Abstract
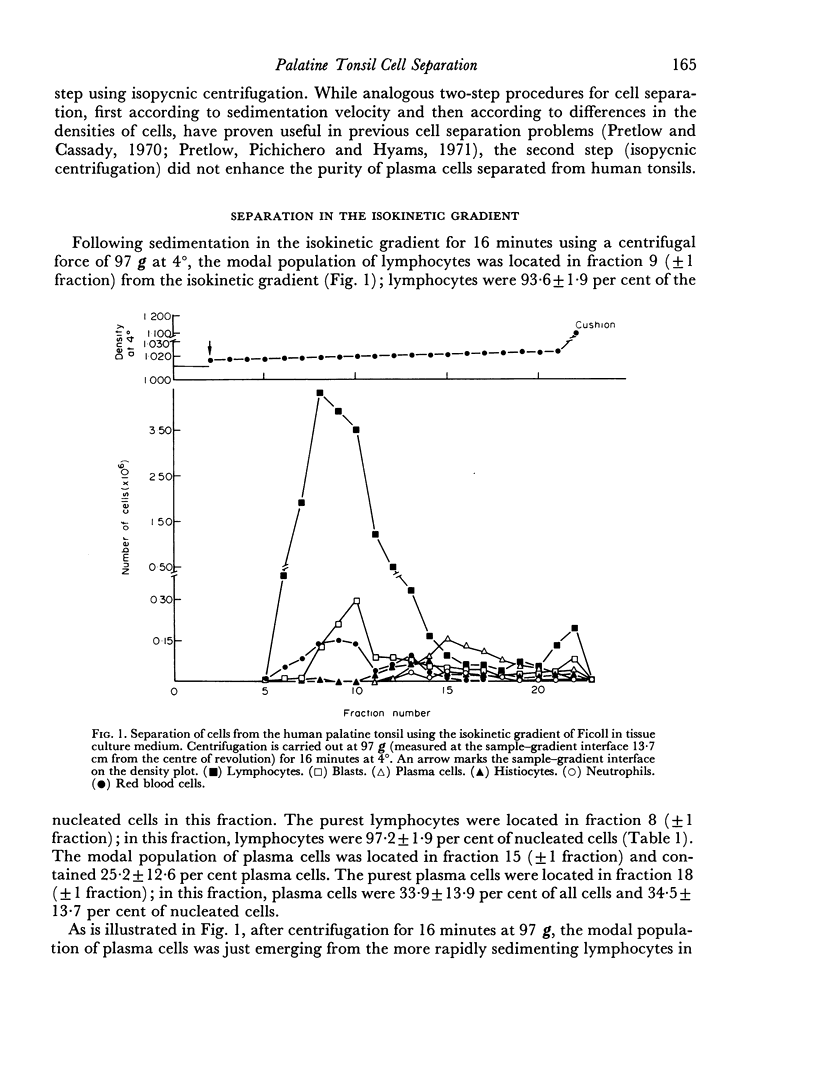
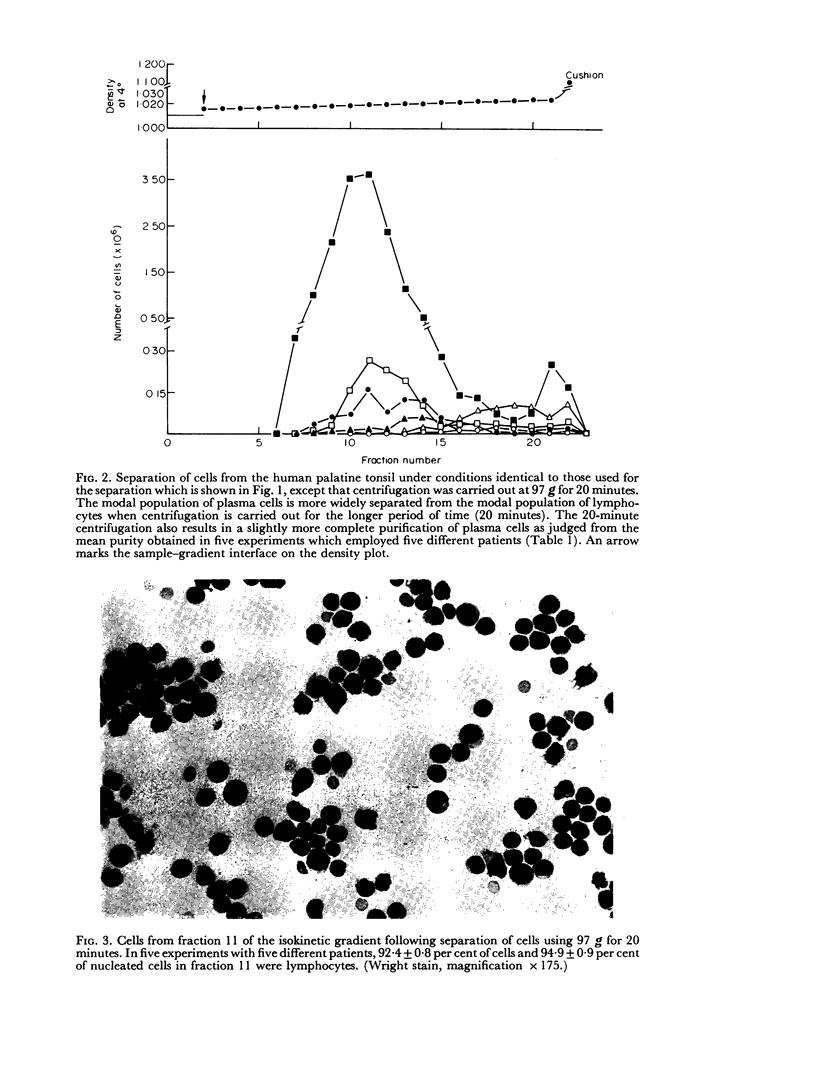
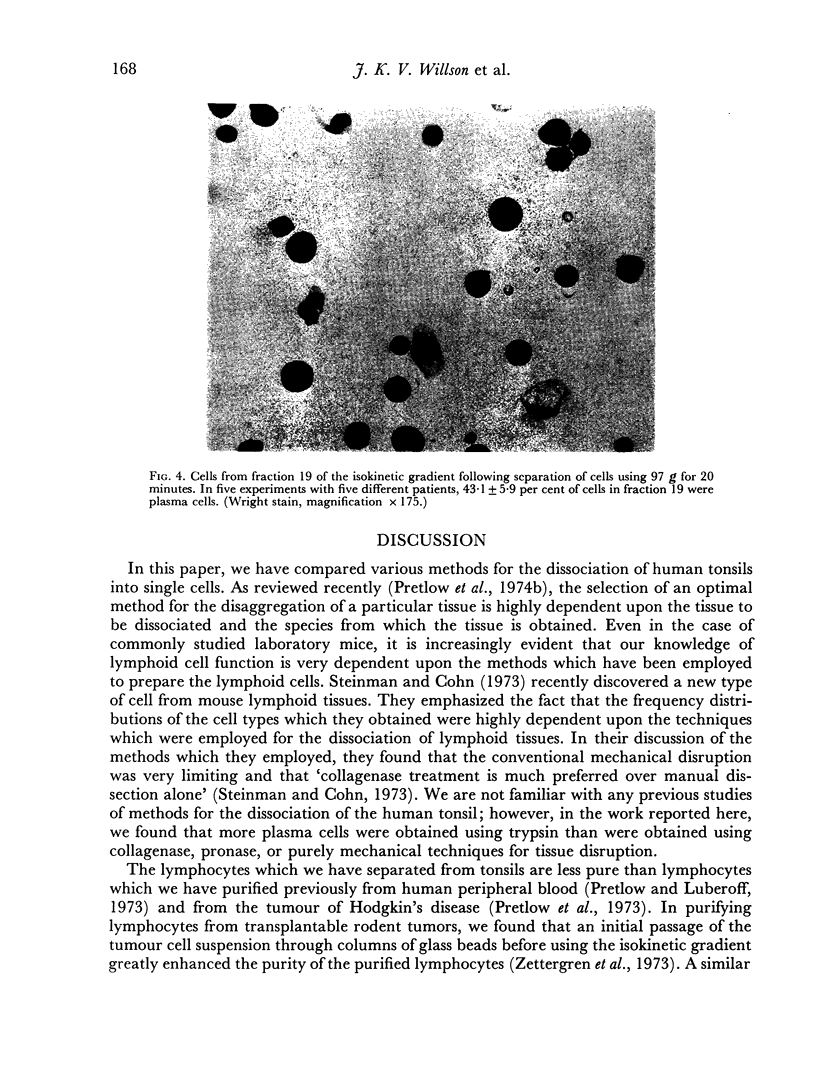
Several methods for the dissociation of human tonsils into cell suspensions were compared. Dissociation of tonsils using 0-25 per cent trypsin gave both the largest number of total cells and the largest number of plasma cells per gram of tonsil. Lymphocytes and plasma cells were separated in a previously described isokinetic gradient of Ficoll in tissue culture medium. In the purest gradient fractions, lymphocytes were 97-2 plus or minus 1-9 per cent of nucleated cells. The purest gradient fractions contained 43-1 plus or minus 5-9 per cent plasma cells. More than 95 per cent of purified lymphocytes and plasma cells excluded Trypan Blue.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlqvist J., Anderson L. Methyl green-pyronin staining: effects of fixation; use in routine pathology. Stain Technol. 1972 Jan;47(1):17–22. doi: 10.3109/10520297209116529. [DOI] [PubMed] [Google Scholar]
- Boone C. W., Harell G. S., Bond H. E. The resolution of mixtures of viable mammalian cells into homogeneous fractions by zonal centrifugation. J Cell Biol. 1968 Feb;36(2):369–378. doi: 10.1083/jcb.36.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum J. N. The biosynthesis, assembly, and secretion of immunoglobulins. Semin Hematol. 1973 Jan;10(1):33–52. [PubMed] [Google Scholar]
- HARRIS S., HARRIS T. N. Studies on the transfer of lymph node cells. III. Effects of variation in the interval between the injection of antigen into the donor and collection of its lymph node cells. J Exp Med. 1954 Sep 1;100(3):269–287. doi: 10.1084/jem.100.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELMREICH E., KERN M., EISEN H. N. The secretion of antibody by isolated lymph node cells. J Biol Chem. 1961 Feb;236:464–473. [PubMed] [Google Scholar]
- Hannig K., Zeiller K. Zur Auftrennung und Charakterisierung immunkompetenter Zellen mit Hilfe der trägerfreien Ablenkungselektrophorese. Hoppe Seylers Z Physiol Chem. 1969 Apr;350(4):467–472. [PubMed] [Google Scholar]
- Hunt S. V. Separation of thymus-derived and marrow-derived rat lymphocytes on glass bead columns. Immunology. 1973 Apr;24(4):699–705. [PMC free article] [PubMed] [Google Scholar]
- Häyry P., Andersson L. C., Nordling S. Electrophoretic fractionation of mouse T and B lymphocytes. Efficiency of the method and purity of separated cells. Transplant Proc. 1973 Mar;5(1):87–90. [PubMed] [Google Scholar]
- MALMGREN R. A. Influence of antigenic factors in the production of antitumor cytotoxic sera. J Natl Cancer Inst. 1958 Feb;20(2):417–429. [PubMed] [Google Scholar]
- Miller H. R., Avrameas S., Ternynck T. Intracellular distribution of antibody in immunocytes responding to primary challenge with horseradish peroxidase. Am J Pathol. 1973 May;71(2):239–260. [PMC free article] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Boone C. W. Centrifugation of mammalian cells on gradients: a new rotor. Science. 1968 Aug 30;161(3844):911–913. doi: 10.1126/science.161.3844.911. [DOI] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Boone C. W. Separation of mammalian cells using programmed gradient sedimentation. Exp Mol Pathol. 1969 Oct;11(2):139–152. doi: 10.1016/0014-4800(69)90003-3. [DOI] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Cassady I. M. Separation of mast cells in successive stages of differentiation using programmed gradient sedimentation. Am J Pathol. 1970 Dec;61(3):323–340. [PMC free article] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Glick M. R., Reddy W. J. Separation of beating cardiac myocytes from suspensions of heart cells. Am J Pathol. 1972 May;67(2):215–226. [PMC free article] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Jones J., Dow S. Separation of cells having histochemically demonstrable glucose-6-phosphatase from suspensions of hamster kidney cells in an isokinetic density gradient of Ficoll in tissue culture medium. Am J Pathol. 1974 Feb;74(2):275–286. [PMC free article] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Luberoff D. E. A new method for separating lymphocytes and granulocytes from human peripheral blood using programmed gradient sedimentation in an isokinetic gradient. Immunology. 1973 Jan;24(1):85–92. [PMC free article] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Luberoff D. E., Hamilton L. J., Weinberger P. C., Maddox W. A., Durant J. R. Pathogenesis of Hodgkin's disease: separation and culture of different kinds of cells from Hodgkin's disease in a sterile isokinetic gradient of Ficoll in tissue culture medium. Cancer. 1973 May;31(5):1120–1126. doi: 10.1002/1097-0142(197305)31:5<1120::aid-cncr2820310513>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Pichichero M. E., Hyams L. Separation of lymphocytes and macrophages from suspensions of guinea pig peritonitis exudate cells using programmed gradient sedimentation. Am J Pathol. 1971 May;63(2):255–276. [PMC free article] [PubMed] [Google Scholar]
- Pretlow T. G. Estimation of experimental conditions that permit cell separations by velocity sedimentation on isokinetic gradients of Ficoll in tissue culture medium. Anal Biochem. 1971 May;41(1):248–255. doi: 10.1016/0003-2697(71)90207-7. [DOI] [PubMed] [Google Scholar]
- ROBERTS J. C., Jr, DIXON F. J. The transfer of lymph node cells in the study of the immune response to foreign proteins. J Exp Med. 1955 Oct 1;102(4):379–392. doi: 10.1084/jem.102.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabolović D., Dumont F. Separation and characterization of cell subpopulations in the thymus. Immunology. 1973 Apr;24(4):601–606. [PMC free article] [PubMed] [Google Scholar]
- Saito K., Uda H., Mori T., Miyamoto K., Takenaka T. Ultrastructural study on antibody-forming cells to horseradish peroxidase. Special reference to thoracic duct lymphocytes. Exp Cell Res. 1973 Jan;76(1):127–135. doi: 10.1016/0014-4827(73)90427-8. [DOI] [PubMed] [Google Scholar]
- Shortman K. Physical procedures for the separation of animal cells. Annu Rev Biophys Bioeng. 1972;1:93–130. doi: 10.1146/annurev.bb.01.060172.000521. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973 May 1;137(5):1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M. J., Pretlow T. G., 2nd, Hiramoto R. Separation of ascites myeloma cells, lymphocytes and macrophages by zonal centrifugation on an isokinetic gradient. Am J Pathol. 1972 Jul;68(1):163–182. [PMC free article] [PubMed] [Google Scholar]
- Vann D. C., Kettman J. R. In vitro cooperation of cells of bone marrow and thymus origins in the generation of antibody-forming cells. J Immunol. 1972 Jan;108(1):73–80. [PubMed] [Google Scholar]
- Zeiller K., Holzberg E., Pascher G., Hannig K. Free flow electrophoretic separation of T and B lymphocytes. Evidence for various subpopulations of B cells. Hoppe Seylers Z Physiol Chem. 1972 Jan;353(1):105–110. [PubMed] [Google Scholar]
- Zeiller K., Pascher G., Hannig K. Free-flow electrophoresis: a new method for the elucidation of immunological events at the cellular level, II. The formation of 19S hemolysin-producing cells in intestinal lymph nodes of the rat. Hoppe Seylers Z Physiol Chem. 1970 Apr;351(4):435–447. doi: 10.1515/bchm2.1970.351.1.435. [DOI] [PubMed] [Google Scholar]
- Zettergren J. G., Luberoff D. E., Pretlow T. G., 2nd Separation of lymphocytes from disaggregated mouse malignant neoplasms by sedimentation in gradients of ficoll in tissue culture medium. J Immunol. 1973 Sep;111(3):836–840. [PubMed] [Google Scholar]
- van Furth R., Schuit R. E., Hijmans W. The formation of immunoglobulins by human tissues in vitro. I. The methods and their specificity. Immunology. 1966 Jul;11(1):1–11. [PMC free article] [PubMed] [Google Scholar]