Abstract
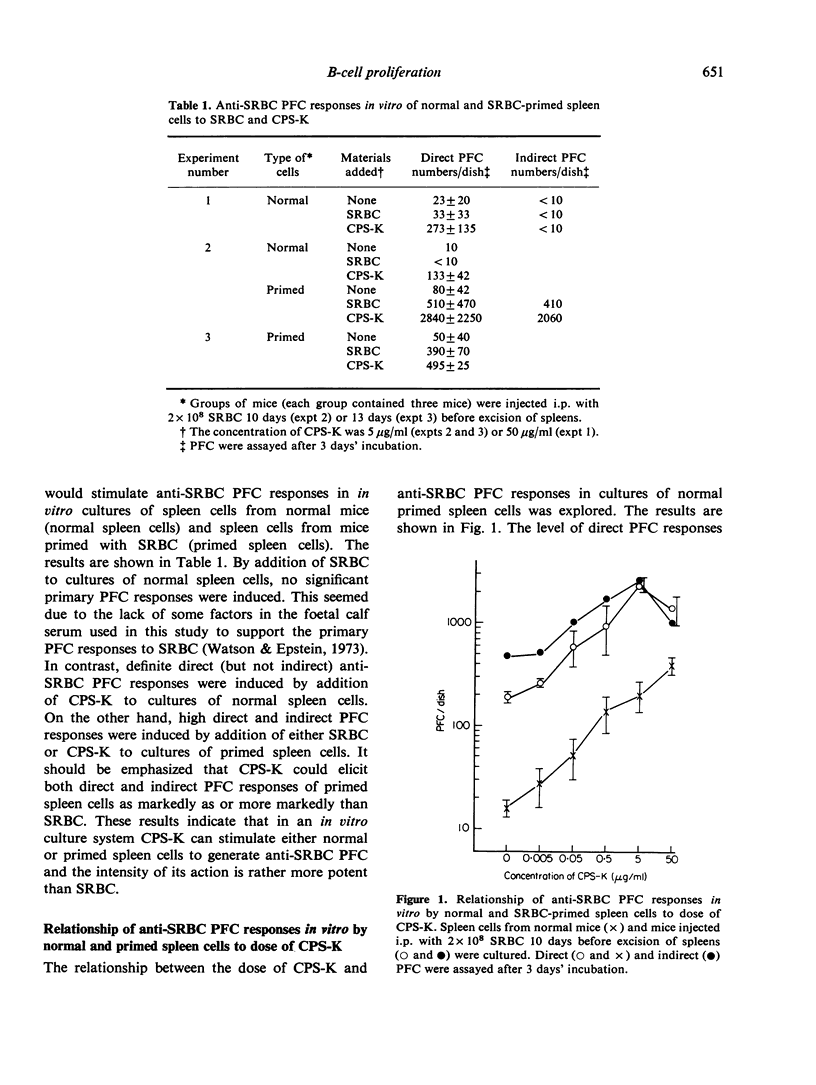
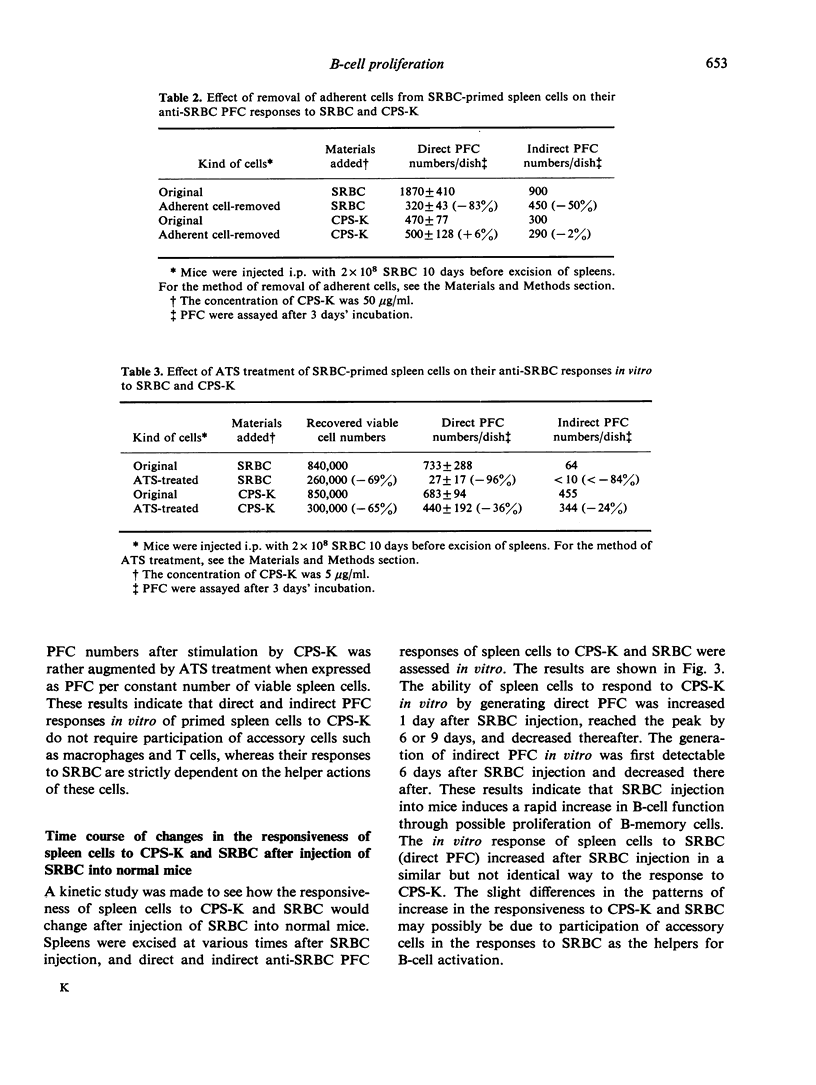
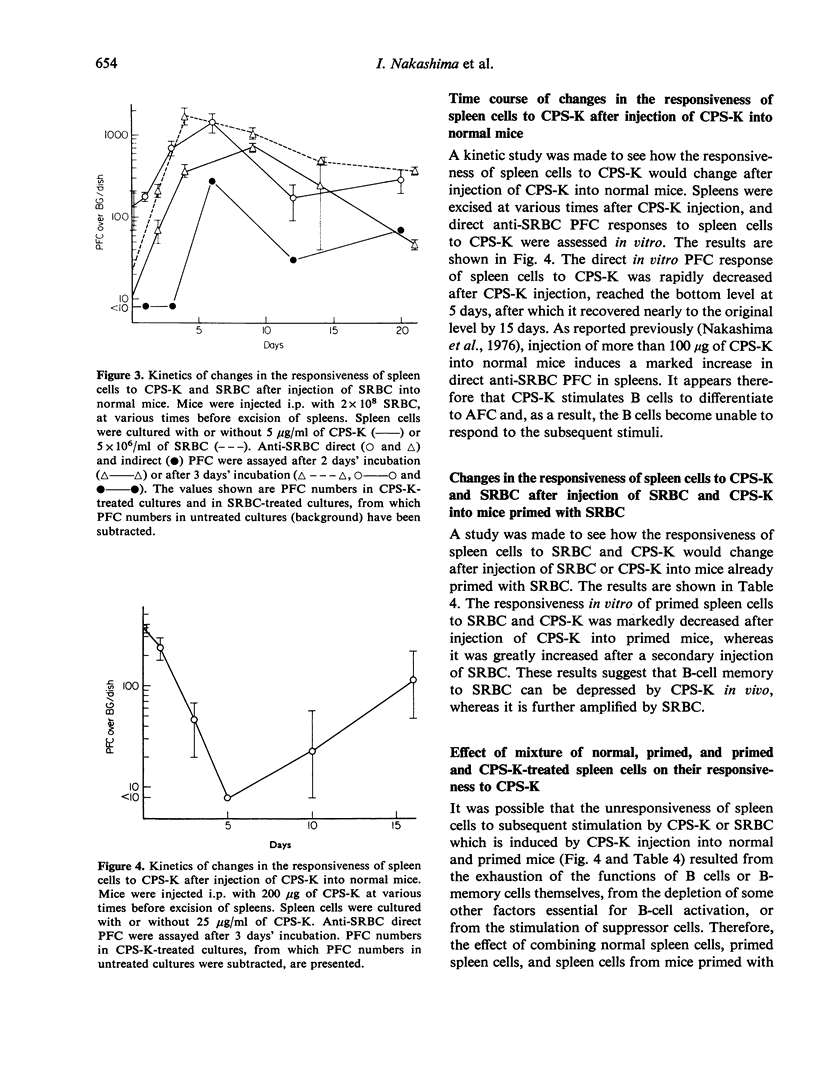
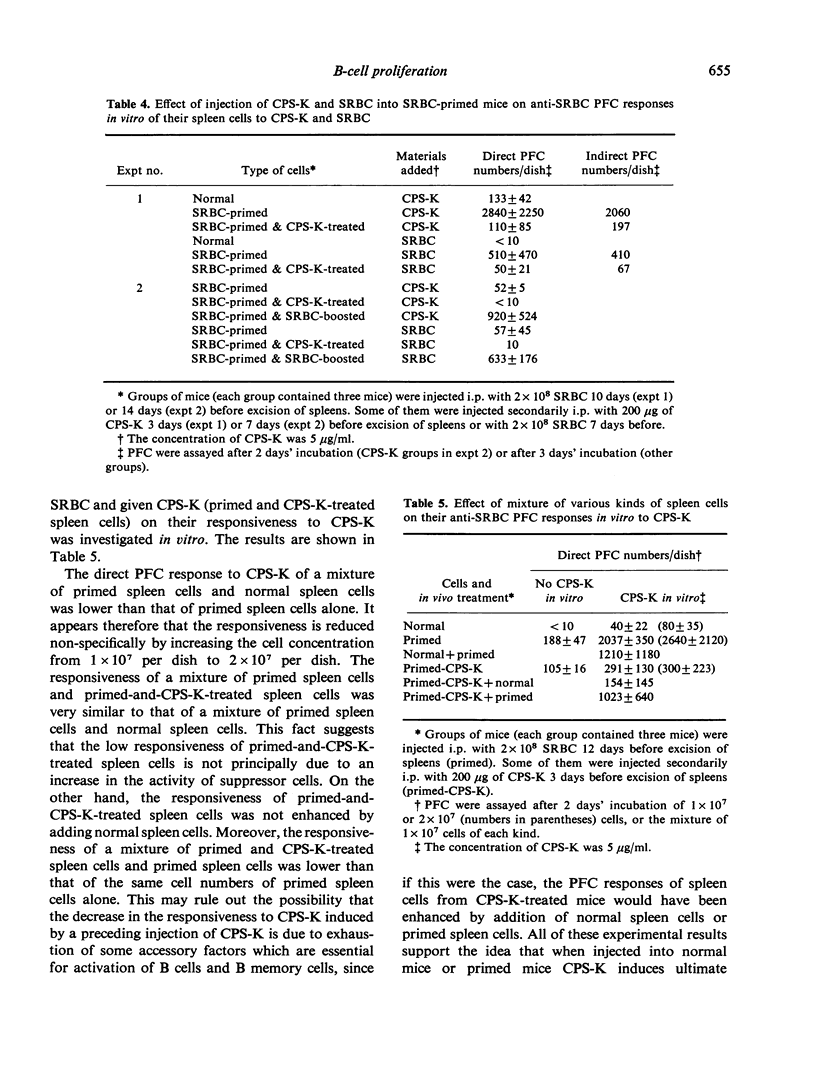
Using the capsular polysaccharide of Klebsiella pneumoniae (CPS-K) as a polyclonal B-cell activator (PBA) and sheep red blood cells (SRBC) as a T cell-dependent antigen, we compared the ability of PBA and antigen to differentiate (generate antibody-forming cells, AFC) and proliferate (generate immunological memory) virgin B cells and B memory cells. In vitro CPS-K induced the differentiation of IgM virgin B cells, IgM B memory cells and IgG B memory cells to AFC, as well as or better than SRBC. The differentiation of B memory cells to AFC by CPS-K did not require the participation of macrophages or T cells, whereas the action of SRBC depended strictly upon the helper actions of these cells. The responsiveness to CPS-K and SRBC of normal and antigen-primed spleen cells as judged by anti-SRBC PFC responses in vitro was markedly decreased after stimulation of virgin B cells and B memory cells in vivo by CPS-K injection into normal or primed mice but greatly increased after the injection of SRBC. The decrease in the responsiveness to CPS-K of spleen cells from mice treated with CPS-K appeared principally due to exhaustion of the functions of B cells and B memory cells. From the present data it has been concluded that the signals required for the differentiation and proliferation of B cells of B memory cells are different from each other, the signal for differentiation being provided by either antigen (SRBC) or PBA (CPS-K), while the signal for proliferation only by antigen.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Sjöberg O., Möller G. Induction of immunoglobulin and antibody synthesis in vitro by lipopolysaccharides. Eur J Immunol. 1972 Aug;2(4):349–353. doi: 10.1002/eji.1830020410. [DOI] [PubMed] [Google Scholar]
- BOYSE E. A., OLD L. J., CHOUROULINKOV I. CYTOTOXIC TEST FOR DEMONSTRATION OF MOUSE ANTIBODY. Methods Med Res. 1964;10:39–47. [PubMed] [Google Scholar]
- Coutinho A., Gronowicz E., Bullock W. W., Möller G. Mechanism of thymus-independent immunocyte triggering. Mitogenic activation of B cells results in specific immune responses. J Exp Med. 1974 Jan 1;139(1):74–92. doi: 10.1084/jem.139.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho A., Gronowicz E. Genetical control of B-cell responses. III. Requirement for functional mitogenicity of the antigen in thymus-independent specific responses. J Exp Med. 1975 Apr 1;141(4):753–760. [PMC free article] [PubMed] [Google Scholar]
- Coutinho A., Möller G. In vitro induction of specific immune responses in the absence of serum: requirement for nonspecific T or B cell mitogens. Eur J Immunol. 1973 Sep;3(9):531–537. doi: 10.1002/eji.1830030902. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Möller G. Thymus-independent B-cell induction and paralysis. Adv Immunol. 1975;21:113–236. doi: 10.1016/s0065-2776(08)60220-5. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A. Selective triggering of B cell subpopulations by mitogens. Eur J Immunol. 1974 Nov;4(11):771–776. doi: 10.1002/eji.1830041113. [DOI] [PubMed] [Google Scholar]
- Kolb C., Di Pauli R., Weiler E. Induction of IgG by lipid A in the newborn mouse. J Exp Med. 1974 Mar 1;139(3):467–478. doi: 10.1084/jem.139.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisler J. M., Möller G. Effect of PPD on the specific immune response to heterologous red cells in vitro. J Immunol. 1974 Jan;112(1):151–161. [PubMed] [Google Scholar]
- Melchers F., Andersson J. IgM in bone marrow-derived lymphocytes. Changes in synthesis, turnover and secretion, and in numbers of molecules on the surface of B cells after mitogenic stimulation. Eur J Immunol. 1974 Mar;4(3):181–188. doi: 10.1002/eji.1830040306. [DOI] [PubMed] [Google Scholar]
- Melchers F., Braun V., Galanos C. The lipoprotein of the outer membrane of Escherichia coli: a B-lymphocyte mitogen. J Exp Med. 1975 Aug 1;142(2):473–482. doi: 10.1084/jem.142.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M., Uchiyama T., Tanabe M. J., Saito K. Nonspecific elicitation of antibody-forming cells in the mouse spleen by bacterial lipopolysaccharide. Jpn J Microbiol. 1975 Apr;19(2):141–148. doi: 10.1111/j.1348-0421.1975.tb00860.x. [DOI] [PubMed] [Google Scholar]
- Nakashima I. Adjuvant action of capsular polysaccharide of Klebsiella pneumoniae on antibody response. I. Intensity of its action. J Immunol. 1972 Apr;108(4):1009–1016. [PubMed] [Google Scholar]
- Nakashima I., Kato N. Adjuvant action of capsular polysaccharide of Klebsiella pneumoniae on antibody response. IV. The roles of antigen and adjuvant for induction of primary and secondary antibody responses and for development of immunological memory to bovine serum albumin. Jpn J Microbiol. 1975 Aug;19(4):277–285. [PubMed] [Google Scholar]
- Nakashima I., Kato N. Non-specific stimulation of immunoglobulin synthesis in mice by capsular polysaccharide of Klebsiella pneumoniae. Immunology. 1974 Aug;27(2):179–193. [PMC free article] [PubMed] [Google Scholar]
- Nakashima I., Kobayashi T., Kato N. Alterations in the antibody response to bovine serum albumin by capsular polysaccharide of Klebsiella pneumoniae. J Immunol. 1971 Oct;107(4):1112–1121. [PubMed] [Google Scholar]
- Nakashima I., Kojima T., Kato N. Cellular aspects of non-specific stimulation of antibody production by capsular polysaccharide of Klebsiella pneumoniae. Immunology. 1976 Feb;30(2):229–240. [PMC free article] [PubMed] [Google Scholar]
- Nilsson B. S., Sultzer B. M., Bullock W. W. Purified protein derivative of tuberculin induces immunoglobulin production in normal mouse spleen cells. J Exp Med. 1973 Jan 1;137(1):127–139. doi: 10.1084/jem.137.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Epstein R. The role of humoral factors in the initiation of in vitro primary immune responses. I. Effects of deficient fetal bovine serum. J Immunol. 1973 Jan;110(1):31–42. [PubMed] [Google Scholar]


