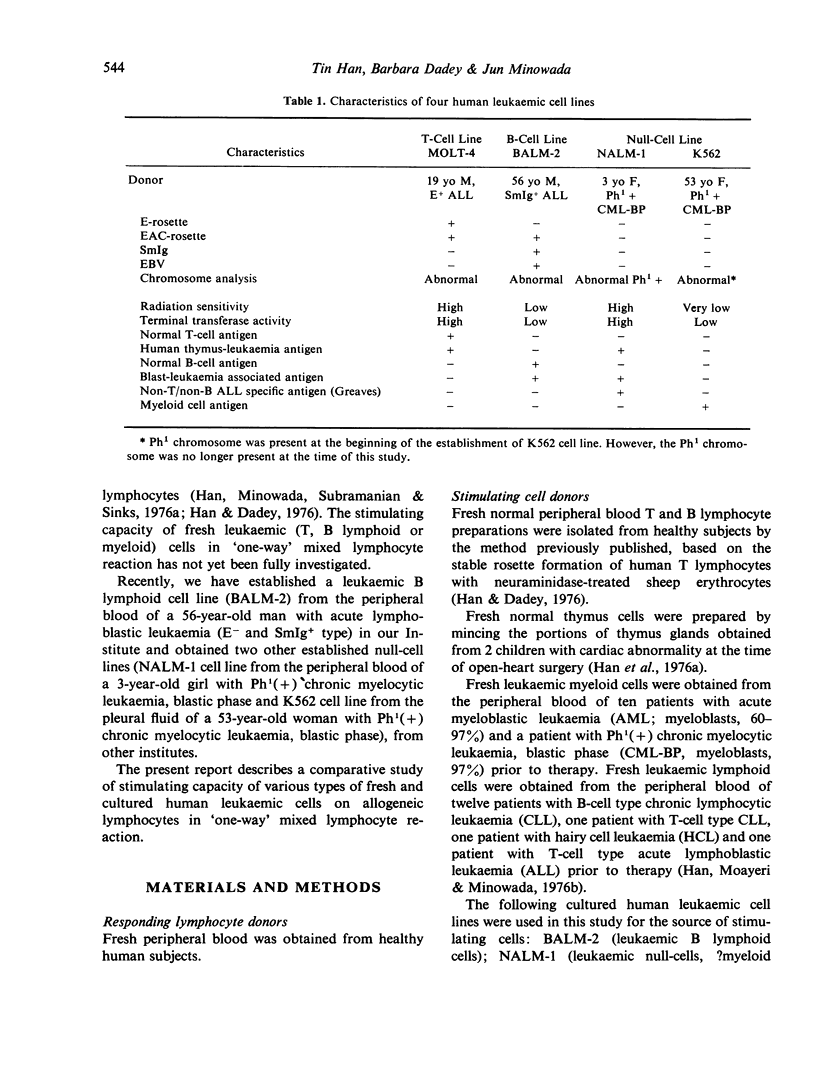
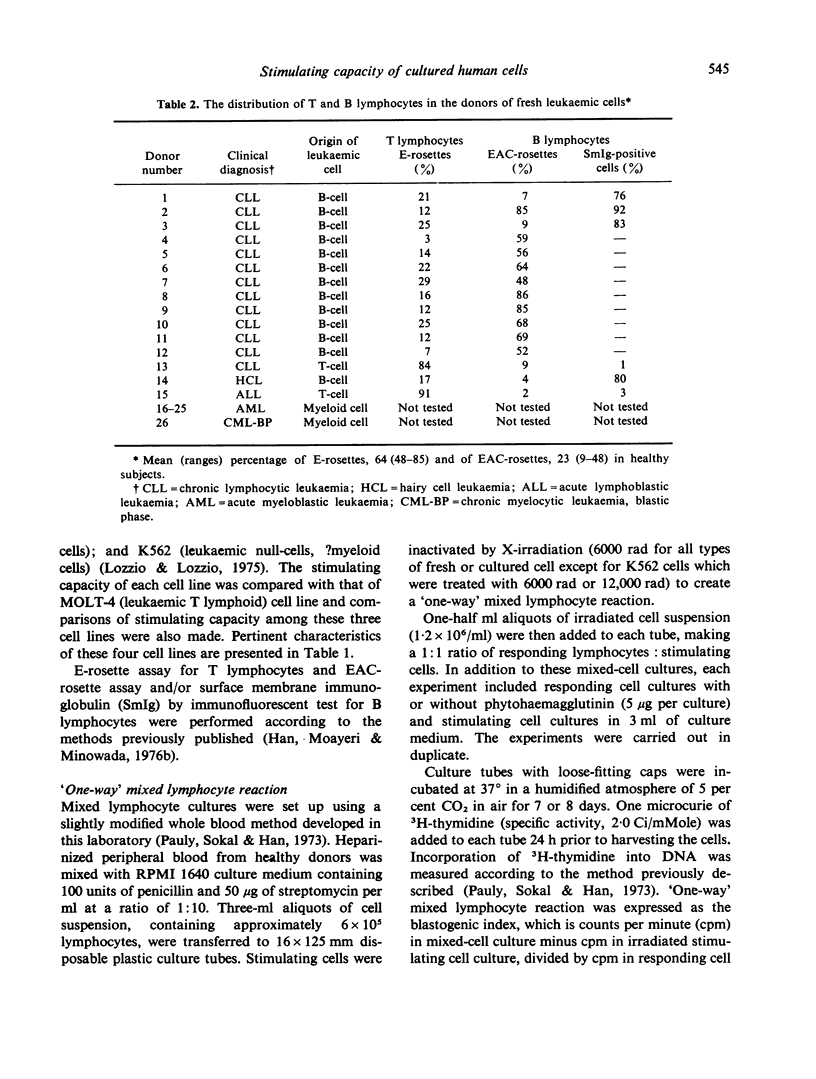
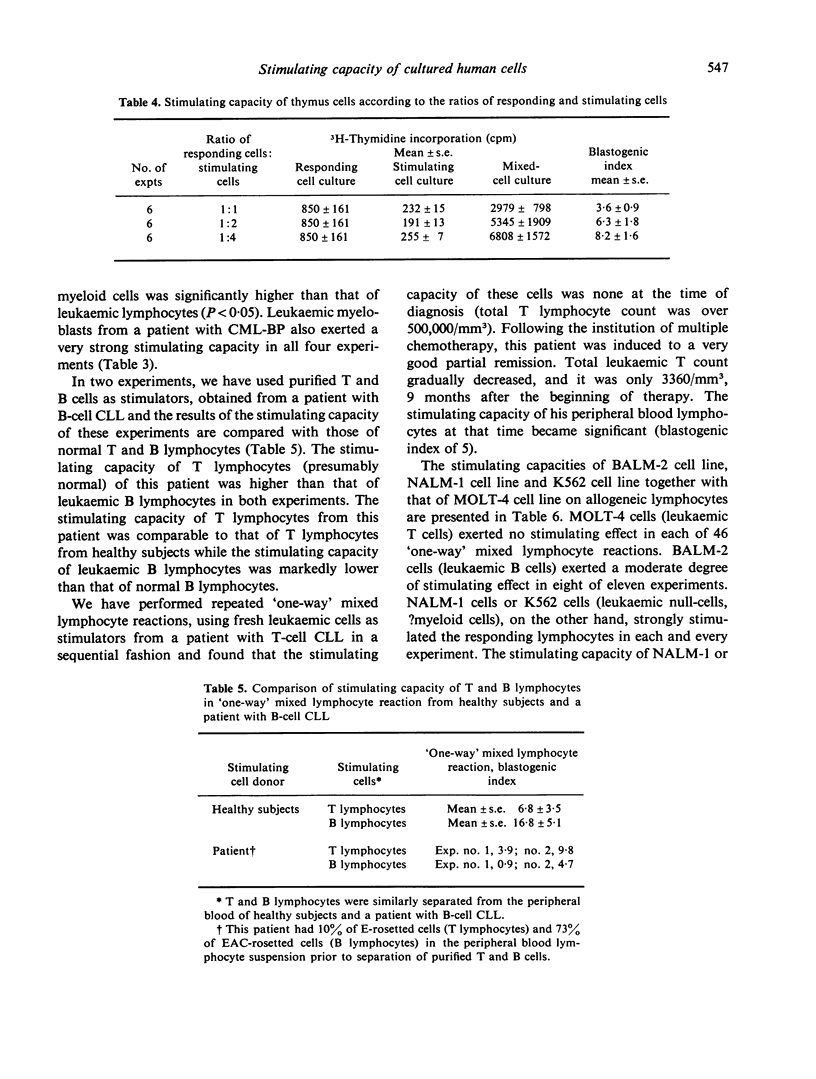
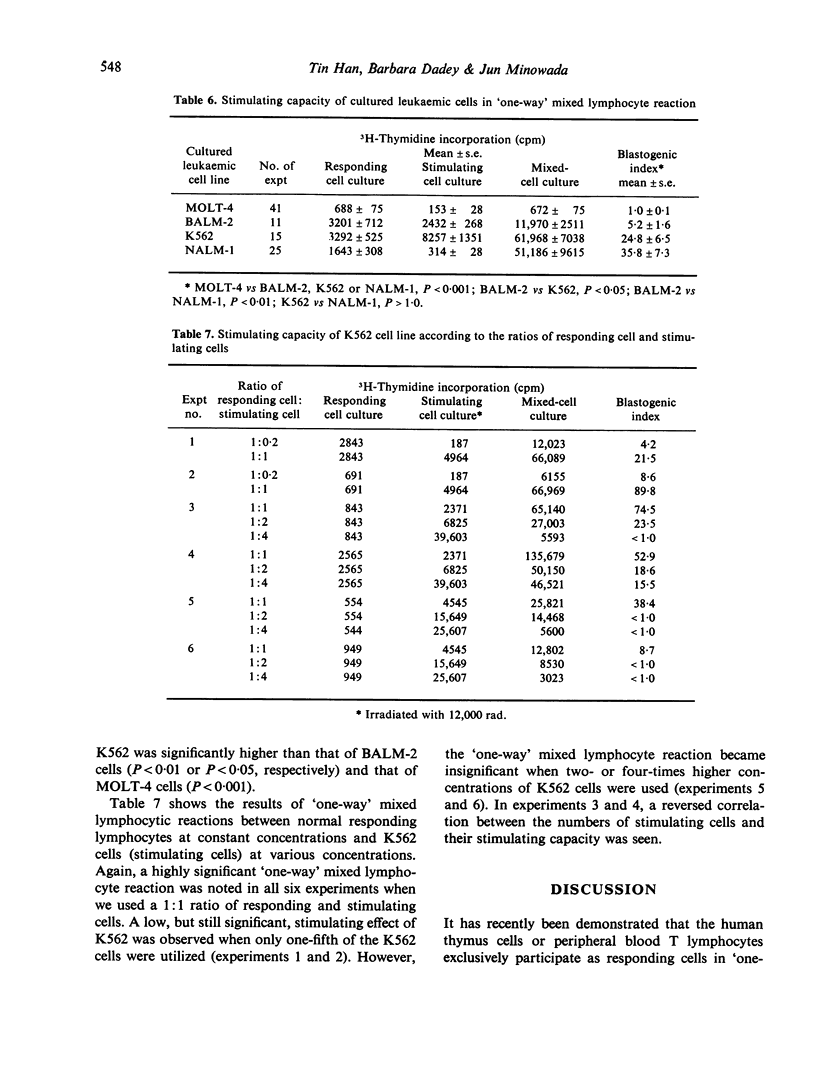
Abstract
Fresh normal peripheral blood B lymphocytes possess a strong stimulating capacity while fresh thymus cells or fresh peripheral T lymphocytes possess a weak, but significant stimulating capacity on allogeneic lymphocytes in `one-way' mixed lymphocyte reaction. Fresh leukaemic T lymphoid cells from patients with T-cell ALL or T-cell CLL exert little or no stimulation on allogeneic lymphocytes. Fresh leukaemic B lymphoid cells from patients with B-cell CLL or B-cell HCL, on the other hand, exert a lesser stimulation on allogeneic lymphocytes, as compared to that of normal B lymphocytes. Leukaemic myeloblasts from patients with AML or Ph1(+) CML-BP exert significantly higher stimulation than leukaemic lymphoid cells in `one-way' mixed lymphocyte reaction (P<0.05). Cultured leukaemic T lymphoid cells (MOLT-4) possess no stimulating capacity, cultured leukaemic B lymphoid cells (BALM-2) possess a moderate degree of stimulating capacity and cultured leukaemic, possibly myeloid, cells (NALM-1 and K562) possess vigorous stimulation on allogeneic lymphocytes. The stimulating capacity of NALM-1 or K562 cells is significantly higher than that of BALM-2 cells (P<0.01 or P<0.05, respectively) and that of MOLT-4 cells (P<0.001). These observations suggest that the stimulating capacity of leukaemic T or B lymphoid cells may have been completely or partially lost during the process of leukaemogenesis. Since we do not have an opportunity to study the stimulating capacity of normal myeloblasts, it is not known whether the stimulating capacity of leukaemic myeloblasts, which is found to be very strong on allogeneic lymphocytes, may have been modified during the process of leukaemogenesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boggs D. R. Editorial: Hematopoietic stem cell theory in relation to possible lymphoblastic conversion of chronic myeloid leukemia. Blood. 1974 Sep;44(3):449–453. [PubMed] [Google Scholar]
- Halterman R. H., Leventhal B. G. Enhanced immune response to leukaemia. Lancet. 1971 Sep 25;2(7726):704–705. doi: 10.1016/s0140-6736(71)92269-0. [DOI] [PubMed] [Google Scholar]
- Han T. Blastogenic response of normal lymphocytes to cultured lymphoid cells and non-lymphoid neoplastic cells. Immunology. 1972 Sep;23(3):355–359. [PMC free article] [PubMed] [Google Scholar]
- Han T., Dadey B. T and B lymphocytes. Exclusive role as responders and stimulators in human one-way mixed lymphocyte reaction. Immunology. 1976 Oct;31(4):643–648. [PMC free article] [PubMed] [Google Scholar]
- Han T., Minowada J. A unique 'leukaemic' T lymphoid cell line: absence of stimulating effect in mixed lymphocyte reaction. Lack of MLR-S in leukaemic T lymphoid cells. Clin Exp Immunol. 1973 Dec;15(4):535–541. [PMC free article] [PubMed] [Google Scholar]
- Han T., Moayeri H., Minowada J. T- and B-lymphocytes in chronic lymphocytic leukemia: correlation with clinical and immunologic status of the disease. J Natl Cancer Inst. 1976 Sep;57(3):477–481. doi: 10.1093/jnci/57.3.477. [DOI] [PubMed] [Google Scholar]
- Han T., Pauly J. L., Minowada J. In vitro preferential effect of irradiation on cultured T lymphoid cell line. Clin Exp Immunol. 1974 Jul;17(3):455–462. [PMC free article] [PubMed] [Google Scholar]
- Han T., Sokal J. E., Moore G. E. "Antigenic" disparity between cultured lymphoid cells and autologous lymphocytes. Am J Med. 1972 Oct;53(4):437–445. doi: 10.1016/0002-9343(72)90139-8. [DOI] [PubMed] [Google Scholar]
- Han T., Wang J. 'Antigenic' disparity between leukaemic lymphoblasts and normal lymphocytes in identical twins. Clin Exp Immunol. 1972 Oct;12(2):171–175. [PMC free article] [PubMed] [Google Scholar]
- Hardy D. A., Ling N. R., Knight S. C. Exceptional lymphocyte stimulating capacity of cells from lymphoid cell lines. Nature. 1969 Aug 2;223(5205):511–512. doi: 10.1038/223511a0. [DOI] [PubMed] [Google Scholar]
- Lohrmann H. P., Novikovs L., Graw R. G., Jr Stimulatory capacity of human T and B lymphocytes in the mixed leukocyte culture. Nature. 1974 Jul 12;250(462):144–146. doi: 10.1038/250144a0. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- McCaffrey R., Harrison T. A., Parkman R., Baltimore D. Terminal deoxynucleotidyl transferase activity in human leukemic cells and in normal human thymocytes. N Engl J Med. 1975 Apr 10;292(15):775–780. doi: 10.1056/NEJM197504102921504. [DOI] [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Pauly J. L., Minowada J., Han T., Moore G. E. Disparity of mixed lymphocyte reactivity to cultured cells of human T and B lymphoid lines. J Natl Cancer Inst. 1975 Mar;54(3):557–562. [PubMed] [Google Scholar]
- Powles R. L., Balchin L. A., Fairley G. H., Alexander P. Recognition of leukaemia cells as foreign before and after autoimmunization. Br Med J. 1971 Feb 27;1(5747):486–489. doi: 10.1136/bmj.1.5747.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston I., Graze P. R., Pitts R. B. Failure of cultured human T-cell lymphoid lines to stimulate in mixed leukocyte culture. J Natl Cancer Inst. 1974 Aug;53(2):361–367. doi: 10.1093/jnci/53.2.361. [DOI] [PubMed] [Google Scholar]


