constant velocity sedimentation. Nature. 1967 Jul 22;215(5099):360–363. doi: 10.1038/215360a0. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Orlans E., Rose M. E. An IgA-like immunoglobulin in the fowl. Immunochemistry. 1972 Aug;9(8):833–838. doi: 10.1016/0019-2791(72)90262-5. [DOI] [PubMed] [Google Scholar]
- Parry S. H., Aitken I. D. Immunoglobulin A in some avian species other than the fowl. Res Vet Sci. 1975 May;18(3):333–334. [PubMed] [Google Scholar]
- Parry S. H., Aitken I. D. Immunoglobulin A in the respiratory tract of the chicken following exposure to Newcastle disease virus. Vet Rec. 1973 Sep 1;93(9):258–260. doi: 10.1136/vr.93.9.258. [DOI] [PubMed] [Google Scholar]
- Porter P., Noakes D. E. Immunoglobulin IgA in bovine serum and external secretions. Biochim Biophys Acta. 1970 Jul 27;214(1):107–116. doi: 10.1016/0005-2795(70)90074-7. [DOI] [PubMed] [Google Scholar]
- Porter P. Studies of porcine secretory IgA and its component chains in relation to intestinal absorption of colostral immunoglobulins by the neonatal pig. Immunology. 1973 Jan;24(1):163–176. [PMC free article] [PubMed] [Google Scholar]
- Rose M. E., Orlans E., Buttress N. Immunoglobulin classes in the hen's egg: their segregation in yolk and white. Eur J Immunol. 1974 Jul;4(7):521–523. doi: 10.1002/eji.1830040715. [DOI] [PubMed] [Google Scholar]
- Vaerman J. P., Heremans J. F., Van Kerckhoven G. Communications. Identification of IgA in several mammalian species. J Immunol. 1969 Dec;103(6):1421–1423. [PubMed] [Google Scholar]
- Watanabe H., Kobayashi K. Peculiar secretory IgA system identified in chickens. J Immunol. 1974 Nov;113(5):1405–1409. [PubMed] [Google Scholar]