Abstract
The present studies reinvestigate the role of the T cell in the development of resistance to Listeria monocytogenes. Doubly thymus-deprived adult thymectomized irradiated bone marrow-reconstituted mice (D-AT × BM) were prepared using anti-theta serum-treated bone marrow from AT × BM donors for reconstitution. The growth of the bacteria in spleens and livers of D-AT × BM was inhibited and such animals survived infection with doses which were lethal to normal mice. Since the D-AT × BM animals were T cell-depleted, as evidenced by (a) absence of theta-positive cells in their spleens; (b) inability to mount a primary humoral response to a T-dependent antigen and (c) failure to reject H-2 incompatible skin allografts, their antibacterial resistance was not due to the presence of a residual T-cell population. Further evidence of T-cell depletion in these animals was furnished by the findings that, despite their resistance to L. monocytogenes, they failed to exhibit a delayed hypersensitivity reaction to Listeria antigens, their splenocytes were unable to transfer resistance to naïve hosts and they did not develop an anamnestic response upon secondary challenge.
We concluded from these findings that primary antibacterial resistance to L. monocytogenes need not necessarily depend on the development of specific cell-mediated immunity although under normal circumstances these two processes develop in chronological association. The increased resistance of D-AT × BM animals is interpreted as being due to the enhancement of bactericidal activity of mononuclear phagocytes, possibly caused by the removal of a regulatory T-cell population. This population seems to be radiosensitive and spleen-seeking and requires an intact spleen to mediate its effect.
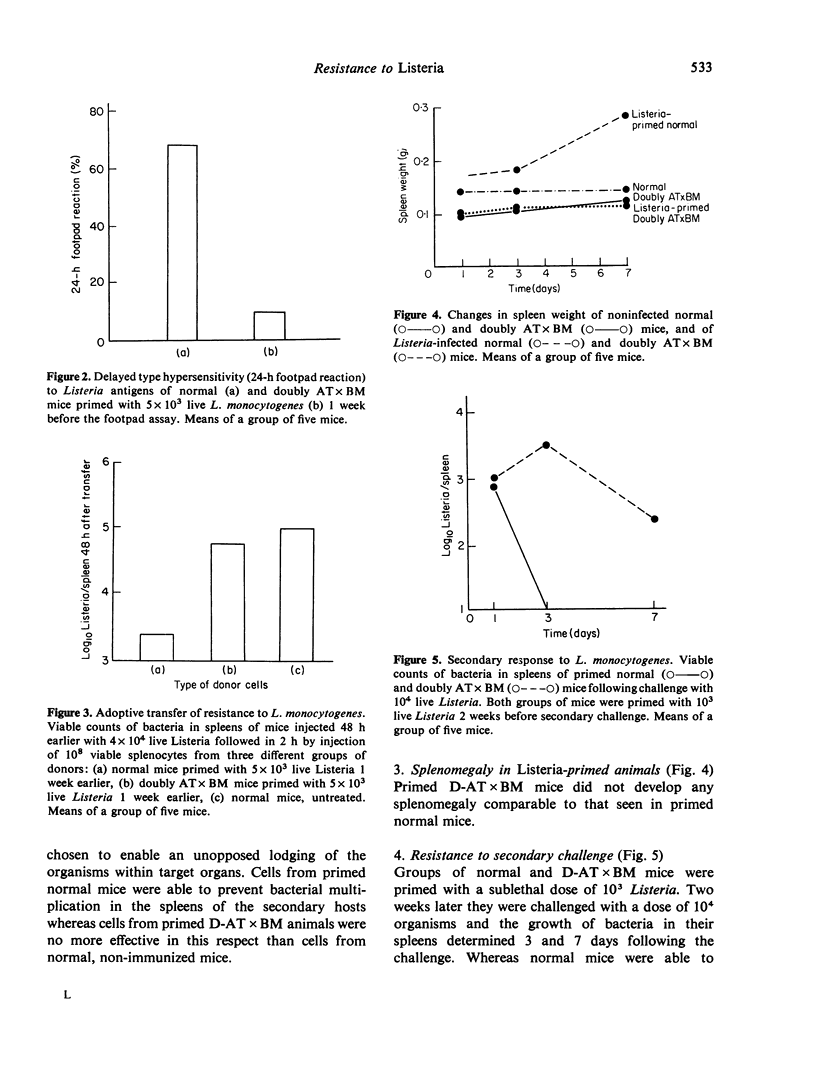
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanden R. V., Langman R. E. Cell-mediated immunity to bacterial infection in the mouse. Thymus-derived cells as effectors of acquired resistance to Listeria monocytogenes. Scand J Immunol. 1972;1(4):379–391. doi: 10.1111/j.1365-3083.1972.tb03304.x. [DOI] [PubMed] [Google Scholar]
- Campbell P. A., Martens B. L., Cooper H. R., McClatchy J. K. Requirement for bone marrow-derived cells in resistance to Listeria. J Immunol. 1974 Apr;112(4):1407–1414. [PubMed] [Google Scholar]
- Cheers C., Waller R. Activated macrophages in congenitally athymic "nude mice" and in lethally irradiate mice. J Immunol. 1975 Sep;115(3):844–847. [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Fauve R. M., Hevin B. Immunostimulation with bacterial phospholipid extracts. Proc Natl Acad Sci U S A. 1974 Feb;71(2):573–577. doi: 10.1073/pnas.71.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Lance E. M., Kondo K. Immuno-regulatory role of spleen localizing thymocytes. J Immunol. 1974 Feb;112(2):546–554. [PubMed] [Google Scholar]
- Halliburton B. L., Blazkovec A. A. Delayed hypersensitivity and acquired cellular resistance in guinea pigs infected with Listeria monocytogenes. Infect Immun. 1975 Jan;11(1):1–7. doi: 10.1128/iai.11.1.1-7.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. H. The allogeneic effect on immune responses: model for regulatory influences of T lymphocytes on the immune system. Transplant Rev. 1972;12:141–179. doi: 10.1111/j.1600-065x.1972.tb00055.x. [DOI] [PubMed] [Google Scholar]
- Kongshavn P. A., Lapp W. S. Immunosuppressive effect of male mouse submandibular gland extracts on plaque-forming cells in mice: abolition by orchiectomy. Immunology. 1972 Feb;22(2):227–230. [PMC free article] [PubMed] [Google Scholar]
- Lane F. C., Petit J. C., Gordon E., Unaue E. R. Increased bactericidal activity by non-specific stimulation of lymphoid cells--experiments in vivo with concanavalin A. Clin Immunol Immunopathol. 1973 Nov;2(1):74–81. doi: 10.1016/0090-1229(73)90038-x. [DOI] [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B., Hill W. C. The effect of anti-lymphocyte globulin on cell-mediated reistance to infection. J Exp Med. 1969 May 1;129(5):993–1012. doi: 10.1084/jem.129.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marbrook J. Primary immune response in cultures of spleen cells. Lancet. 1967 Dec 16;2(7529):1279–1281. doi: 10.1016/s0140-6736(67)90393-5. [DOI] [PubMed] [Google Scholar]
- Medina S., Vas S. I., Robson H. G. Effect of nonspecific stimulation on the defense mechanisms of inbred mice. J Immunol. 1975 Jun;114(6):1720–1725. [PubMed] [Google Scholar]
- North R. J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973 Apr;7(1):166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- Osebold J. W., Pearson L. D., Medin N. I. Relationship of antimicrobial cellular immunity to delayed hypersensitivity in Listeriosis. Infect Immun. 1974 Feb;9(2):354–362. doi: 10.1128/iai.9.2.354-362.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjpe M. S., Boone C. W. Kinetics of the anti-tumor delayed hypersensitivity response in mice with progressively growing tumors: stimulation followed by specific suppression. Int J Cancer. 1974 Feb 15;13(2):179–186. doi: 10.1002/ijc.2910130205. [DOI] [PubMed] [Google Scholar]
- Prabhakaran K., Harris E. B., Kirchheimer W. F. Hairless mice, human leprosy and thymus-derived-lymphocytes. Experientia. 1975 Jul 15;31(7):784–785. doi: 10.1007/BF01938464. [DOI] [PubMed] [Google Scholar]
- Raff M. Theta isoantigen as a marker of thymus-derived lymphocytes in mice. Nature. 1969 Oct 25;224(5217):378–379. doi: 10.1038/224378a0. [DOI] [PubMed] [Google Scholar]
- Schlesinger M., Hurvitz D. Serological analysis of thymus and spleen grafts. J Exp Med. 1968 Jun 1;127(6):1127–1137. doi: 10.1084/jem.127.6.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya K., Mori R., Imaizumi N. Suppressed multiplication of Listeria monocytogenes within macrophages derived from thymectomized mice. Nature. 1968 Jun 22;218(5147):1174–1174. doi: 10.1038/2181174a0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Blanden R. V. Macrophage activation in mice lacking thymus-derived (T) cells. Experientia. 1975 May 15;31(5):591–593. doi: 10.1007/BF01932477. [DOI] [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968 Sep 1;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]


