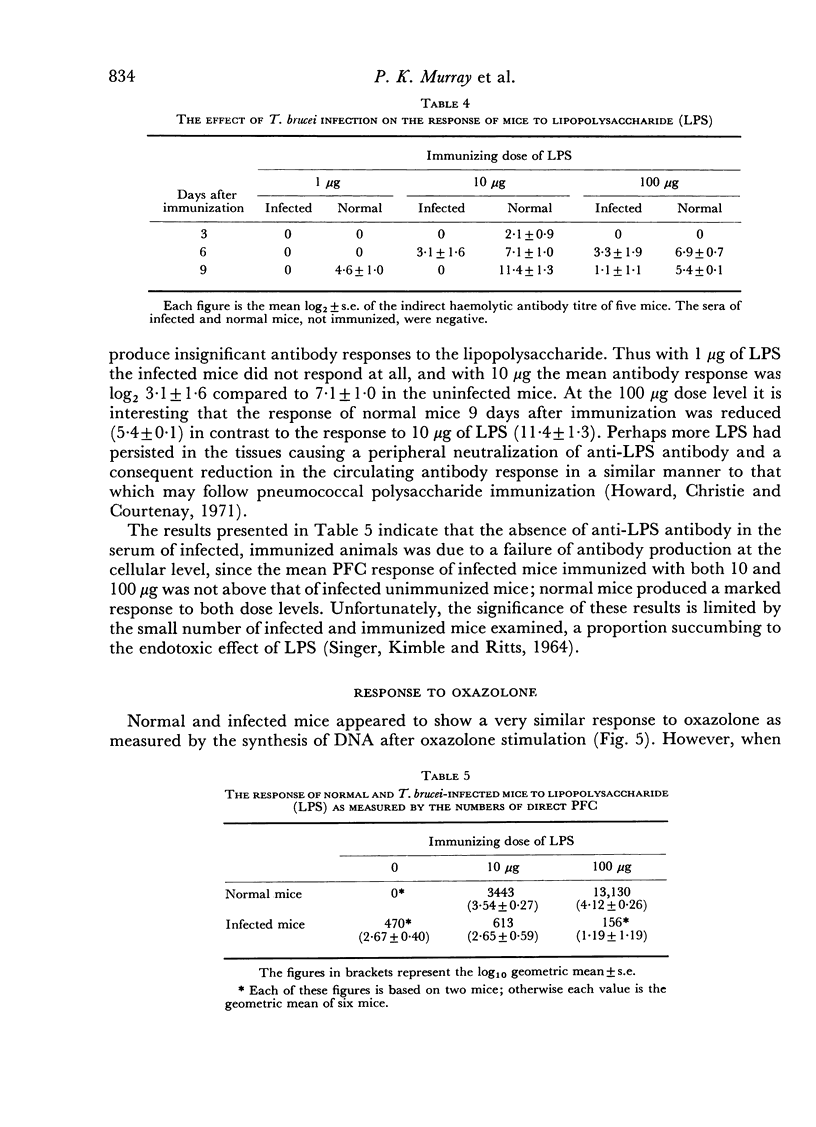
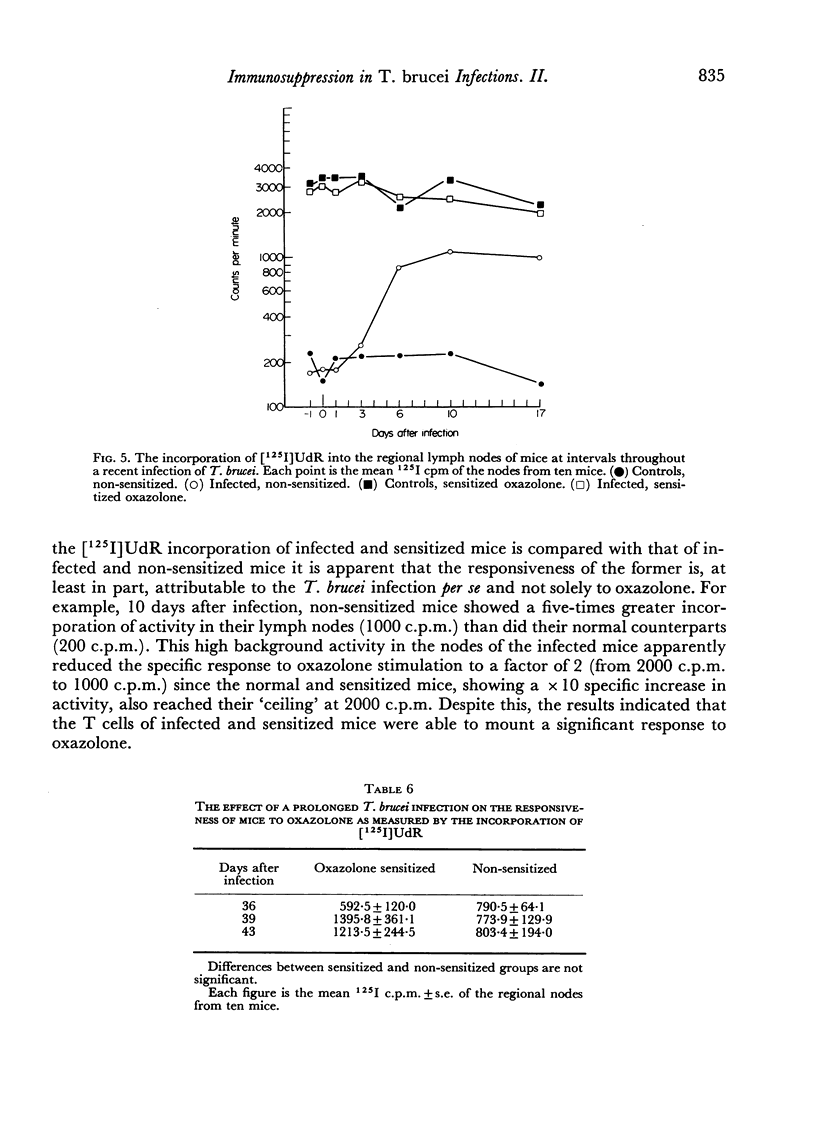
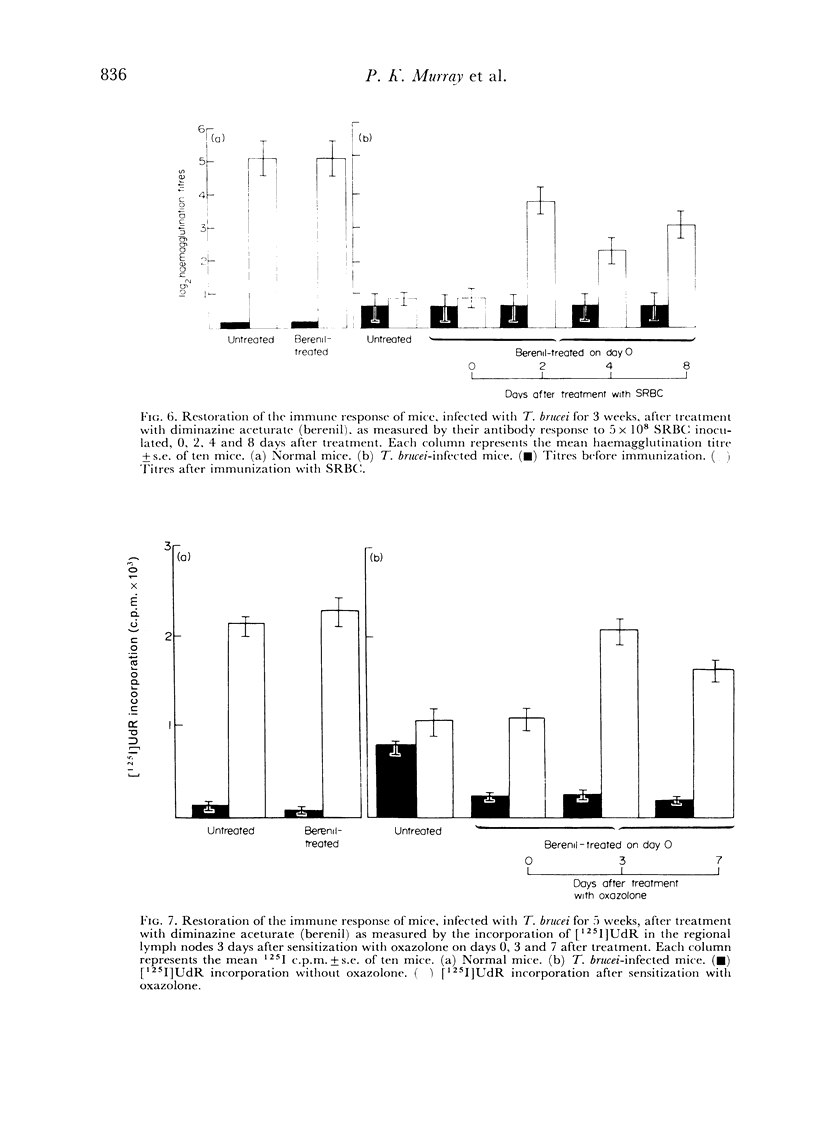
Abstract
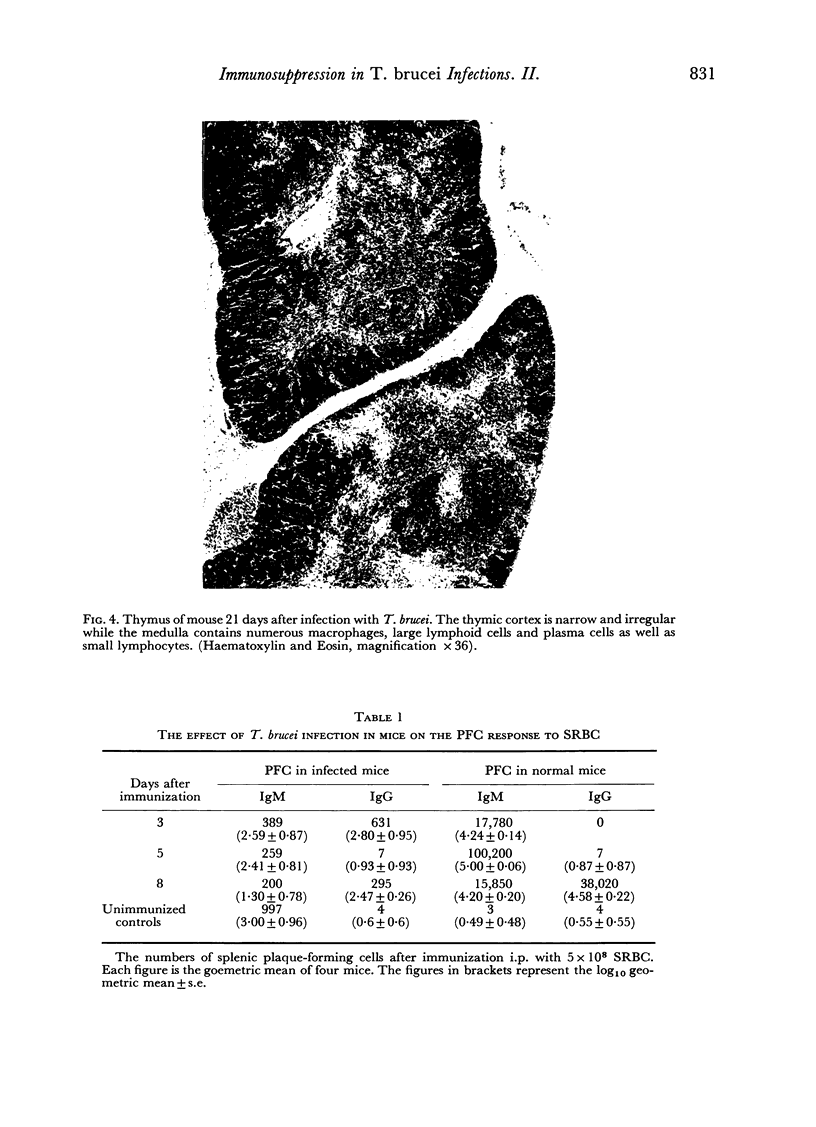
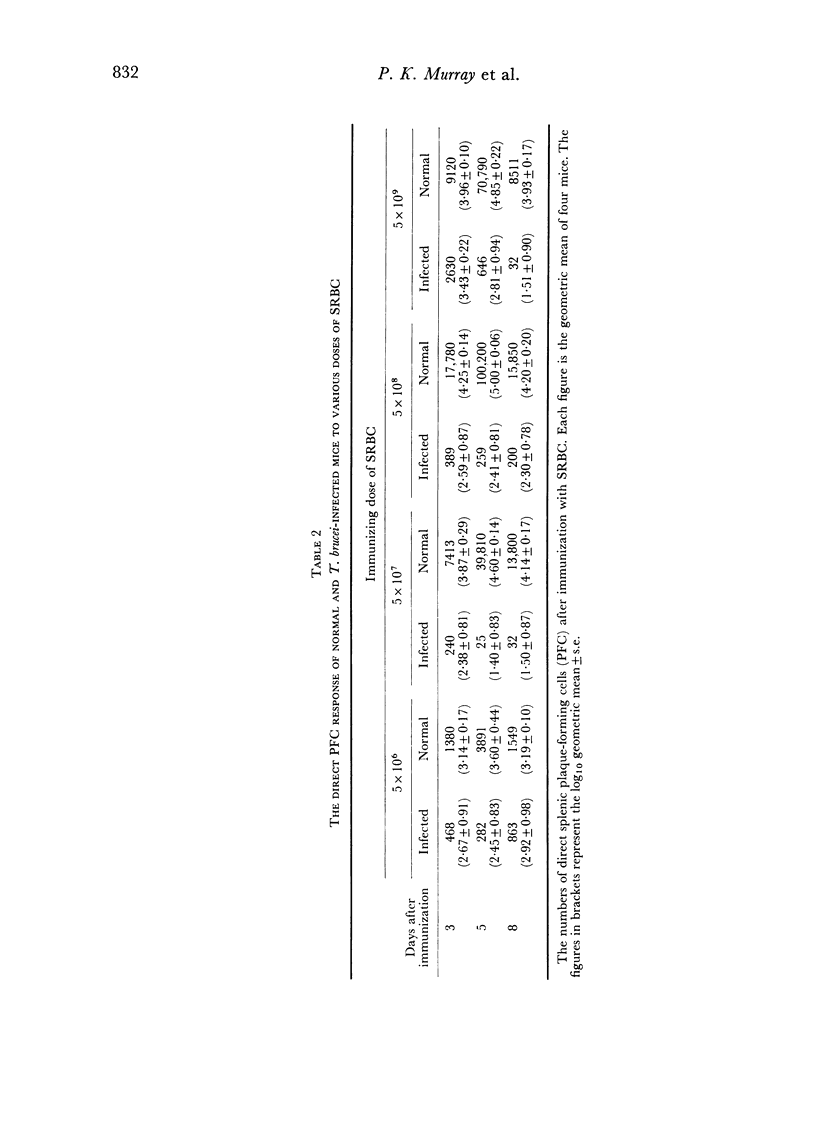
In an investigation of the immunosuppression associated with trypanosomiasis, the thymus-derived lymphocytes (T cells) and the thymus-independent lymphocytes (B cells) in mice with subacute Trypanosoma brucei infections were studied. It was shown that: (a) there was a massive plasma cell response in lymph nodes and spleen which replaced the thymus-dependent areas; (b) a failure of antibody production at the cellular level occurred as shown by the absence of IgM PFC responses to SRBC and lipopolysaccharide; (c) T cells appeared relatively normal as judged by their ability to proliferate following a primary stimulus with oxazolone unless measured during the terminal stages of the disease; (d) immune competence was rapidly restored after treatment with a trypanocidal drug.
It appeared that immunosuppression was closely associated with the presence of living trypanosomes, possibly mediated through a B-cell defect. The mechanism whereby this might occur is discussed.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allt G., Evans E. M., Evans D. H., Targett G. A. Effect of infection with trypanosomes on the development of experimental allergic neuritis in rabbits. Nature. 1971 Sep 17;233(5316):197–199. doi: 10.1038/233197b0. [DOI] [PubMed] [Google Scholar]
- Andersson B., Blomgren H. Evidence for thymus-independent humoral antibody production in mice against polyvinylpyrrolidone and E. coli lipopolysaccharide. Cell Immunol. 1971 Oct;2(5):411–424. doi: 10.1016/0008-8749(71)90052-9. [DOI] [PubMed] [Google Scholar]
- Davies A. J., Carter R. L., Leuchars E., Wallis V. The morphology of immune reactions in normal, thymectomized and reconstituted mice. II. The response to oxazolone. Immunology. 1969 Jul;17(1):111–126. [PMC free article] [PubMed] [Google Scholar]
- Goodwin L. G. The pathology of African trypanosomiasis. Trans R Soc Trop Med Hyg. 1970;64(6):797–817. doi: 10.1016/0035-9203(70)90096-9. [DOI] [PubMed] [Google Scholar]
- Houba V., Brown K. N., Allison A. C. Heterophile antibodies, M-antiglobulins and immunoglobulins in experimental trypanosomiasis. Clin Exp Immunol. 1969 Jan;4(1):113–123. [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Christie G. H., Courtenay B. M. Studies on immunological paralysis. IV. The relative contributions of continuous antibody neutralization and central inhibition to paralysis with type 3 pneumococcal polysaccharide. Proc R Soc Lond B Biol Sci. 1971 Sep 28;178(1053):417–438. doi: 10.1098/rspb.1971.0073. [DOI] [PubMed] [Google Scholar]
- Jennings F. W., Murray P. K., Murray M., Urquhart G. M. Protein catabolism in trypanosomiasis. Trans R Soc Trop Med Hyg. 1973;67(2):277–277. doi: 10.1016/0035-9203(73)90191-0. [DOI] [PubMed] [Google Scholar]
- Longstaffe J. A., Freeman J., Hudson K. M. Immunosuppression in trypanosomiasis: some thymus dependent and thymus independent responses. Trans R Soc Trop Med Hyg. 1973;67(2):264–265. doi: 10.1016/0035-9203(73)90169-7. [DOI] [PubMed] [Google Scholar]
- Murray P. K., Jennings F. W., Murray M., Urquhart G. M. The nature of immunosuppression in Trypanosoma brucei infections in mice. I. The role of the macrophage. Immunology. 1974 Nov;27(5):815–824. [PMC free article] [PubMed] [Google Scholar]
- Murray P. K., Urquhart G. M., Murray M., Jennings F. W. The response of mice infected with T. brucei to the administration of sheep erythrocytes. Trans R Soc Trop Med Hyg. 1973;67(2):267–267. doi: 10.1016/0035-9203(73)90173-9. [DOI] [PubMed] [Google Scholar]
- Pantelouris E. M., Flisch P. A. Estimation of PFC and serum haemolysin response to SRBC in 'nude' mice. Immunology. 1972 Jan;22(1):159–164. [PMC free article] [PubMed] [Google Scholar]
- Pritchard H., Micklem H. S. Immune responses in congenitally thymus-less mice. I. Absence of response to oxazolone. Clin Exp Immunol. 1972 Jan;10(1):151–161. [PMC free article] [PubMed] [Google Scholar]
- Rich R. R., Pierce C. W. Biological expressions of lymphocyte activation. II. Generation of a population of thymus-derived suppressor lymphocytes. J Exp Med. 1973 Mar 1;137(3):649–659. doi: 10.1084/jem.137.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart G. M., Murray M., Murray P. K., Jennings F. W., Bate E. Immunosuppression in Trypanosoma brucei infections in rats and mice. Trans R Soc Trop Med Hyg. 1973;67(4):528–535. doi: 10.1016/0035-9203(73)90083-7. [DOI] [PubMed] [Google Scholar]