Abstract
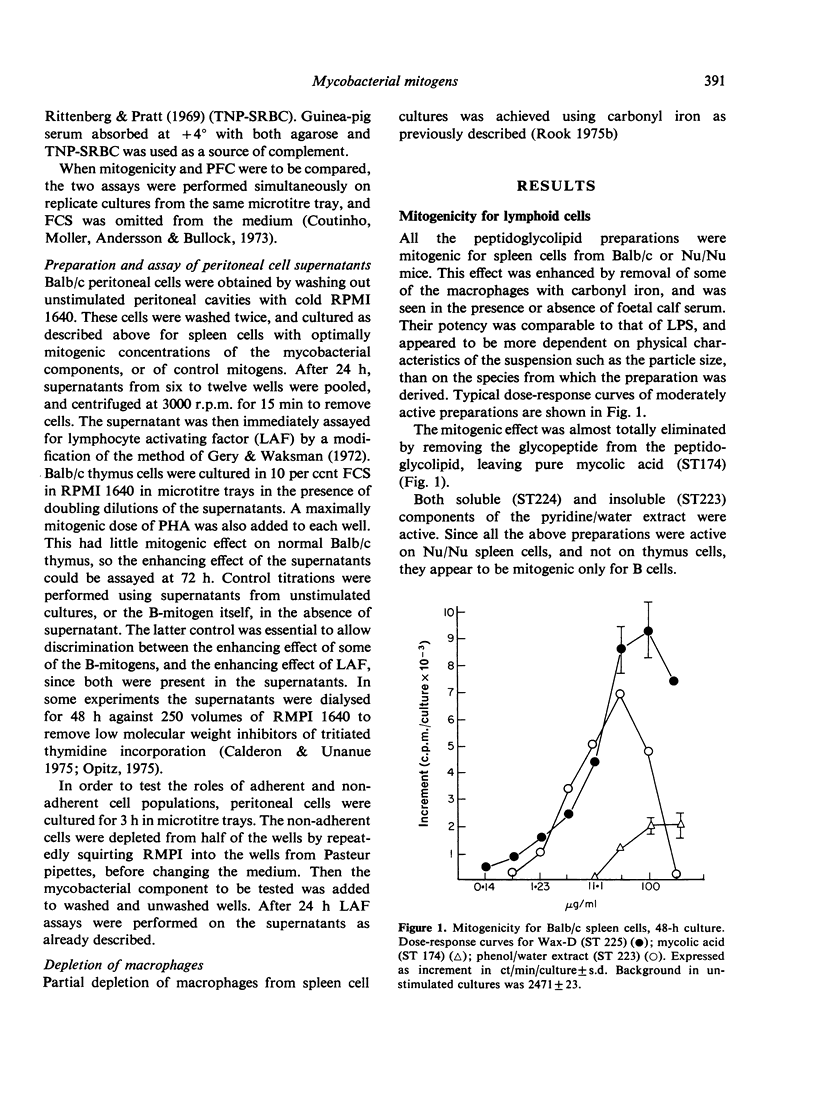
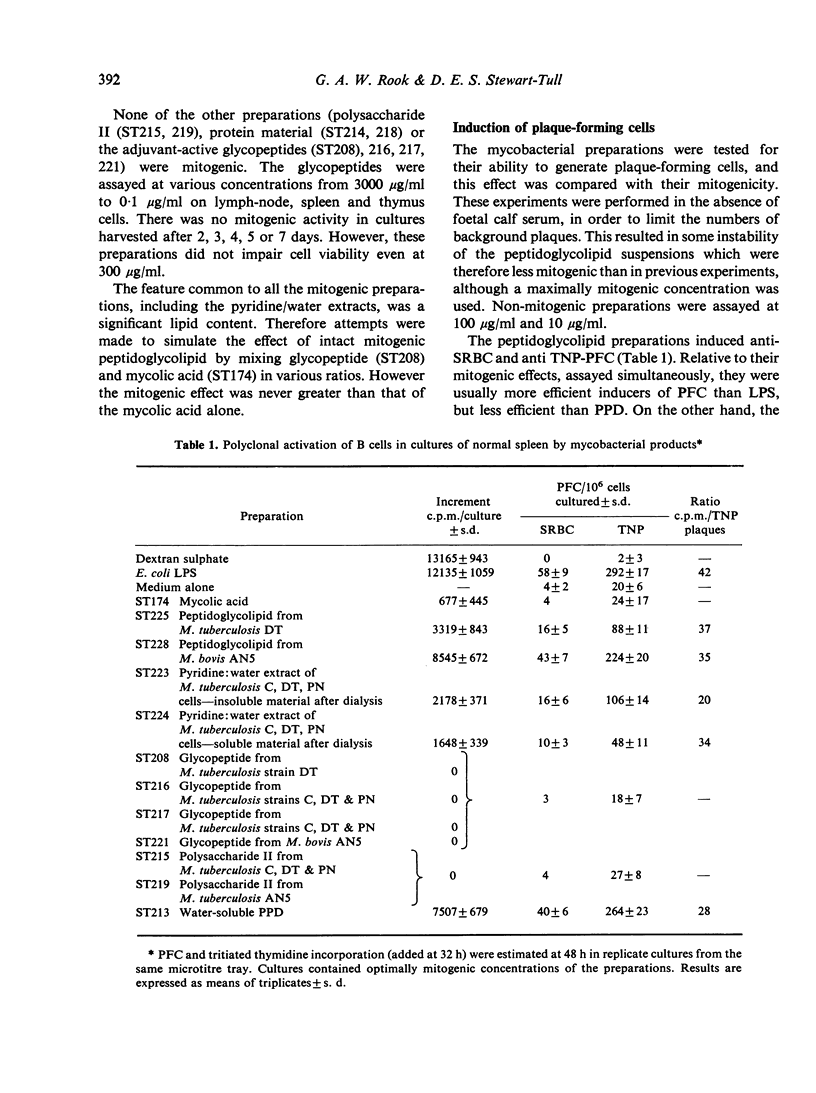
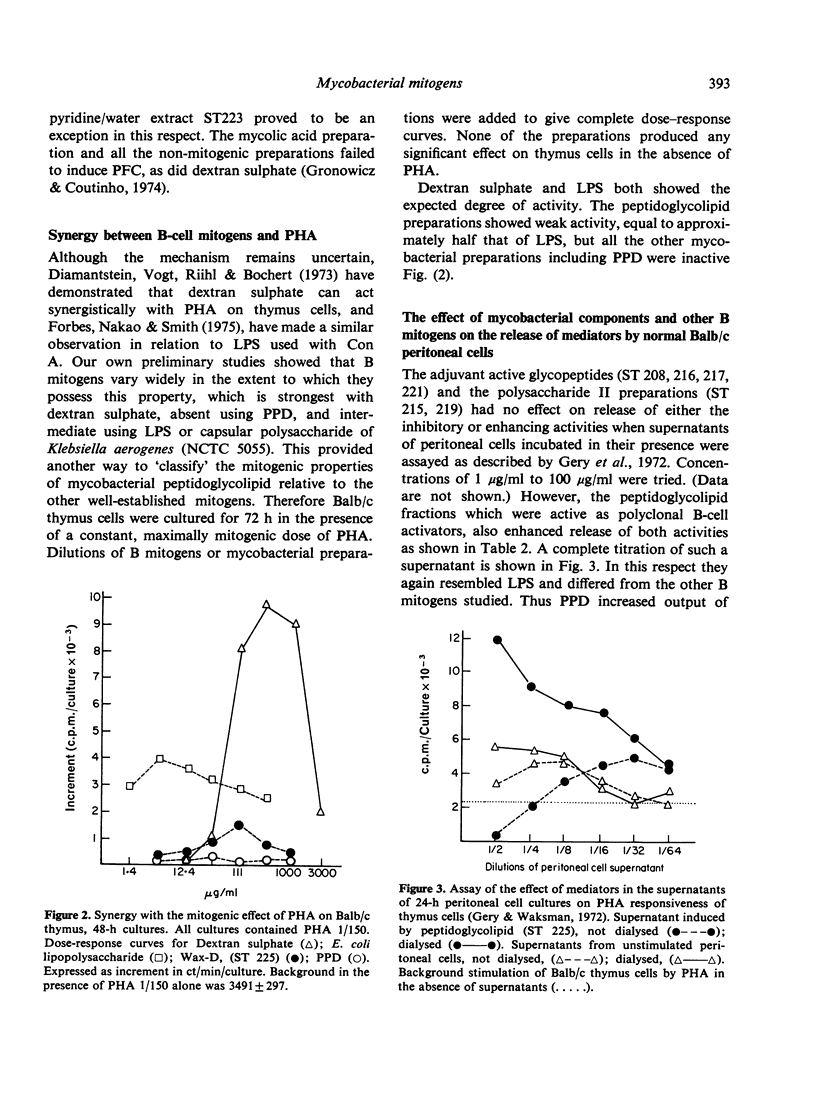
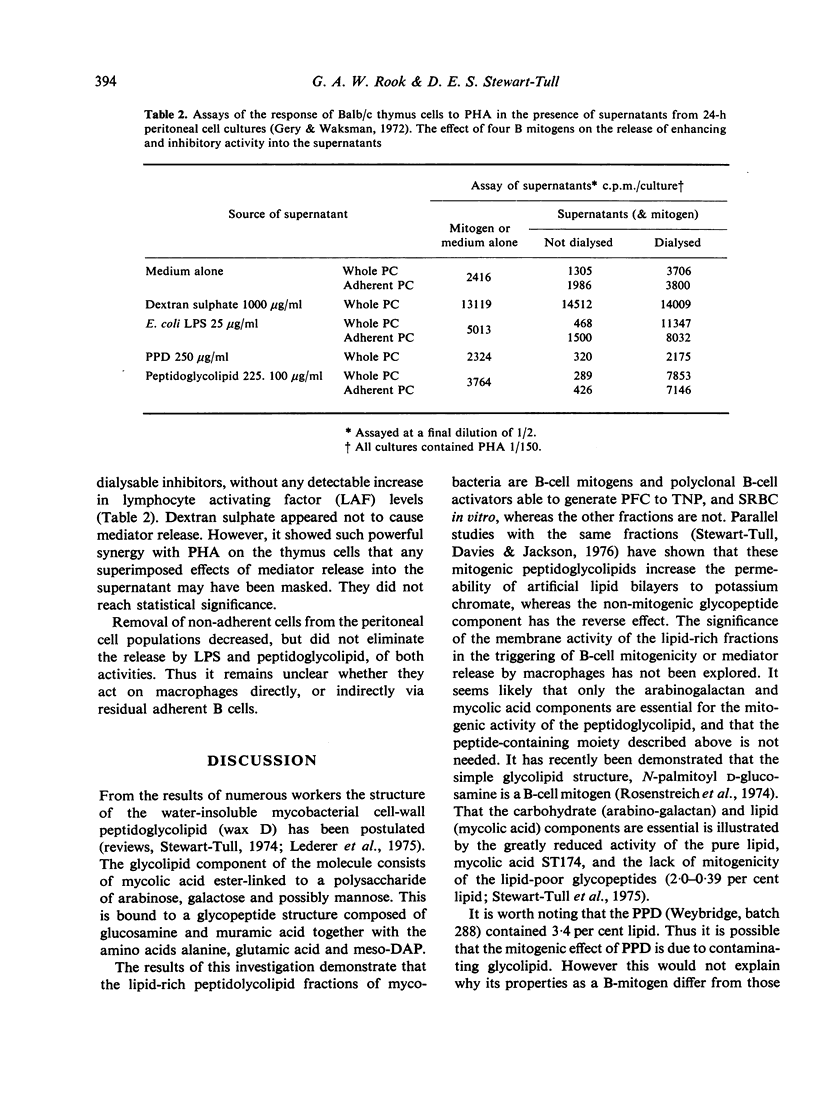
Twelve preparations from mycobacterial cell walls and culture supernatant fluids were tested for their ability to activate lymphocytes from Balb/c or Nu/Nu mice, and to increase the release of mediators from macrophages in vitro. The peptidoglycolipids (wax D) were B-cell mitogens and induced plaque-forming cells. These properties were lost if the glycopeptide component was removed, leaving the pure lipid, mycolic acid. Neither the glycopeptide fractions, with well-documented adjuvant properties, nor mycobacterial polysacchardie II-activated B cells. Only intact peptidoglycolipid showed synergy with the mitogenic effect of phytohaemagglutinin (PHA) on thymocytes from Balb/c mice. The effect was much smaller than with E. coli lipopolysacharide (LPS) or dextran sulphate. The peptidoglycolipid also enhanced the release of factors from macrophages able to modify the response of Balb/c thymus cells to PHA. In this respect it resembled E. coli LPS. Adjuvant active glycopeptides did not share this property.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASSELINEAU J., BUC H., JOLLES P., LEDERER E. Sur la structure chimique d'une fraction peptido-glycolipidique (cire D) isolée de Mycobacterium tuberculosis var. hominis. Bull Soc Chim Biol (Paris) 1958;40(12):1953–1964. [PubMed] [Google Scholar]
- Adam A., Ciorbaru R., Petit J. F., Lederer E. Isolation and properties of a macromolecular, water-soluble, immuno-adjuvant fraction from the cell wall of Mycobacterium smegmatis. Proc Natl Acad Sci U S A. 1972 Apr;69(4):851–854. doi: 10.1073/pnas.69.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon J., Unanue E. R. Two biological activities regulating cell proliferation found in cultures of peritoneal exudate cells. Nature. 1975 Jan 31;253(5490):359–361. doi: 10.1038/253359a0. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Möller G., Anderson J., Bullock W. W. In vitro activation of mouse lymphocytes in serum-free medium: effect of T and B cell mitogens on proliferation and antibody synthesis. Eur J Immunol. 1973 May;3(5):299–306. doi: 10.1002/eji.1830030509. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Diamantstein T., Vogt W., Rühl H., Bochert G. Stimulation of DNA synthesis in mouse lymphoid cells by polyanions in vitro. I. Target cells and possible mode of action. Eur J Immunol. 1973 Aug;3(8):488–493. doi: 10.1002/eji.1830030807. [DOI] [PubMed] [Google Scholar]
- Forbes J. T., Nakao Y., Smith R. T. T mitogens trigger LPS responsiveness in mouse thymus cells. J Immunol. 1975 Mar;114(3):1004–1007. [PubMed] [Google Scholar]
- Gery I., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. II. The cellular source of potentiating mediator(s). J Exp Med. 1972 Jul 1;136(1):143–155. doi: 10.1084/jem.136.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A. Selective triggering of B cell subpopulations by mitogens. Eur J Immunol. 1974 Nov;4(11):771–776. doi: 10.1002/eji.1830041113. [DOI] [PubMed] [Google Scholar]
- Hiu I. J. Water-soluble and lipid-free fraction from BCG with adjuvant and antitumour activity. Nat New Biol. 1972 Aug 23;238(86):241–242. doi: 10.1038/newbio238241a0. [DOI] [PubMed] [Google Scholar]
- Lederer E., Adam A., Ciorbaru R., Petit J. F., Wietzerbin J. Cell walls of Mycobacteria and related organisms; chemistry and immunostimulant properties. Mol Cell Biochem. 1975 May 30;7(2):87–104. doi: 10.1007/BF01792076. [DOI] [PubMed] [Google Scholar]
- Modolell M., Luckenbach G. A., Parant M., Munder P. G. The adjuvant activity of a mycobacterial water soluble adjuvant (WSA) in vitro. I. The requirement of macrophages. J Immunol. 1974 Jul;113(1):395–403. [PubMed] [Google Scholar]
- Myrvang B., Godal T., Ridley D. S., Fröland S. S., Song Y. K. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and histopathological spectrum of leprosy. Clin Exp Immunol. 1973 Aug;14(4):541–553. [PMC free article] [PubMed] [Google Scholar]
- Opitz H. G., Niethammer D., Lemke H., Flad H. D., Huget R. Inhibition of 3H-thymidine incorporation of lymphocytes by a soluble factor from macrophages. Cell Immunol. 1975 Apr;16(2):379–388. doi: 10.1016/0008-8749(75)90126-4. [DOI] [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Rook G. A. The immunological consequences of antigen overload in experimental mycobacterial infections of mice. Clin Exp Immunol. 1975 Jan;19(1):167–177. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A. The potentiating, mitogenic and inhibitory effects on lymphocytes in vitro, of macrophages in the lymph nodes of mice 'overloaded' with mycobacterial products. Clin Exp Immunol. 1975 Jul;21(1):163–172. [PMC free article] [PubMed] [Google Scholar]
- Rosenstreich D. L., Asselineau J., Mergenhagen S. E., Nowotny A. A synthetic glycolipid with B-cell mitogenic activity. J Exp Med. 1974 Nov 1;140(5):1404–1409. doi: 10.1084/jem.140.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART-TULL D. E., WHITE R. G. THE OCCURRENCE OF MURAMIC ACID IN WAX D PREPARATIONS OF MYCOBACTERIA. J Gen Microbiol. 1964 Jan;34:43–49. doi: 10.1099/00221287-34-1-43. [DOI] [PubMed] [Google Scholar]
- Stewart-Tull D. E., White R. G. The influence of age of culture on the production of adjuvant-active peptidoglycolipids by saprophytic mycobacteria. Immunology. 1967 Mar;12(3):349–359. [PMC free article] [PubMed] [Google Scholar]
- WHITE R. G., JOLLES P., SAMOUR D., LEDERER E. CORRELATION OF ADJUVANT ACTIVITY AND CHEMICAL STRUCTURE OF WAX D FRACTIONS OF MYCOBACTERIA. Immunology. 1964 Mar;7:158–171. [PMC free article] [PubMed] [Google Scholar]
- Wilton J. M., Rosenstreich D. L., Oppenheim J. J. Activation of guinea pig macrophages by bacterial lipopolysaccharide requires bone marrow-derived lymphocytes. J Immunol. 1975 Jan;114(1 Pt 2):388–393. [PubMed] [Google Scholar]


