Abstract
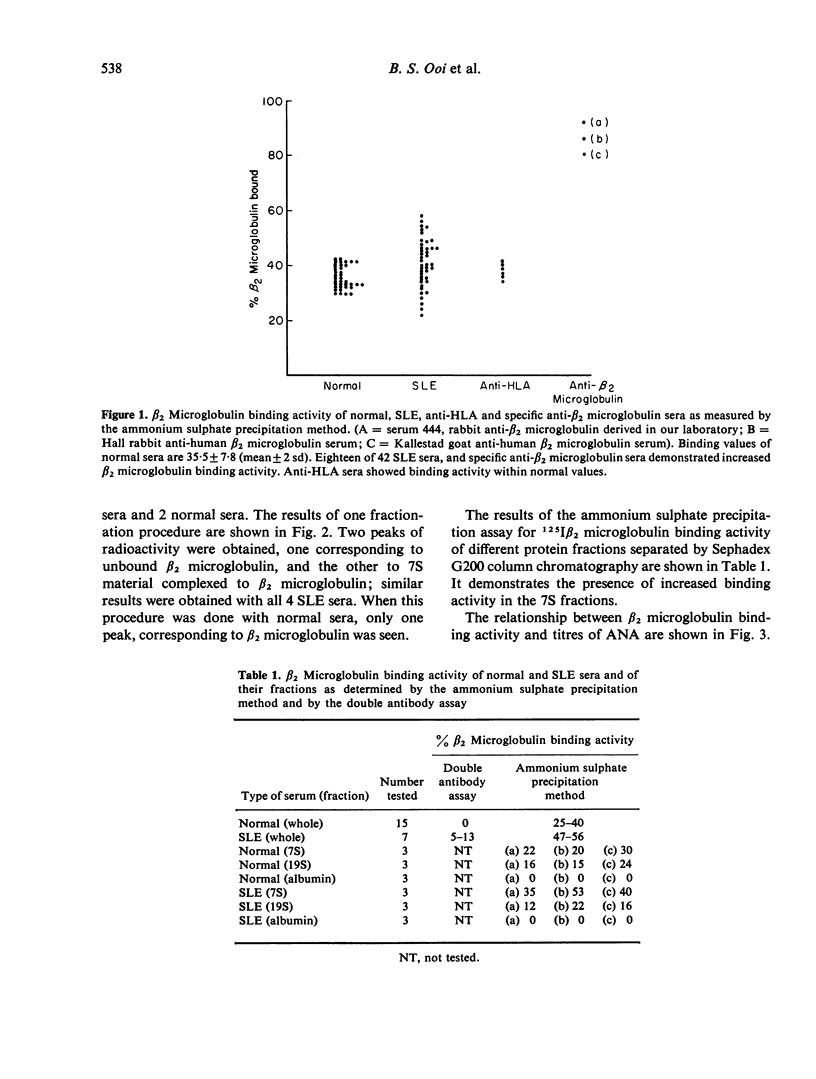
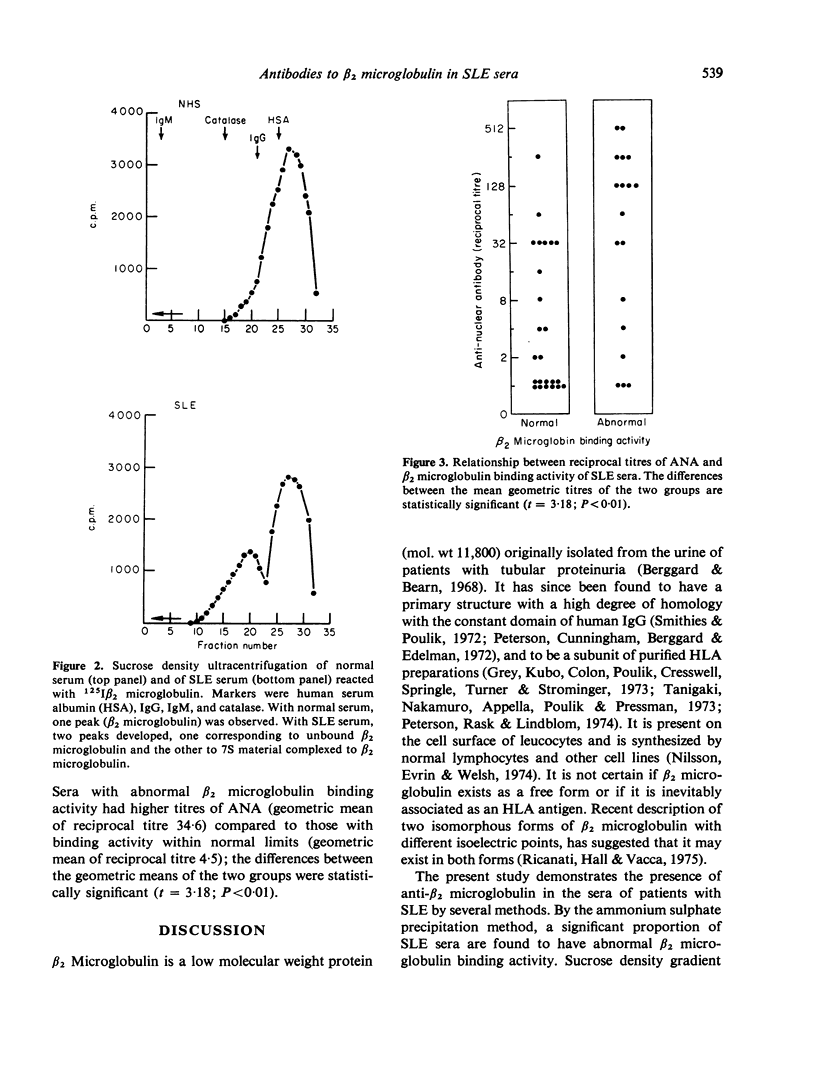
The present study reports the detection of antibodies to β2 microglobulin in the sera of patients with systemic lupus erythematosus (SLE). Using a Farr-type ammonium sulphate precipitation assay, test sera were reacted with 125Iβ2 microglobulin, and immunoglobulins precipitated by 50% saturated ammonium sulphate. Increased β2 microglobulin binding activity (normal values: mean±2 sd = 35.5 ±7.8) was detected in 18 of 42 SLE sera. Anti-HLA sera did not reveal increased binding activity, suggesting that the antibody in SLE serum was directed toward free β2 microglobulin. Direct validation was done by reacting 125Iβ2 microglobulin with 4 SLE sera having increased 125Iβ2 microglobulin binding activity, and subjecting the reactants to sucrose density gradient ultracentrifugation. Two peaks were obtained, one corresponding to free β2 microglobulin, and the other to 7S material complexed to β2 microglobulin. Normal sera demonstrated only one peak corresponding to unbound β2 microglobulin. Assays of β2 microglobulin binding activity on protein fractions obtained by Sephadex G200 column chromatography also showed the presence of increased binding activity with 7S fractions. Using a double antibody assay, the 7S material reactive to β2 microglobulin was demonstrated to be IgG. It was also shown that sera with abnormal β2 microglobulin binding activity had higher titres of antinuclear antibody compared to those lacking such activity (t = 3.18; P<0.01), indicating the pathogenetic relationship of this antibody to increased disease activity. This antibody may be responsible for some of the abnormalities of cell-mediated function previously described in SLE patients.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Berggård I., Bearn A. G. Isolation and properties of a low molecular weight beta-2-globulin occurring in human biological fluids. J Biol Chem. 1968 Aug 10;243(15):4095–4103. [PubMed] [Google Scholar]
- Grey H. M., Kubo R. T., Colon S. M., Poulik M. D., Cresswell P., Springer T., Turner M., Strominger J. L. The small subunit of HL-A antigens is beta 2-microglobulin. J Exp Med. 1973 Dec 1;138(6):1608–1612. doi: 10.1084/jem.138.6.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz D. A. Impaired delayed hypersensitivity in systemic lupus erythematosus. Arthritis Rheum. 1972 Jul-Aug;15(4):353–359. doi: 10.1002/art.1780150406. [DOI] [PubMed] [Google Scholar]
- Lewis R. M., Tannenberg W., Smith C., Schwartz R. S. C-type viruses in systemic lupus erythematosus. Nature. 1974 Nov 1;252(5478):78–79. doi: 10.1038/252078a0. [DOI] [PubMed] [Google Scholar]
- McCalmon R. T., Kubo R. T., Grey H. M. Effect of anti-beta2-microglobulin on antigen and allogeneic lymphocyte-induced proliferation of human lymphocytes. J Immunol. 1975 Jun;114(6):1766–1770. [PubMed] [Google Scholar]
- Mintz U., Sachs L. Membrane difference in peripheral blood lymphocytes from patients with chronic lymphocytic leukemia and Hodgkin's disease. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2428–2432. doi: 10.1073/pnas.72.6.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal K. K., Rossen R. D., Sharp J. T., Lidsky M. D., Butler W. T. Lymphocyte cytotoxic antibodies in systemic lupus erythematosus. Nature. 1970 Mar 28;225(5239):1255–1256. doi: 10.1038/2251255a0. [DOI] [PubMed] [Google Scholar]
- Nilsson K., Evrin P. E., Welsh K. I. Production of beta 2-microglobulin by normal and malignant human cell lines and peripheral lymphocytes. Transplant Rev. 1974;21(0):53–84. doi: 10.1111/j.1600-065x.1974.tb01546.x. [DOI] [PubMed] [Google Scholar]
- Ooi B. S., Orlina A. R., Pesce A. J., Mendoza N., Masaitis L., Pollak V. E. Lymphocytotoxic antibodies in patients with systemic lupus erythematosus. Clin Exp Immunol. 1974 Jun;17(2):237–243. [PMC free article] [PubMed] [Google Scholar]
- POLLAK V. E. ANTINUCLEAR ANTIBODIES IN FAMILIES OF PATIENTS WITH SYSTEMIC LUPUS ERYTHEMATOSUS. N Engl J Med. 1964 Jul 23;271:165–171. doi: 10.1056/NEJM196407232710401. [DOI] [PubMed] [Google Scholar]
- Peterson P. A., Cunningham B. A., Berggård I., Edelman G. M. 2 -Microglobulin--a free immunoglobulin domain. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1697–1701. doi: 10.1073/pnas.69.7.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. A., Rask L., Lindblom J. B. Highly purified papain-solubilized HL-A antigens contain beta2-microglobulin. Proc Natl Acad Sci U S A. 1974 Jan;71(1):35–39. doi: 10.1073/pnas.71.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithies O., Poulik M. D. Initiation of protein synthesis at an unusual position in an immunoglobulin gene? Science. 1972 Jan 14;175(4018):187–189. doi: 10.1126/science.175.4018.187. [DOI] [PubMed] [Google Scholar]
- Stastny P., Ziff M. Antibodies against cell membrane constituents in systemic lupus erythematosus and related diseases. I. Cytotoxic effect of serum from patients with systemic lupus erythematosus (SLE) for allogeneic and for autologous lymphocytes. Clin Exp Immunol. 1971 Apr;8(4):543–550. [PMC free article] [PubMed] [Google Scholar]
- Tanigaki N., Nakamuro K., Appella E., Poulik M. D., Pressman D. Identity of the HL-A common portion fragment and human beta2-microglobulin. Biochem Biophys Res Commun. 1973 Dec 19;55(4):1234–1239. doi: 10.1016/s0006-291x(73)80026-9. [DOI] [PubMed] [Google Scholar]
- Terasaki P. I., Mottironi V. D., Barnett E. V. Cytotoxins in disease. Autocytotoxins in lupus. N Engl J Med. 1970 Oct 1;283(14):724–728. doi: 10.1056/NEJM197010012831403. [DOI] [PubMed] [Google Scholar]
- Wernet P., Kunkel H. G. Demonstration of specific T-lymphocyte membrane antigens associated with antibodies inhibiting the mixed leukocyte culture in man. Transplant Proc. 1973 Dec;5(4):1875–1881. [PubMed] [Google Scholar]
- Williams R. C., Jr, Lies R. B., Messner R. P. Inhibition of mixed leukocyte culture responses by serum and gamma-globulin fractions from certain patients with connective tissue disorders. Arthritis Rheum. 1973 Sep-Oct;16(5):597–605. doi: 10.1002/art.1780160504. [DOI] [PubMed] [Google Scholar]
- Winfield J. B., Winchester R. J., Wernet P., Fu S. M., Kunkel H. G. Nature of cold-reactive antibodies to lymphocyte surface determinants in systemic lupus erythematosus. Arthritis Rheum. 1975 Jan-Feb;18(1):1–8. doi: 10.1002/art.1780180101. [DOI] [PubMed] [Google Scholar]


