Abstract
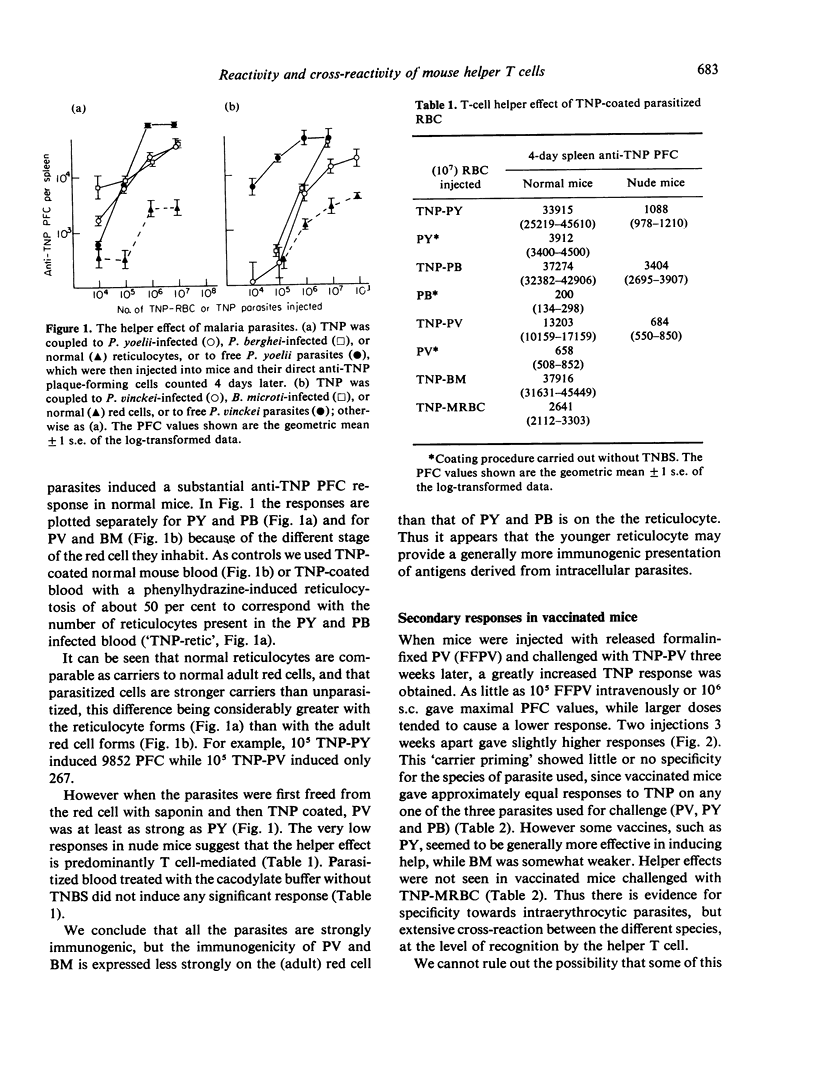
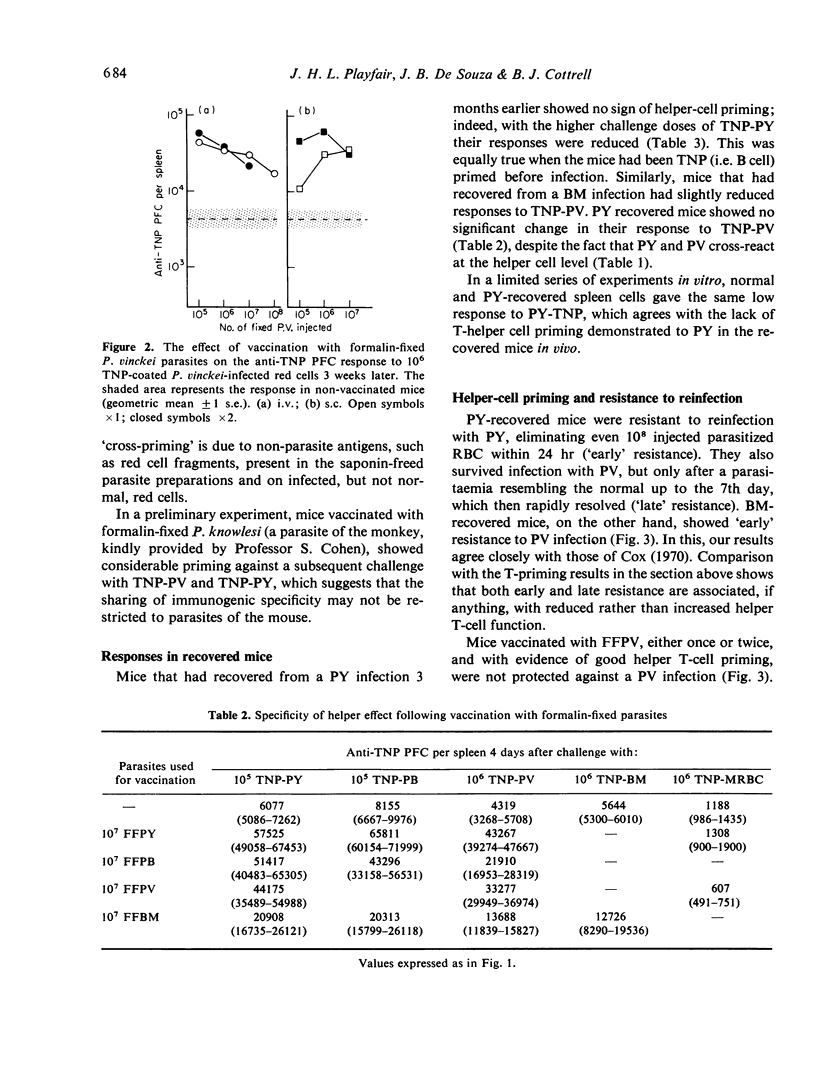
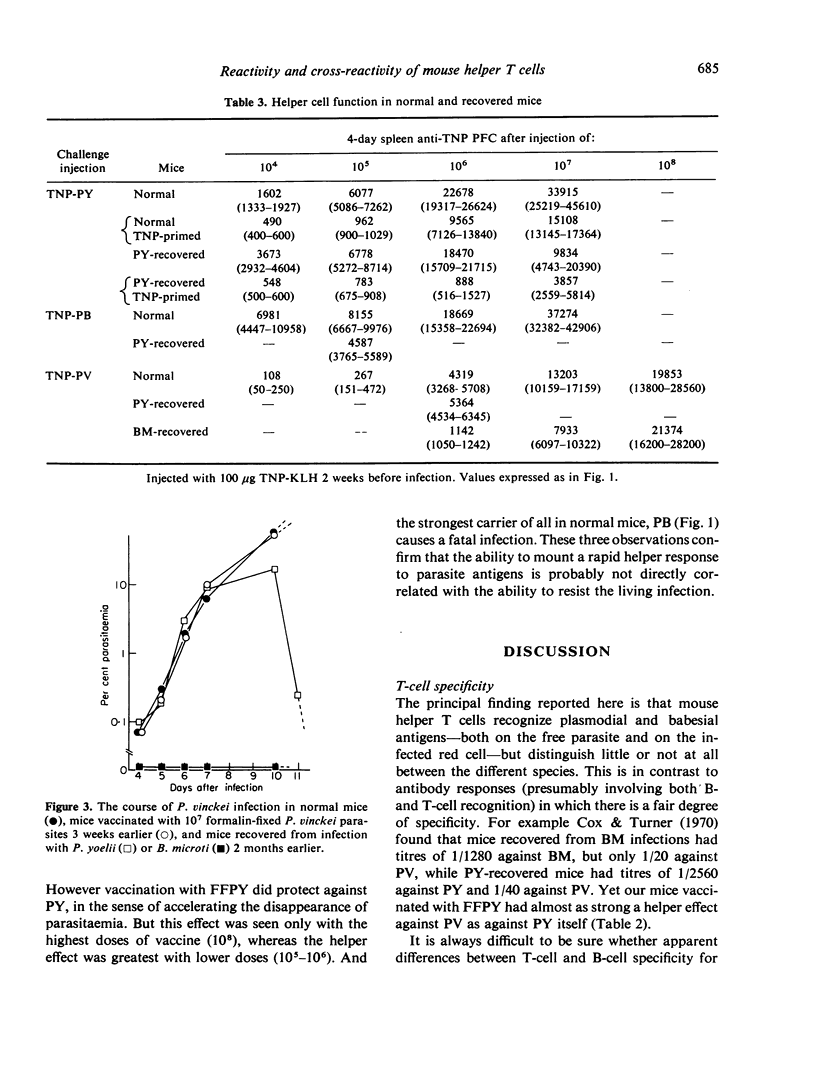
The mouse helper T-cell response to four plasmodial and babesial parasites was measured by using them as carriers for a standard hapten (TNP). Helper T cells appeared to recognize all the parasites, but not to be able to distinguish between them. Helper T-cell responses could be augmented by vaccination with formalin-fixed parasites. However vaccination did not always confer protection against infection. Conversely, mice resistant to infection because of prior recovery from a homologous or heterologous infection had normal or reduced helper T-cell responses. It is concluded that resistance to infection with these parasites, though dependent on T cells, may not only involve the helper T-cell subpopulation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binz H., Kimura A., Wigzell H. Idiotype-positive T lymphocytes. Scand J Immunol. 1975 Sep;4(5-6):413–420. doi: 10.1111/j.1365-3083.1975.tb02646.x. [DOI] [PubMed] [Google Scholar]
- Brown K. N., Jarra W., Hills L. A. T cells and protective immunity to Plasmodium berghei in rats. Infect Immun. 1976 Oct;14(4):858–871. doi: 10.1128/iai.14.4.858-871.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Allison A. C. Babesia microti and Plasmodium berghei yoelii infections in nude mice. Nature. 1974 Nov 22;252(5481):328–329. doi: 10.1038/252328a0. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Allison A. C., Cox F. E. Protection of mice against Babesia and Plasmodium with BCG. Nature. 1976 Jan 29;259(5541):309–311. doi: 10.1038/259309a0. [DOI] [PubMed] [Google Scholar]
- Cox F. E. Protective immunity between malaria parasites and piroplasms in mice. Bull World Health Organ. 1970;43(2):325–336. [PMC free article] [PubMed] [Google Scholar]
- Cox F. E., Turner S. A. Antigenic relationships between the malaria parasites and piroplasms of mice as determined by the fluorescent-antibody technique. Bull World Health Organ. 1970;43(2):337–340. [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Dennert G. Thymus derived killer cells: specificity of function, and antigen recognition. Transplant Rev. 1976;29:59–88. doi: 10.1111/j.1600-065x.1976.tb00197.x. [DOI] [PubMed] [Google Scholar]
- Greenwood B. M., Playfair J. H., Torrigiani G. Immunosuppression in murine malaria. I. General characteristics. Clin Exp Immunol. 1971 Mar;8(3):467–478. [PMC free article] [PubMed] [Google Scholar]
- Greenwood B. M., Vick R. M. Evidence for a malaria mitogen in human malaria. Nature. 1975 Oct 16;257(5527):592–594. doi: 10.1038/257592a0. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Targett G. A., Leuchars E., Carter R. L., Doenhoff M. J., Davies A. J. T-cell activation in murine malaria. Nature. 1975 Nov 13;258(5531):149–151. doi: 10.1038/258149a0. [DOI] [PubMed] [Google Scholar]
- Jerusalem C., Eling W. Active immunization against Plasmodium berghei malaria in mice, using different preparations of plasmodial antigen and different pathways of administration. Bull World Health Organ. 1969;40(6):807–818. [PMC free article] [PubMed] [Google Scholar]
- Ohmichi Y., Nomoto K., Yamada H., Takeya K. Relationships among differentiated T-cell subpopulations. I. Dissociated development of tuberculin type hypersensitivity, Jones-Mote type hypersensitivity and activation of helper function. Immunology. 1976 Jul;31(1):101–110. [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H., Marshall-Clarke S. Cross-reactions between erythrocytes at the T-cell level. Immunology. 1973 Mar;24(3):579–588. [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H. The role of antibody in T-cell responses. Clin Exp Immunol. 1974 May;17(1):1–18. [PMC free article] [PubMed] [Google Scholar]
- Ramalho-Pinto F. J., De Souza J. B., Playfair J. H. Stimulation and suppression of response of mouse T cells to the schistosomules of Schistosoma mansoni during infection. Nature. 1976 Feb 19;259(5544):603–604. doi: 10.1038/259603a0. [DOI] [PubMed] [Google Scholar]
- Rank R. G., Weidanz W. P., Bondi A. Nonsterilizing immunity in avian malaria: an antibody-independent phenomenon. Proc Soc Exp Biol Med. 1976 Feb;151(2):257–259. doi: 10.3181/00379727-151-39186. [DOI] [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Rubin B. Regulation of helper cell activity by specifically adsorbable T lymphocytes. J Immunol. 1976 Jan;116(1):80–85. [PubMed] [Google Scholar]
- SPIRAD, ZUCKERMAN A. Antigenic structure of Plasmodium vinckei. Science. 1962 Aug 17;137(3529):536–537. doi: 10.1126/science.137.3529.536. [DOI] [PubMed] [Google Scholar]
- Tada T., Taniguchi M., Takemori T. Properties of primed suppressor T cells and their products. Transplant Rev. 1975;26:106–129. doi: 10.1111/j.1600-065x.1975.tb00177.x. [DOI] [PubMed] [Google Scholar]
- Weinbaum F. I., Evans C. B., Tigelaar R. E. An in vitro assay for T cell immunity to malaria in mice. J Immunol. 1976 May;116(5):1280–1283. [PubMed] [Google Scholar]


