Abstract
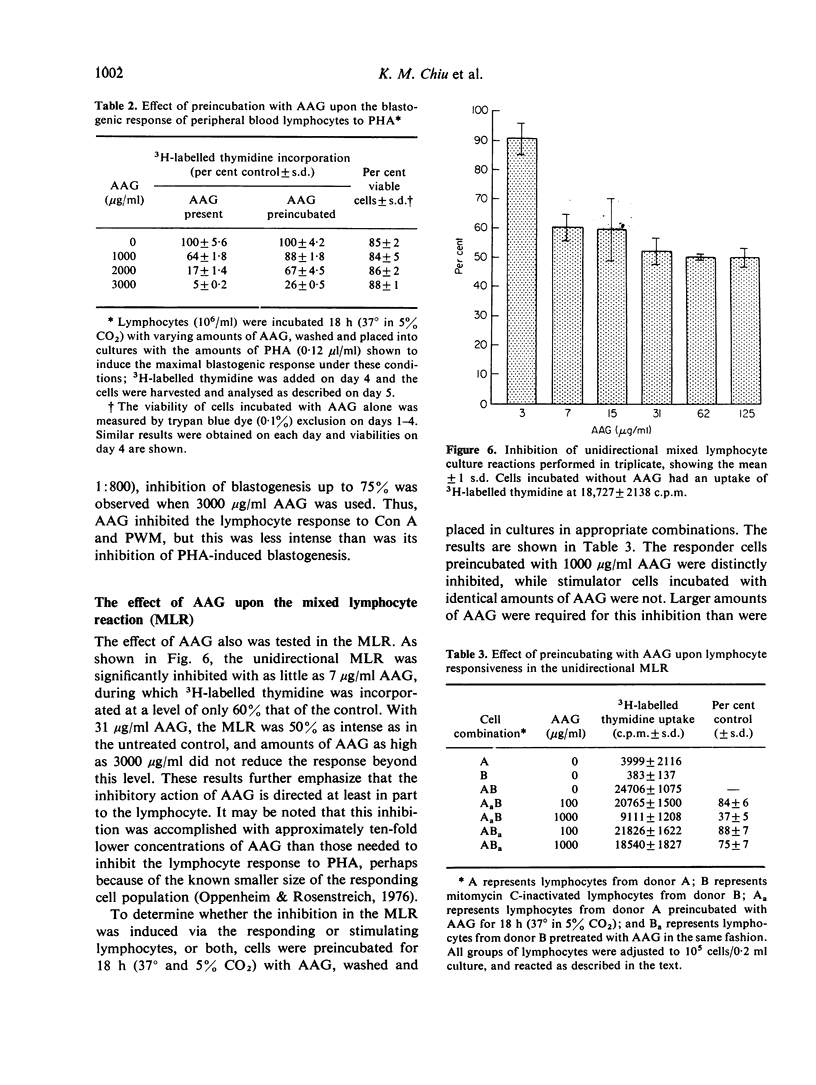
Alpha1-acid glycoprotein (AAG) is a constituent of normal serum which is elevated in concentration in the acute phase of inflammation; its physical and chemical properties have been defined but its biological function is uncertain. In the present study, the effect of AAG on the proliferative response of lymphocytes to several different stimuli was determined. For this purpose AAG was prepared by precipitation of human ascites fluid with sulphosalicylic acid and passage of the supernate through SP-Sephadex; the eluted protein migrated as a single band during immunoelectrophoresis, polyacrylamide gel electrophoresis and chromatography on Bio-Gel-A-1-5 m in 6 M guanidine HCl. This AAG was found to markedly inhibit the proliferative response of human peripheral blood lymphocytes to PHA; it also inhibited blastogenesis induced by Con A and PWM, but to a lesser extent. AAG was not cytotoxic to lymphocytes, and its inhibitory effects were reversed at higher mitogen concentrations. Lymphocytes preincubated with AAG remained less reactive to PHA indicating that, although AAG appeared to react with PHA, its effect was directed predominantly to the cell. Further, AAG markedly inhibited the mixed lymphocyte response, and this effect was directed to the responder cells. Thus, AAG is another acute phase reactant with the ability to modulate lymphocyte responsiveness.
Full text
PDF

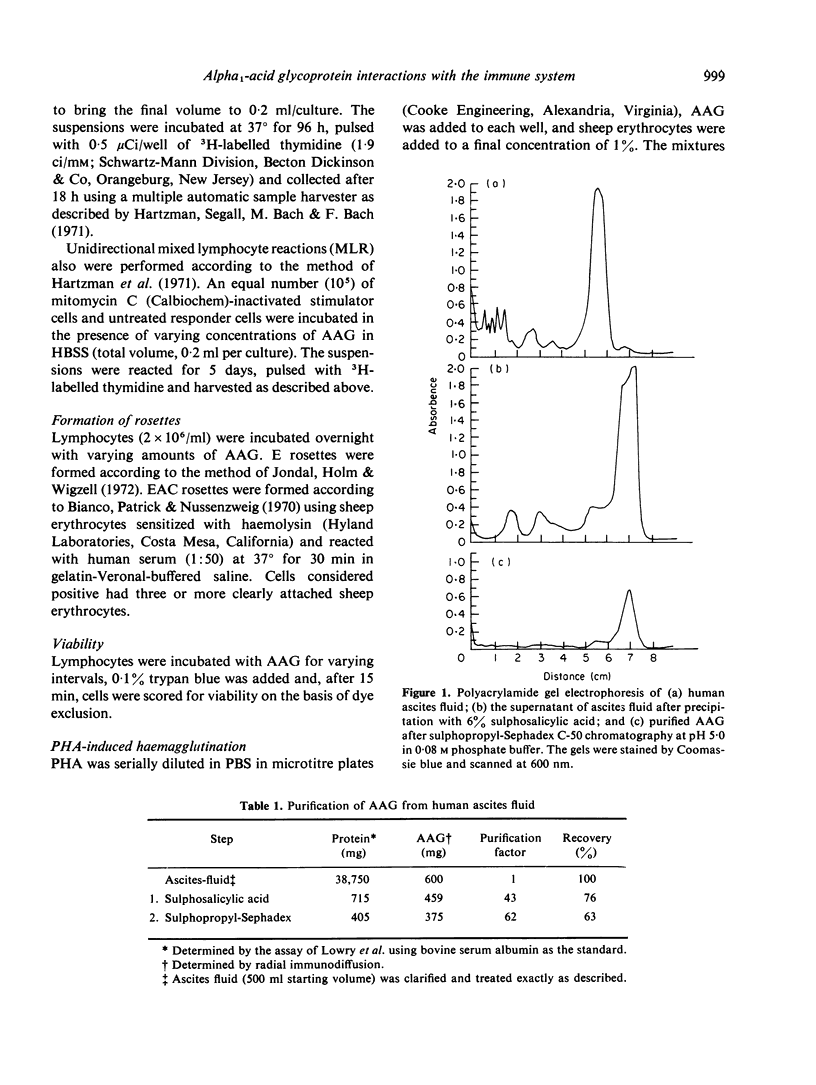
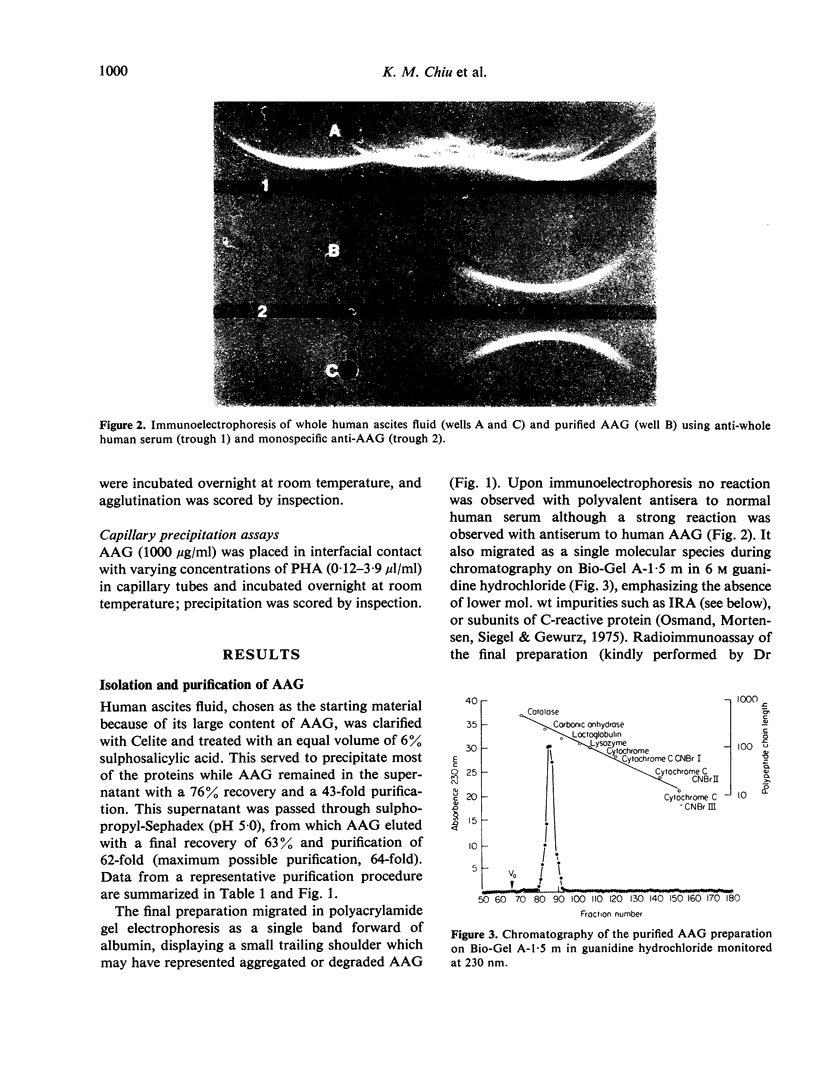
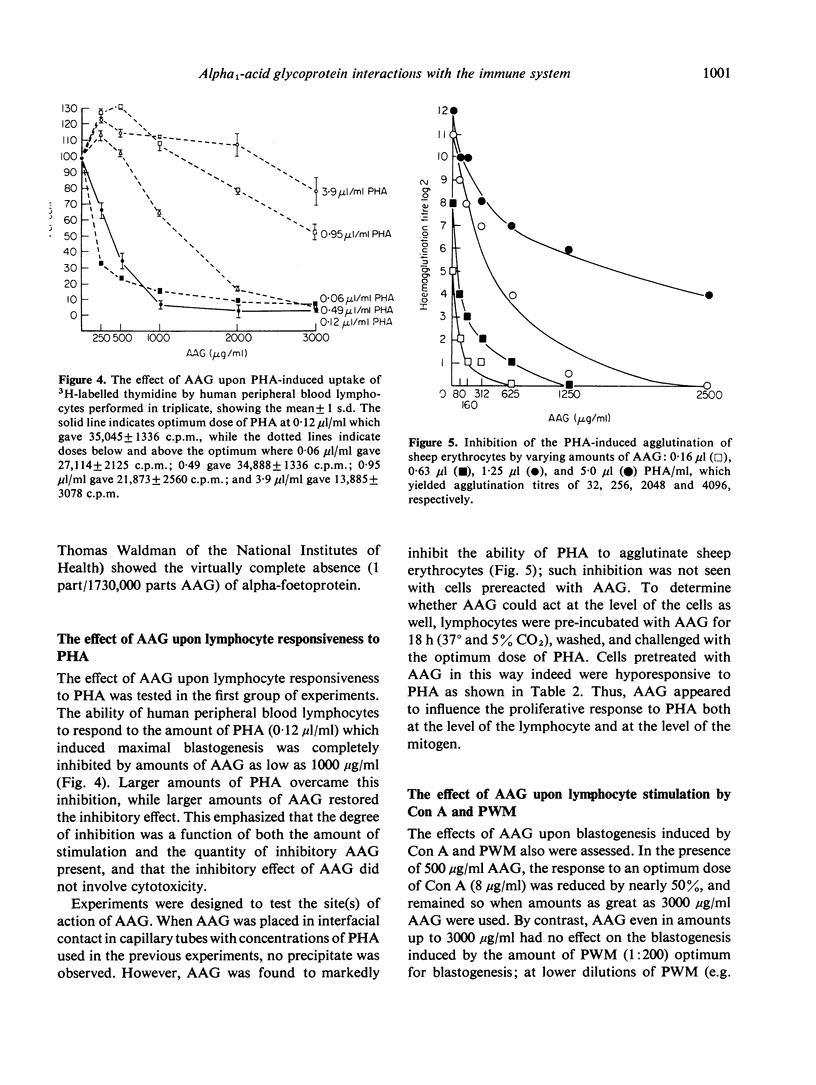



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATHINEOS E., THORNTON M., WINZLER R. J. Comparative antigenicity of native and "desialized" orosomucoid in rabbits. Proc Soc Exp Biol Med. 1962 Nov;111:353–356. doi: 10.3181/00379727-111-27790. [DOI] [PubMed] [Google Scholar]
- Apffel C. A., Peters J. H. Tumors and serum glycoproteins. The 'symbodies'. Prog Exp Tumor Res. 1969;12:1–54. [PubMed] [Google Scholar]
- Bianco C., Patrick R., Nussenzweig V. A population of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. I. Separation and characterization. J Exp Med. 1970 Oct 1;132(4):702–720. doi: 10.1084/jem.132.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase P. S. The effects of human serum fractions on phytohemagglutinin- and concanavalin A-stimulated human lymphocyte cultures. Cell Immunol. 1972 Dec;5(4):544–554. doi: 10.1016/0008-8749(72)90104-9. [DOI] [PubMed] [Google Scholar]
- Cooperband S. R., Badger A. M., Davis R. C., Schmid K., Mannick J. A. The effect of immunoregulatory globulin (IRA) upon lymphocytes in vitro. J Immunol. 1972 Jul;109(1):154–163. [PubMed] [Google Scholar]
- Curtiss L. K., Edgington T. S. Regulatory serum lipoproteins: regulation of lymphocyte stimulation by a species of low density lipoprotein. J Immunol. 1976 May;116(5):1452–1458. [PubMed] [Google Scholar]
- Emura J., Ikenaka T., Collins J. H., Schmid K. The constant and variable regions of the carboxyl-terminal CNBr fragment of alpha-acid glycoprotein. J Biol Chem. 1971 Dec 25;246(24):7821–7823. [PubMed] [Google Scholar]
- Fiedel B. A., Gewurz H. Effects of C-reactive protein on platelet function. I. Inhibition of platelet aggregation and release reactions. J Immunol. 1976 May;116(5):1289–1294. [PubMed] [Google Scholar]
- Ganguly M., Westphal U. Steroid-protein interactions. XX. Effect of chemical modifications on progesterone-binding affinity of alpha 1-acid glycoprotein. Biochim Biophys Acta. 1968 Dec 23;170(2):309–323. doi: 10.1016/0304-4165(68)90011-1. [DOI] [PubMed] [Google Scholar]
- Interactions of C-reactive protein with the complement system. III. Complement-dependent passive hemolysis initiated by CRP. J Exp Med. 1975 Nov 1;142(5):1065–1077. doi: 10.1084/jem.142.5.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krotoski W. A., Weimer H. E. Effects of dilute acids on the chemical and immunochemical properties of human orosomucoid. Clin Chim Acta. 1966 Mar;13(3):329–340. doi: 10.1016/0009-8981(66)90212-9. [DOI] [PubMed] [Google Scholar]
- Menzoian J. O., Glasgow A. H., Nimberg R. D., Cooperband S. R., Schmid K., Saporoschetz I., Mannick J. A. Regulation of T lymphocyte function by immunoregulatory alphaglobulin (IRA). J Immunol. 1974 Jul;113(1):266–273. [PubMed] [Google Scholar]
- Miller F. Serum-derived immunosuppressive substances. I. Partial purification and range of action. Transplantation. 1976 Mar;21(3):179–187. doi: 10.1097/00007890-197603000-00001. [DOI] [PubMed] [Google Scholar]
- Mortensen R. F., Gewurz H. Effects of C-reactive protein on the lymphoid system. II. Inhibition of mixed lymphocyte reactivity and generation of cytotoxic lymphocytes. J Immunol. 1976 May;116(5):1244–1250. [PubMed] [Google Scholar]
- Mortensen R. F., Osmand A. P., Gewurz H. Effects on C-reactive protein on the lymphoid system. I. Binding to thymus-dependent lymphocytes and alteration of their functions. J Exp Med. 1975 Apr 1;141(4):821–839. [PMC free article] [PubMed] [Google Scholar]
- Munthe-Kaas A. C., Berg T., Seglen P. O., Seljelid R. Mass isolation and culture of rat kupffer cells. J Exp Med. 1975 Jan 1;141(1):1–10. doi: 10.1084/jem.141.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILSSON I. M., YAMASHINA I. Effect of human alpha 1-acid glycoprotein on blood coagulation. Nature. 1958 Mar 8;181(4610):711–712. doi: 10.1038/181711a0. [DOI] [PubMed] [Google Scholar]
- Occhino J. C., Glasgow A. H., Cooperband S. R., Mannick J. A., Schmid K. Isolation of an immunosuppressive peptide fraction from human plasma. J Immunol. 1973 Mar;110(3):685–694. [PubMed] [Google Scholar]
- Rudman D., Treadwell P. E., Vogler W. R., Howard C. H., Hollins B. An abnormal orosomucoid in the plasma of patients with neoplastic disease. Cancer Res. 1972 Sep;32(9):1951–1959. [PubMed] [Google Scholar]
- WEIMER H. E., MEHL J. W., WINZLER R. J. Studies on the mucoproteins of human plasma. V. Isolation and characterization of a homogeneous mucoprotein. J Biol Chem. 1950 Aug;185(2):561–568. [PubMed] [Google Scholar]
- WESTPHAL U., ASHLEY B. D., SELDEN G. L. Steroid-protein interactions. VII. Interactions of progesterone and corticosteroids with human plasma proteins determined by multiple equilibrium dialysis. Arch Biochem Biophys. 1961 Mar;92:441–448. doi: 10.1016/0003-9861(61)90383-6. [DOI] [PubMed] [Google Scholar]
- Yachnin S. Demonstration of the inhibitory effect of human alpha-fetoprotein on in vitro transformation of human lymphocytes. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2857–2861. doi: 10.1073/pnas.73.8.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachnin S. Fetuin, an inhibitor of lymphocyte transformation. The interaction of fetuin with phytomitogens and a possible role for fetuin in fetal development. J Exp Med. 1975 Jan 1;141(1):242–256. doi: 10.1084/jem.141.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]