Abstract
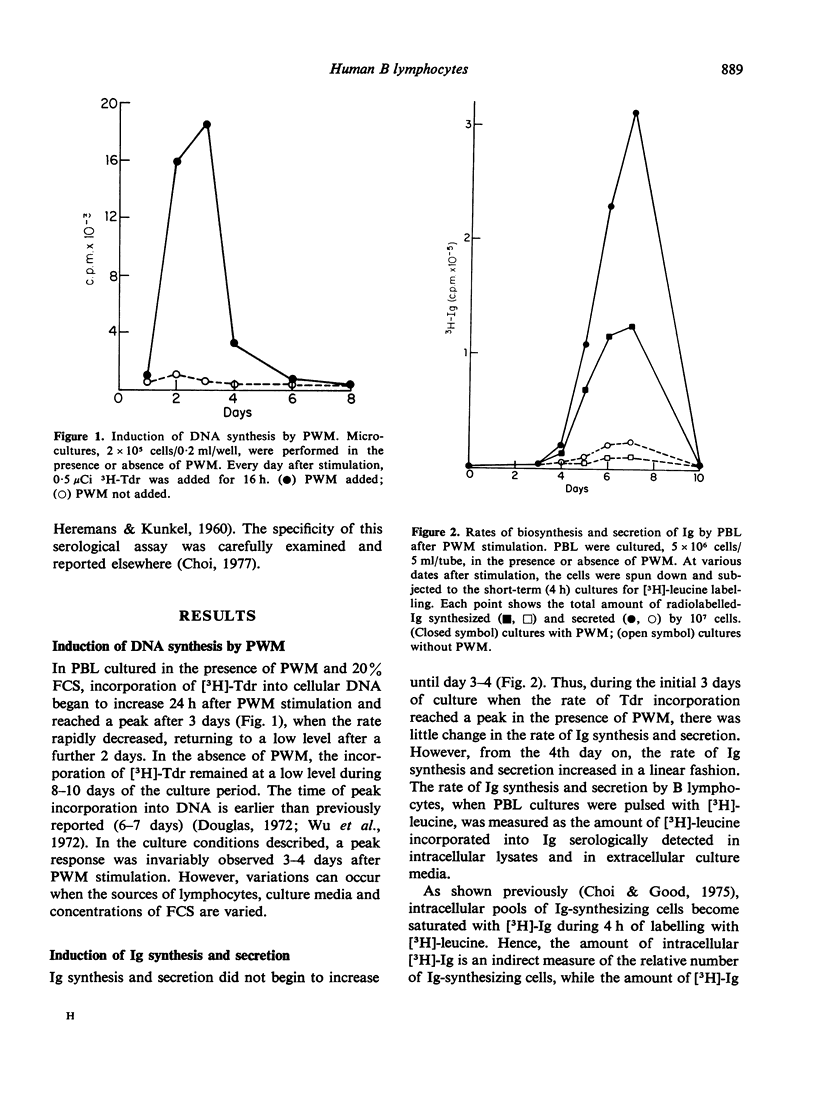
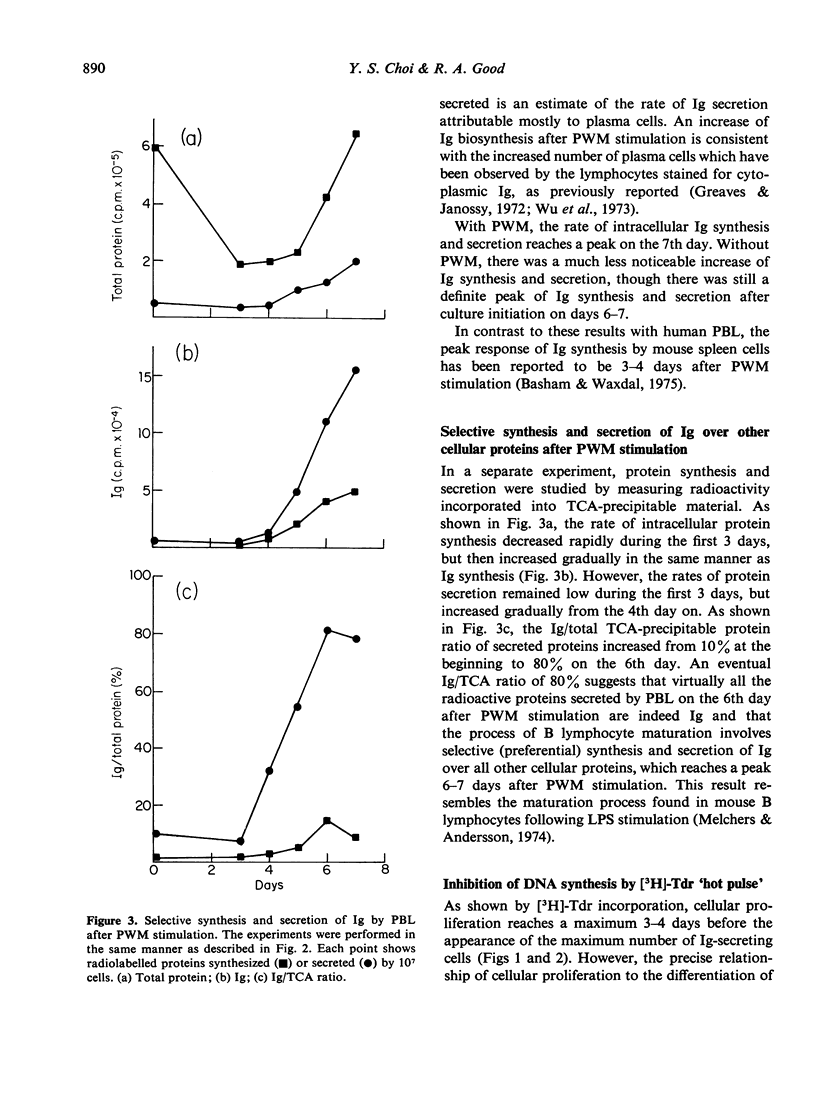
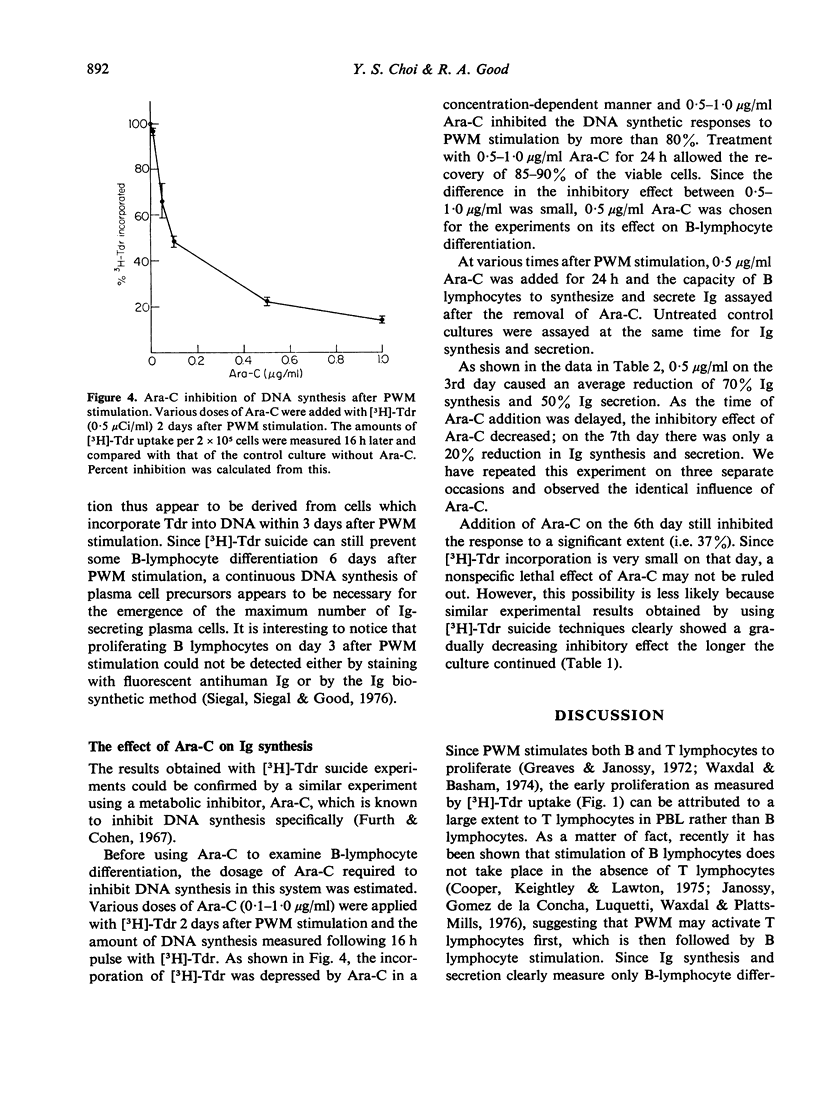
Human B-lymphocyte differentiation was studied by measuring the capacity of such cells, isolated from peripheral blood, to synthesize and secrete Ig after pokeweed stimulation. Results show that a maximum incorporation of [3H]-thymidine took place 2 days before the appearance of detectable Ig-secreting cells. On the 7th day after pokeweed stimulation, when Ig synthesis and secretion are at a maximum, [3H]-thymidine uptake was low. Since inhibition of DNA synthesis 3 days after pokeweed stimulation completely prevents the generation of Ig-secreting plasma cells, initial DNA synthesis is apparently essential before Ig-secreting plasma cells can develop in response to pokeweed stimulation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askonas B. A., Roelants G. E., Mayor-Withey K. S., Welstead J. L. Dual pathway of B lymphocyte differentiation in vitro. Eur J Immunol. 1976 Apr;6(4):250–256. doi: 10.1002/eji.1830060404. [DOI] [PubMed] [Google Scholar]
- Basham T. Y., Waxdal M. J. The stimulation of immunoglobulin production in murine spleen cells by the pokeweed mitogens. J Immunol. 1975 Feb;114(2 Pt 1):715–716. [PubMed] [Google Scholar]
- Chessin L. N., Börjeson J., Welsh P. D., Douglas S. D., Cooper H. L. Studies on human peripheral blood lymphocytes in vitro. II. Morphological and biochemical studies on the transformation of lymphocytes by pokeweed mitogen. J Exp Med. 1966 Nov 1;124(5):873–884. doi: 10.1084/jem.124.5.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. S., Biggar W. D., Good R. A. Biosynthesis and secretion of immunoglobulins by peripheral-blood lymphocytes in severe hypogammaglobulinaemia. Lancet. 1972 May 27;1(7761):1149–1152. doi: 10.1016/s0140-6736(72)91374-8. [DOI] [PubMed] [Google Scholar]
- Choi Y. S., Knopf P. M., Lennox E. S. Subcellular fractionation of mouse myeloma cells. Biochemistry. 1971 Feb 16;10(4):659–667. doi: 10.1021/bi00780a018. [DOI] [PubMed] [Google Scholar]
- Douglas S. D. Electron microscopic and functional aspects of human lymphocyte response to mitogens. Transplant Rev. 1972;11:39–59. doi: 10.1111/j.1600-065x.1972.tb00045.x. [DOI] [PubMed] [Google Scholar]
- Dutton R. W., Mishell R. I. Cell populations and cell proliferation in the in vitro response of normal mouse spleen to heterologous erythrocytes. Analysis by the hot pulse technique. J Exp Med. 1967 Sep 1;126(3):443–454. doi: 10.1084/jem.126.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELMAN G. M., HEREMANS J. F., HEREMANS M. T., KUNKEL H. G. Immunological studies of human gamma-globulin. Relation of the precipitin lines of whole gamma-globulin to those of the fragments produced by papain. J Exp Med. 1960 Jul 1;112:203–223. doi: 10.1084/jem.112.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth J. J., Cohen S. S. Inhibition of mammalian DNA polymerase by the 5'-triphosphate of 9-beta-D-arabinofuranosyladenine. Cancer Res. 1967 Sep;27(9):1528–1533. [PubMed] [Google Scholar]
- Greaves M., Janossy G. Elicitation of selective T and B lymphocyte responses by cell surface binding ligands. Transplant Rev. 1972;11:87–130. doi: 10.1111/j.1600-065x.1972.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Janossy G., Gomez De La Concha E., Waxdal M. J., Platts-Mills T. The effects of purified mitogenic proteins (Pa-1 and Pa-2) from pokeweed on human T and B lymphocytes in vitro. Clin Exp Immunol. 1976 Oct;26(1):108–117. [PMC free article] [PubMed] [Google Scholar]
- Melchers F., Andersson J. Synthesis, surface deposition and secretion of immunoglobulin M in bone marrow-derived lymphocytes before and after mitogenic stimulation. Transplant Rev. 1973;14:76–130. doi: 10.1111/j.1600-065x.1973.tb00103.x. [DOI] [PubMed] [Google Scholar]
- Melchers F., Andersson J. The kinectics of proliferation and maturation of mitogen-activated bone marrow-derived lymphocytes. Eur J Immunol. 1974 Oct;4(10):687–691. doi: 10.1002/eji.1830041010. [DOI] [PubMed] [Google Scholar]
- Siegal F. P., Siegal M., Good R. A. Suppression of B-cell differentiation by leukocytes from hypogammaglobulinemic patients. J Clin Invest. 1976 Jul;58(1):109–122. doi: 10.1172/JCI108439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Durm M., Broder S., Blackman M., Blaese R. M., Strober W. Role of suppressor T cells in pathogenesis of common variable hypogammaglobulinaemia. Lancet. 1974 Sep 14;2(7881):609–613. doi: 10.1016/s0140-6736(74)91940-0. [DOI] [PubMed] [Google Scholar]
- Waxdal M. J., Basham T. Y. B and T-cell stimulatory activities of multiple mitogens from pokeweed. Nature. 1974 Sep 13;251(5471):163–164. doi: 10.1038/251163a0. [DOI] [PubMed] [Google Scholar]
- Wu L. Y., Lawton A. R., Cooper M. D. Differentiation capacity of cultured B lymphocytes from immunodeficient patients. J Clin Invest. 1973 Dec;52(12):3180–3189. doi: 10.1172/JCI107518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XEROS N. Deoxyriboside control and synchronization of mitosis. Nature. 1962 May 19;194:682–683. doi: 10.1038/194682a0. [DOI] [PubMed] [Google Scholar]


