Abstract
In an effort to identify factors important in the pathogenesis of filarial disease, studies of the cellular and humoral immune responses to the parasitic nematode Wuchereria bancrofti were carried out on thirty-nine individuals in a region of the Pacific where filariasis is endemic.
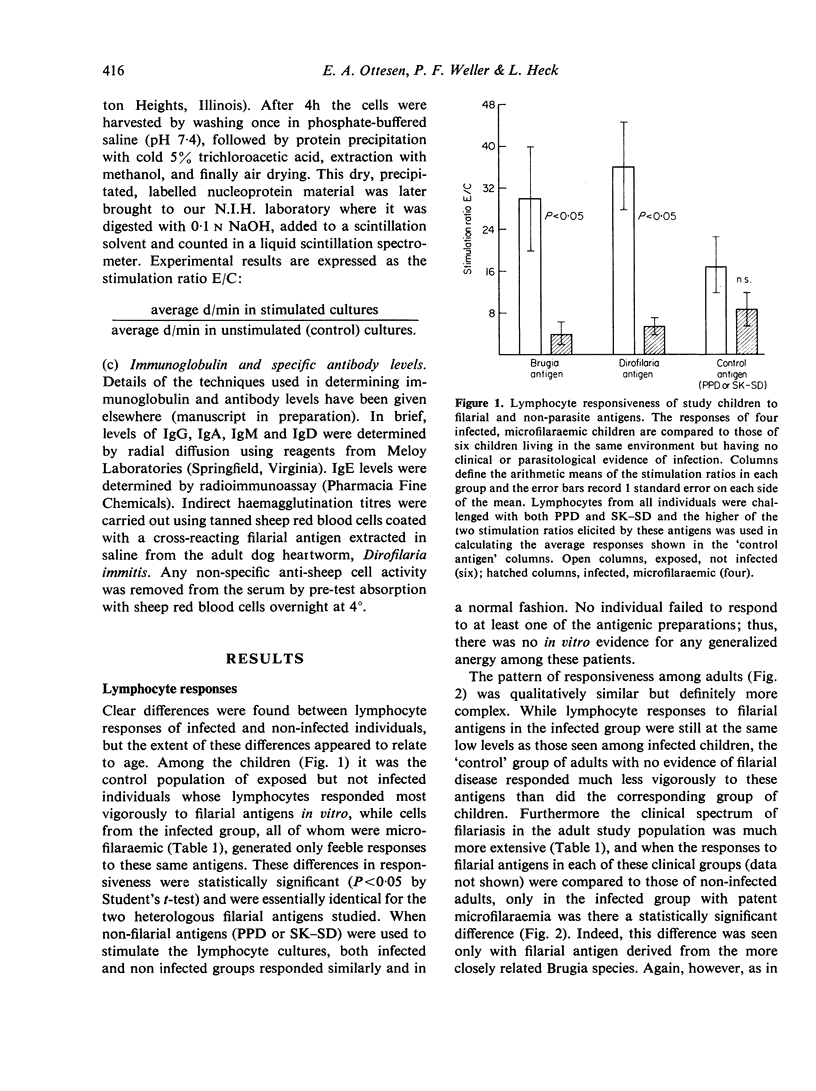
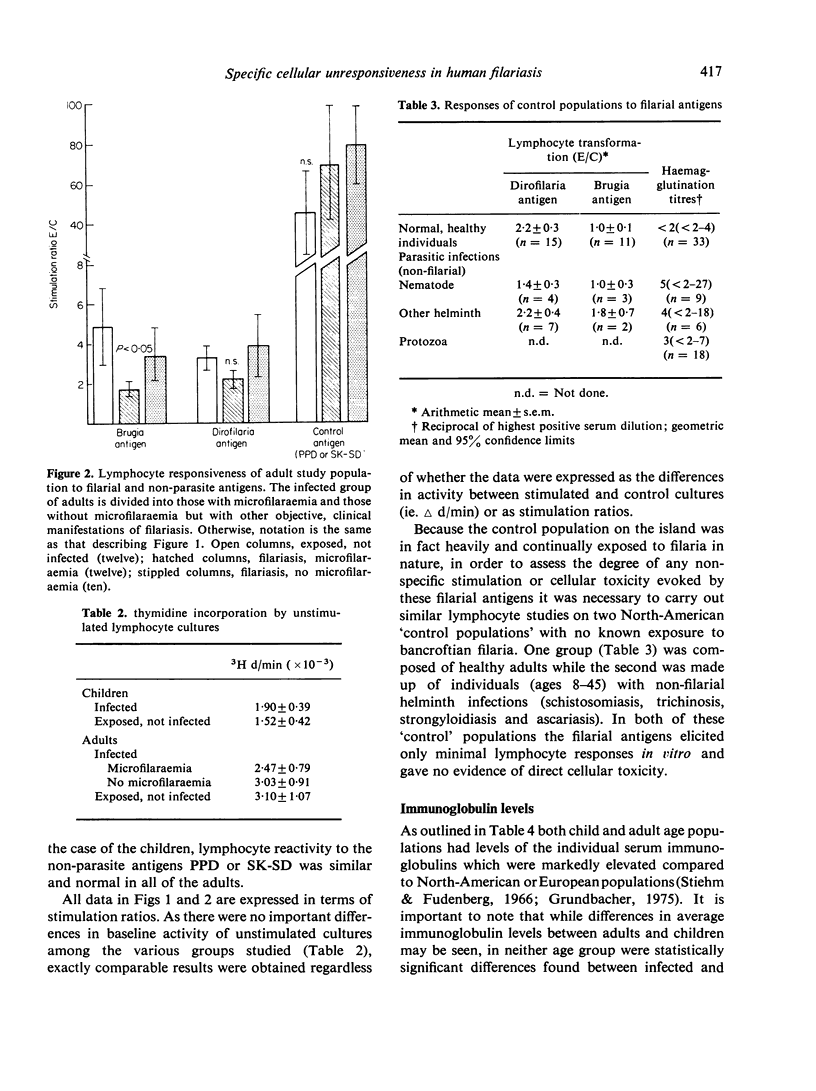
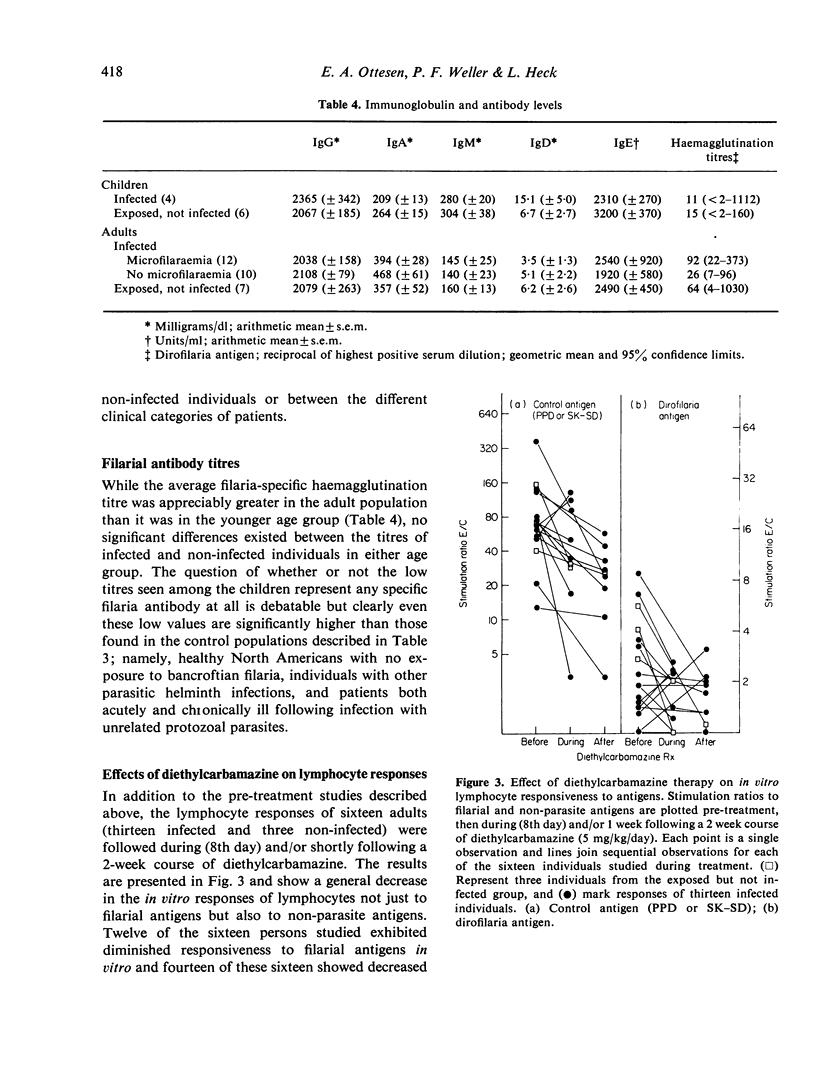
Blood lymphocytes from twenty-six patients with chronic filariasis (especially that associated with persistent microfilaraemia) gave only minimal responses to filarial antigens when challenged in vitro in a lymphocyte transformation assay. In contrast, brisk responses to these same antigens were elicited in cultures of cells from a control population of thirteen exposed but not infected individuals living in the same area, the greatest responses being found in uninfected children less than 10 years old. The poor cellular responsiveness of infected individuals was filaria antigen-specific, as reactivity to tuberculin (PPD) and streptococcal (SK—SD) antigens was normal and equal in all groups. Furthermore, the immunologic deficit was limited to cell-mediated immune responses and appeared unchanged 2 weeks after treatment of the patients with the anti-filarial drug diethylcarbamazine.
We interpret our findings as evidence for a state of antigen-specific cellular unresponsiveness in patients with this chronic parasitic infection and speculate that this immunologic deficit may be of fundamental importance in the pathogenesis of filarial disease.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dennis D. T., McConnell E., White G. B. Bancroftian filariasis and membrane filters: are night surveys necessary? Am J Trop Med Hyg. 1976 Mar;25(2):257–262. doi: 10.4269/ajtmh.1976.25.257. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Disseminated cryptococcosis in man: decreased lymphocyte transformation in response to Cryptococcus neoformans. J Infect Dis. 1973 Jun;127(6):694–697. doi: 10.1093/infdis/127.6.694. [DOI] [PubMed] [Google Scholar]
- Finkel A., Dent P. B. Abnormalities in lymphocyte proliferation in classical and atypical measles infection. Cell Immunol. 1973 Jan;6(1):41–48. doi: 10.1016/0008-8749(73)90004-x. [DOI] [PubMed] [Google Scholar]
- Godal T., Myklestad B., Samuel D. R., Myrvang B. Characterization of the cellular immune defect in lepromatous leprosy: a specific lack of circulating Mycobacterium leprae-reactive lymphocytes. Clin Exp Immunol. 1971 Dec;9(6):821–831. [PMC free article] [PubMed] [Google Scholar]
- Goldstein R. A., Ang U. H., Foellmer J. W., Janicki B. W. Rifampin and cell-mediated immune responses in tuberculosis. Am Rev Respir Dis. 1976 Feb;113(2):197–202. doi: 10.1164/arrd.1976.113.2.197. [DOI] [PubMed] [Google Scholar]
- Grundbacher F. J. Causes of variation in serum IgE levels in normal populations. J Allergy Clin Immunol. 1975 Aug;56(2):104–111. doi: 10.1016/0091-6749(75)90114-1. [DOI] [PubMed] [Google Scholar]
- Jones T. C., Mott K., Pedrosa L. C. A technique for isolating and concentrating microfilariae from peripheral blood by gradient centrifugation. Trans R Soc Trop Med Hyg. 1975;69(2):243–246. doi: 10.1016/0035-9203(75)90162-5. [DOI] [PubMed] [Google Scholar]
- KAGAN I. G. A REVIEW OF IMMUNOLOGIC METHODS FOR THE DIAGNOSIS OF FILARIASIS. J Parasitol. 1963 Oct;49:773–798. [PubMed] [Google Scholar]
- Kirkpatrick C. H., Rich R. R., Bennett J. E. Chronic mucocutaneous candidiasis: model-building in cellular immunity. Ann Intern Med. 1971 Jun;74(6):955–978. doi: 10.7326/0003-4819-74-6-955. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Murgita R. A., Tomasi T. B., Jr Suppression of the immune response by alpha-fetoprotein on the primary and secondary antibody response. J Exp Med. 1975 Feb 1;141(2):269–286. doi: 10.1084/jem.141.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neva F. A., Kaplan A. P., Pacheco G., Gray L., Danaraj T. J. Tropical eosinophilia. A human model of parasitic immunopathology, with observations on serum IgE levels before and after treatment. J Allergy Clin Immunol. 1975 Jun;55(6):422–429. doi: 10.1016/0091-6749(75)90081-0. [DOI] [PubMed] [Google Scholar]
- Pacheco G., Danaraj T. J. Indirect hemagglutination with extracts of various helminths in eosinophilic lung (tropical eosinophilia). Am J Trop Med Hyg. 1966 May;15(3):355–358. doi: 10.4269/ajtmh.1966.15.355. [DOI] [PubMed] [Google Scholar]
- RIDLEY D. S. The complement-fixation test in filariasis. Trans R Soc Trop Med Hyg. 1956 May;50(3):255–257. doi: 10.1016/0035-9203(56)90032-3. [DOI] [PubMed] [Google Scholar]
- Stiehm E. R., Fudenberg H. H. Serum levels of immune globulins in health and disease: a survey. Pediatrics. 1966 May;37(5):715–727. [PubMed] [Google Scholar]
- Stobo J. D., Paul S., Van Scoy R. E., Hermans P. E. Suppressor thymus-derived lymphocytes in fungal infection. J Clin Invest. 1976 Feb;57(2):319–328. doi: 10.1172/JCI108283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagnerová M., Stafpvá J., Wagner V., Vacek V. Transformation of lymphocytes in patients with certain bacterial infections. Med Microbiol Immunol. 1975 Sep 19;161(4):273–277. doi: 10.1007/BF02122715. [DOI] [PubMed] [Google Scholar]
- Webster L. T., Jr, Butterworth A. E., Mahmoud A. A., Mngola E. N., Warren K. S. Suppression of delayed hypersensitivity in schistosome-infected patients by niridazole. N Engl J Med. 1975 May 29;292(22):1144–1147. doi: 10.1056/NEJM197505292922202. [DOI] [PubMed] [Google Scholar]


