Abstract
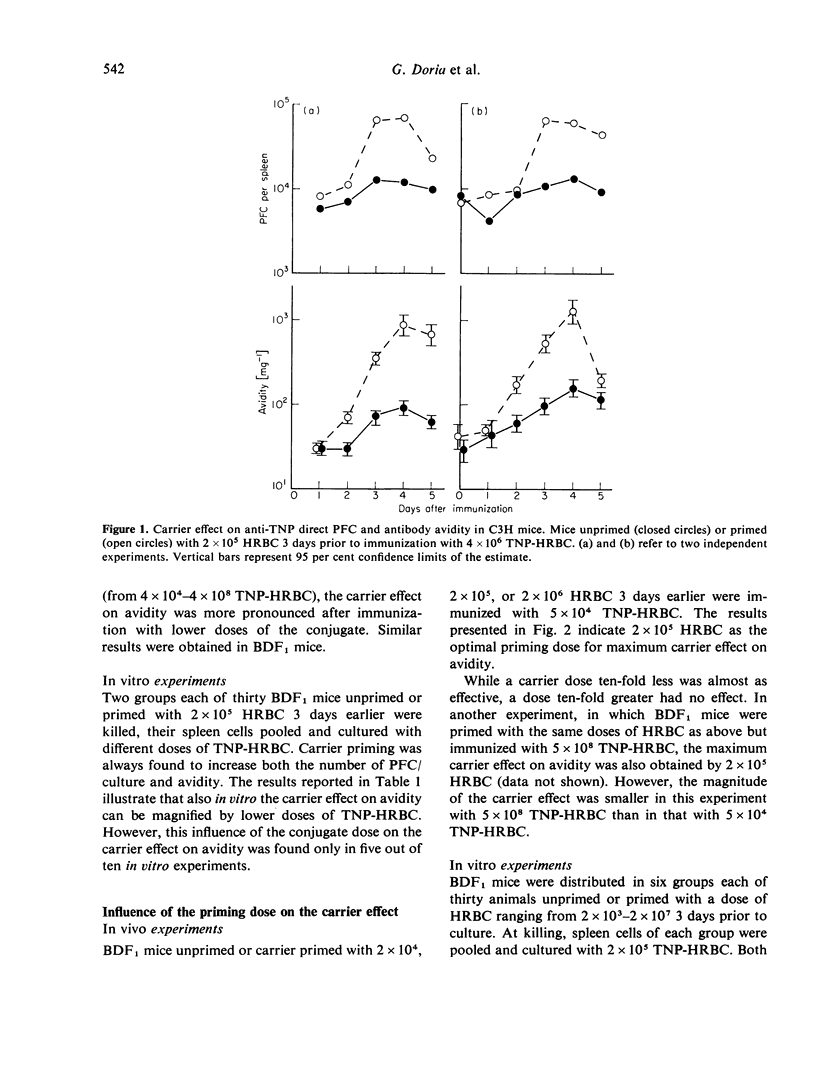
The effect of carrier priming on antibody avidity was investigated under several experimental conditions. Basically, mice were carrier primed with HRBC (horse red blood cells) prior to immunization with TNP (2,4,6-trinitrophenyl) conjugated to HRBC. Immunization was performed either in vivo or in spleen cell culture, and avidity of anti-TNP antibodies was estimated from inhibition of direct PFC (plaque-forming cells) by free TNP-BSA (-bovine serum albumin).
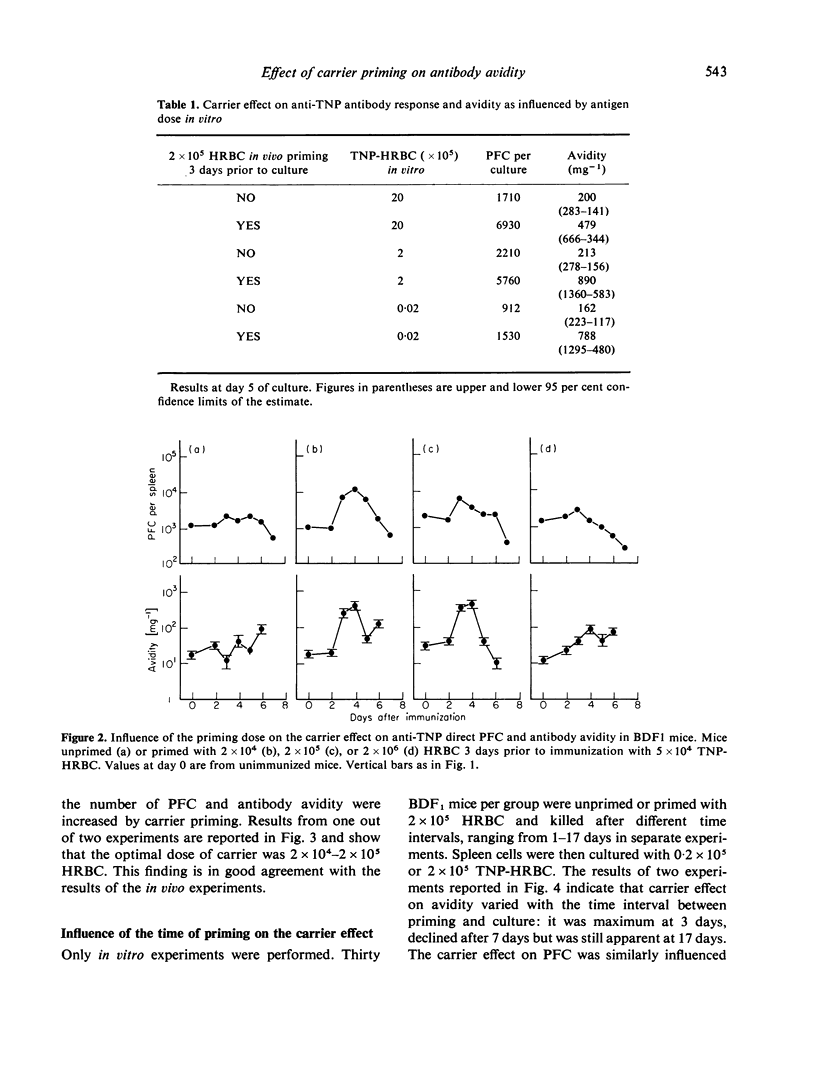
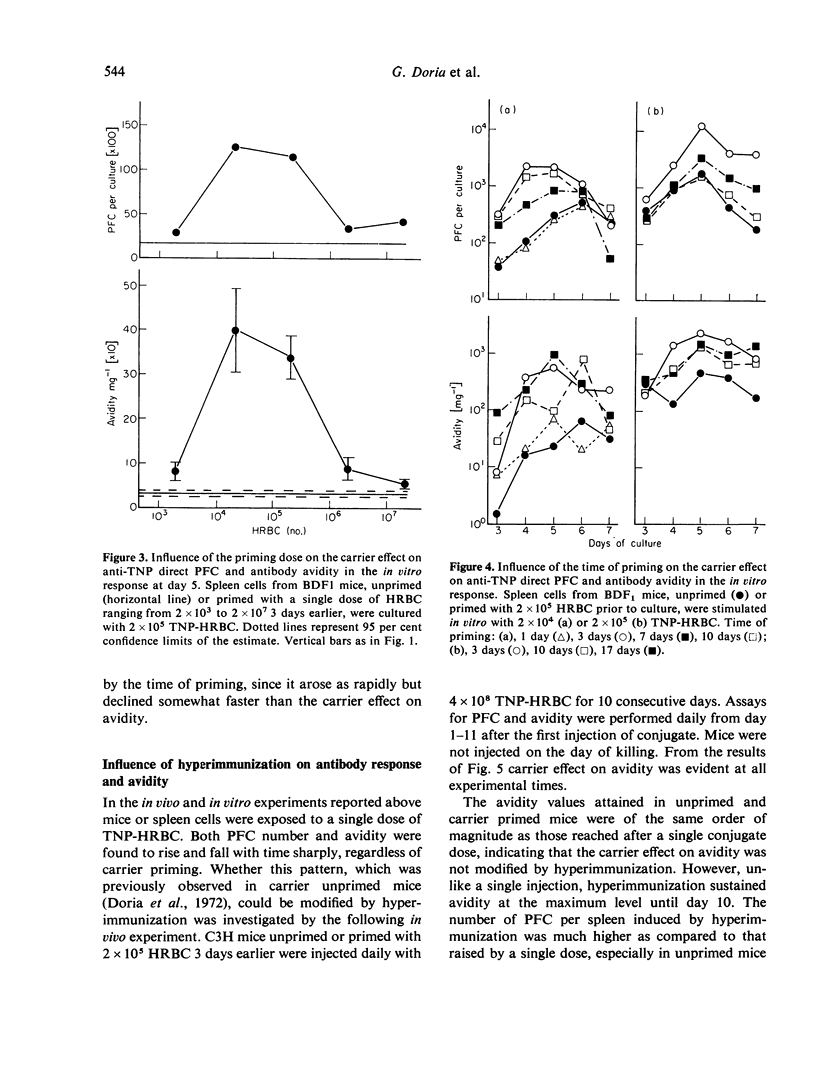
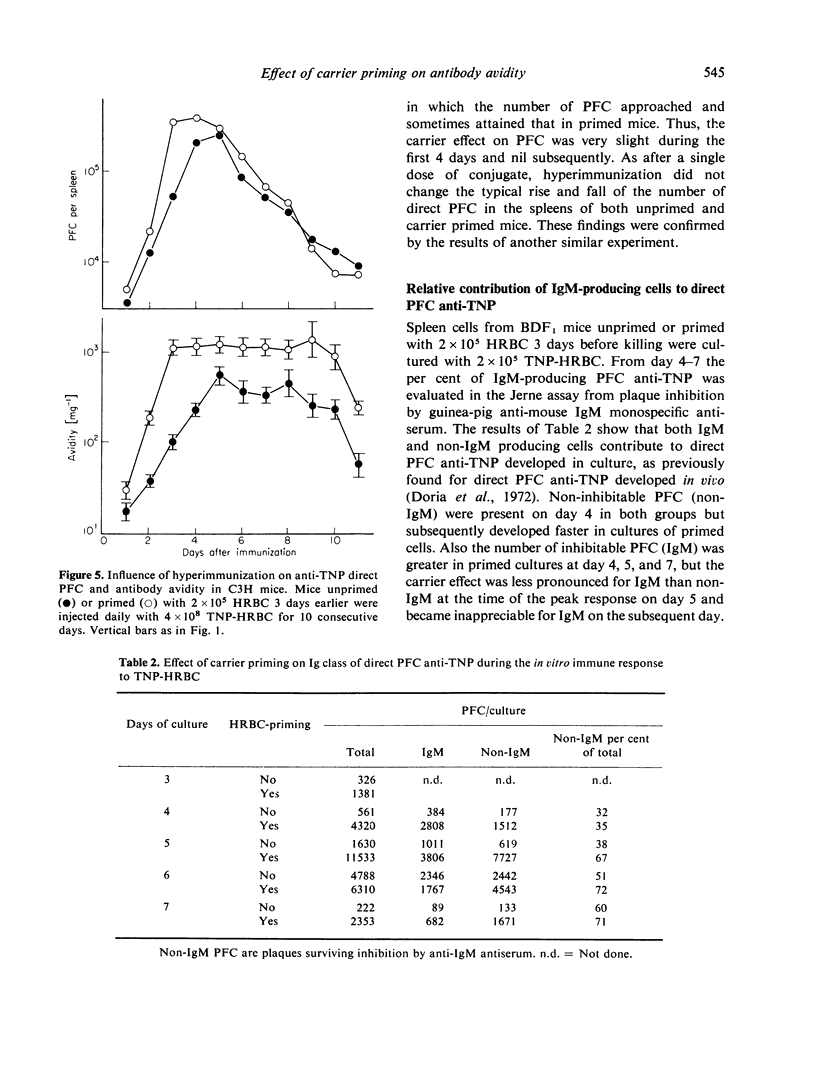
The data indicate the appropriate conditions under which carrier priming can enhance antibody avidity. The carrier effect is maximized by priming the animals with 104-105 HRBC 3-7 days before immunization with a low dose of TNP-HRBC. Hyper-immunization by repeated injections of a high dose of the conjugate does not modify the carrier effect on avidity but it delays the fall of avidity in both carrier primed and unprimed animals. These results are interpreted in terms of T- and B-cell co-operation within the framework of the maturation theory of antibody affinity.
Carrier priming was also found to increase the number of direct PFC of the IgM and, mostly, of the non-IgM classes, a finding in agreement with the notion that T cells can help IgM production and the shift to IgG.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aird J. Thymus dependence of the immune response: response to the haptenic determinant NIP in mice. Immunology. 1971 Apr;20(4):617–624. [PMC free article] [PubMed] [Google Scholar]
- Andersson B. Studies on the regulation of avidity at the level of the single antibody-forming cell. The effect of antigen dose and time after immunization. J Exp Med. 1970 Jul 1;132(1):77–88. doi: 10.1084/jem.132.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson B., Wigzell H. Studies on antibody avidity at the cellular level. Effects of immunological paralysis and administered antibody. Eur J Immunol. 1971 Nov;1(5):384–390. doi: 10.1002/eji.1830010516. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Barth R. F., Stashak P. W., Amsbaugh D. F. Enhancement of the antibody response to type 3 pneumococcal polysaccharide in mice treated with antilymphocyte serum. J Immunol. 1970 May;104(5):1313–1315. [PubMed] [Google Scholar]
- Britton S., Möller G. Regulation of antibody synthesis against Escherichia coli endotoxin. I. Suppressive effect of endogenously produced and passively transferred antibodies. J Immunol. 1968 Jun;100(6):1326–1334. [PubMed] [Google Scholar]
- Doria G., Agarossi G., Di Pietro S. Enhancing activity of thymocyte culture cell-free medium on the in vitro immune response of spleen cells from neonatally thymectomized mice to sheep RBC. J Immunol. 1972 Jan;108(1):268–270. [PubMed] [Google Scholar]
- Doria G., Schiaffini G., Garavini M., Mancini C. The rise and fall of antibody avidity at the level of single immunocytes. J Immunol. 1972 Dec;109(6):1245–1253. [PubMed] [Google Scholar]
- Falkoff R., Kettman J. Differential stimulation of precursor cells and carrier-specific thymus-derived cell activity in the in vivo reponse to heterologous erythrocytes in mice. J Immunol. 1972 Jan;108(1):54–58. [PubMed] [Google Scholar]
- Feldmann M., Nossal G. J. Tolerance, enhancement and the regulation of interactions between T cells, B cells and macrophages. Transplant Rev. 1972;13:3–34. doi: 10.1111/j.1600-065x.1972.tb00058.x. [DOI] [PubMed] [Google Scholar]
- Gershon R. K., Paul W. E. Effect of thymus-derived lymphocytes on amount and affinity of anti-hapten antibody. J Immunol. 1971 Mar;106(3):872–874. [PubMed] [Google Scholar]
- Hurme M., Kontiainen S., Seppälä I. J., Mäkelä O. Affinity and Ig classes of anti-hapten antibodies in carrier-preimmunized rats. Eur J Immunol. 1973 Apr;3(4):191–195. doi: 10.1002/eji.1830030403. [DOI] [PubMed] [Google Scholar]
- Jerne N. K., Nordin A. A. Plaque Formation in Agar by Single Antibody-Producing Cells. Science. 1963 Apr 26;140(3565):405–405. doi: 10.1126/science.140.3565.405. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Benacerraf B. The regulatory influence of activated T cells on B cell responses to antigen. Adv Immunol. 1972;15:1–94. doi: 10.1016/s0065-2776(08)60683-5. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Paul W. E., Goidl E. A., Benacerraf B. Carrier function in anti-hapten antibody responses. 3. Stimulation of antibody synthesis and facilitation of hapten-specific secondary antibody responses by graft-versus-host reactions. J Exp Med. 1971 Feb 1;133(2):169–186. doi: 10.1084/jem.133.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettman J., Dutton R. W. An in vitro primary immune response to 2,4,6-trinitrophenyl substituted erythrocytes: response against carrier and hapten. J Immunol. 1970 Jun;104(6):1558–1561. [PubMed] [Google Scholar]
- Kontiainen S. The effect of carrier-primed cells on an adoptive anti-hapten response. Eur J Immunol. 1971 Aug;1(4):276–280. doi: 10.1002/eji.1830010412. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Basten A., Sprent J., Cheers C. Interaction between lymphocytes in immune responses. Cell Immunol. 1971 Oct;2(5):469–495. doi: 10.1016/0008-8749(71)90057-8. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Grumet F. C., McDevitt H. O. Genetic control of the immune response. The effect of thymectomy on the primary and secondary antibody response of mice to poly-L(tyr, glu)-poly-D, L-ala--poly-L-lys. J Exp Med. 1972 Jan;135(1):126–135. doi: 10.1084/jem.135.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison N. A. The carrier effect in the secondary response to hapten-protein conjugates. I. Measurement of the effect with transferred cells and objections to the local environment hypothesis. Eur J Immunol. 1971 Jan;1(1):10–17. doi: 10.1002/eji.1830010103. [DOI] [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Siskind G. W., Benacerraf B. Cell selection by antigen in the immune response. Adv Immunol. 1969;10:1–50. doi: 10.1016/s0065-2776(08)60414-9. [DOI] [PubMed] [Google Scholar]
- Siskind G. W., Dunn P., Walker J. G. Studies on the control of antibody synthesis. II. Effect of antigen dose and of suppression by passive antibody on the affinity of antibody synthesized. J Exp Med. 1968 Jan 1;127(1):55–66. doi: 10.1084/jem.127.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J., Miller J. F., Mitchell G. F. Antigen-induced selective recruitment of circulating lymphocytes. Cell Immunol. 1971 Apr;2(2):171–181. doi: 10.1016/0008-8749(71)90036-0. [DOI] [PubMed] [Google Scholar]
- Tada T., Taniguchi M., Takemori T. Properties of primed suppressor T cells and their products. Transplant Rev. 1975;26:106–129. doi: 10.1111/j.1600-065x.1975.tb00177.x. [DOI] [PubMed] [Google Scholar]
- Takemori T., Tada T. Selective roles of thymus-derived lymphocytes in the antibody response. II. Preferential suppression of high-affinity antibody-forming cells by carrier-primed suppressor T cells. J Exp Med. 1974 Jul 1;140(1):253–266. doi: 10.1084/jem.140.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M., Tada T. Dual regulatory role of the thymus in the maturation of immune response in the rabbit. J Exp Med. 1974 Jan 1;139(1):108–127. doi: 10.1084/jem.139.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbain J., Van Acker A., De Vos-Cloetens C., Urbain-Vansanten G. Increase and decrease in binding affinity of antibodies during the immune response. Immunochemistry. 1972 Feb;9(2):121–136. doi: 10.1016/0019-2791(72)90033-x. [DOI] [PubMed] [Google Scholar]
- Werblin T. P., Kim Y. T., Quagliata F., Siskind G. W. Studies on the control of antibody synthesis. 3. Changes in heterogeneity of antibody affinity during the course of the immune response. Immunology. 1973 Mar;24(3):477–492. [PMC free article] [PubMed] [Google Scholar]


