Abstract
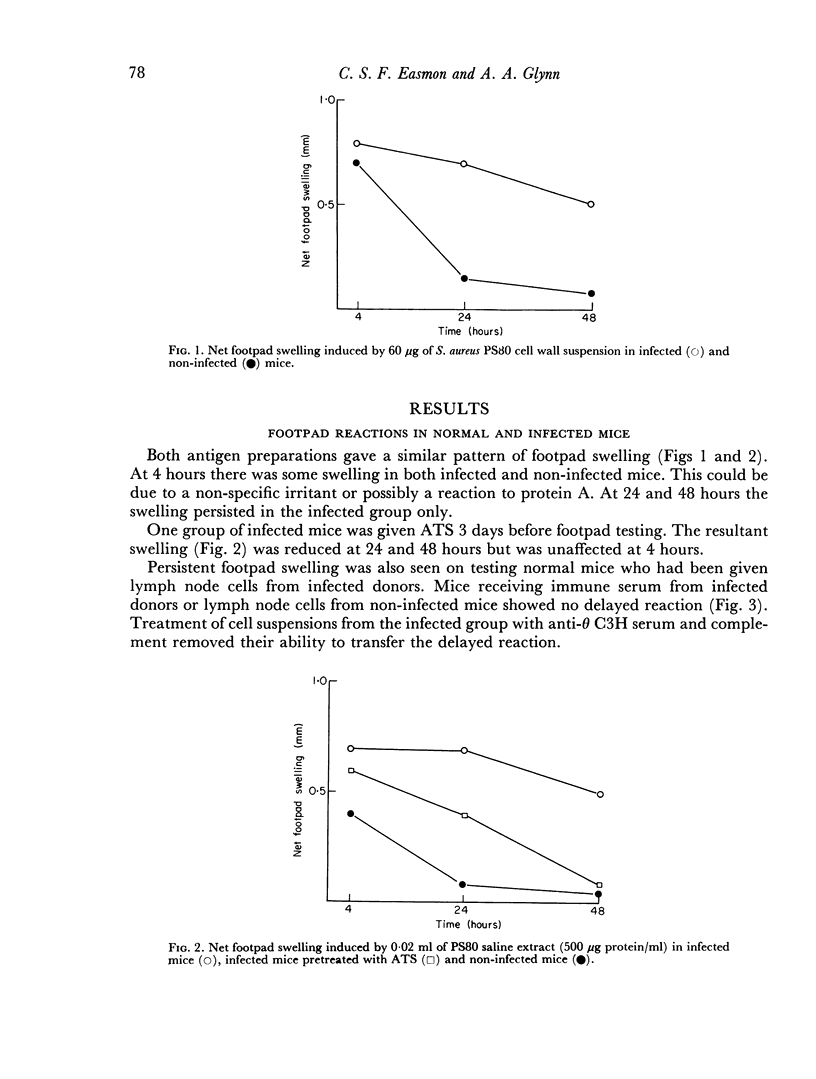
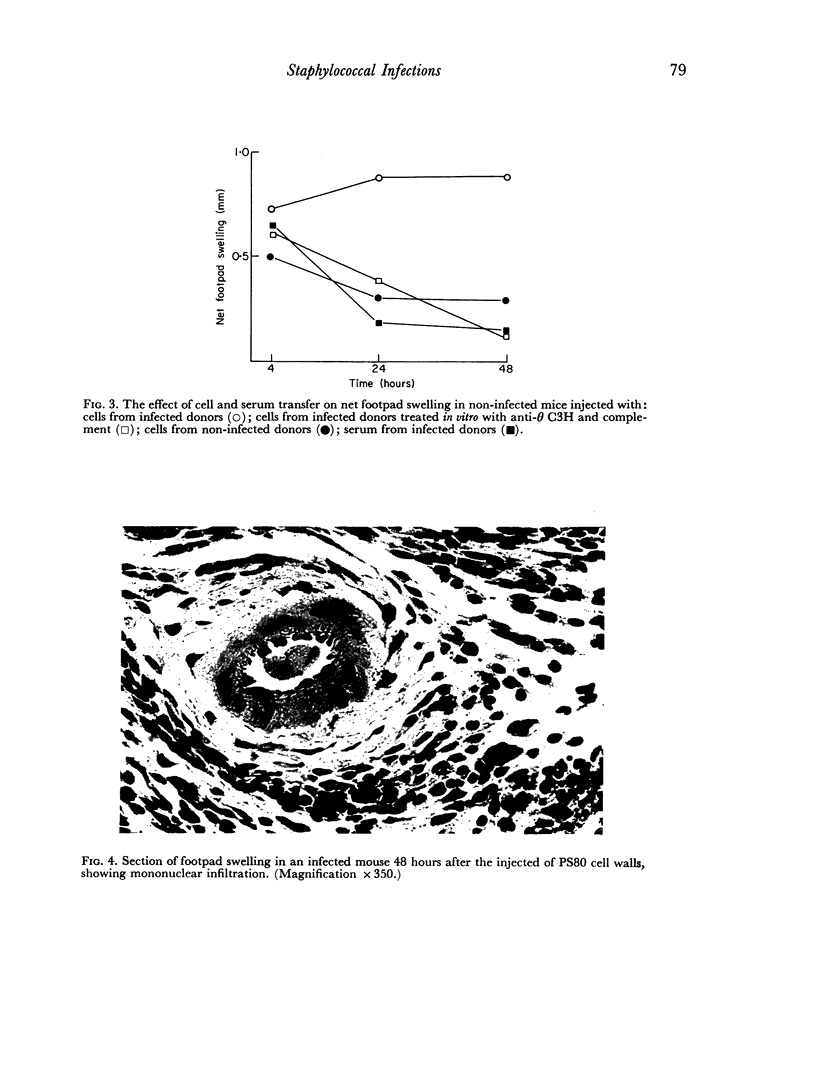
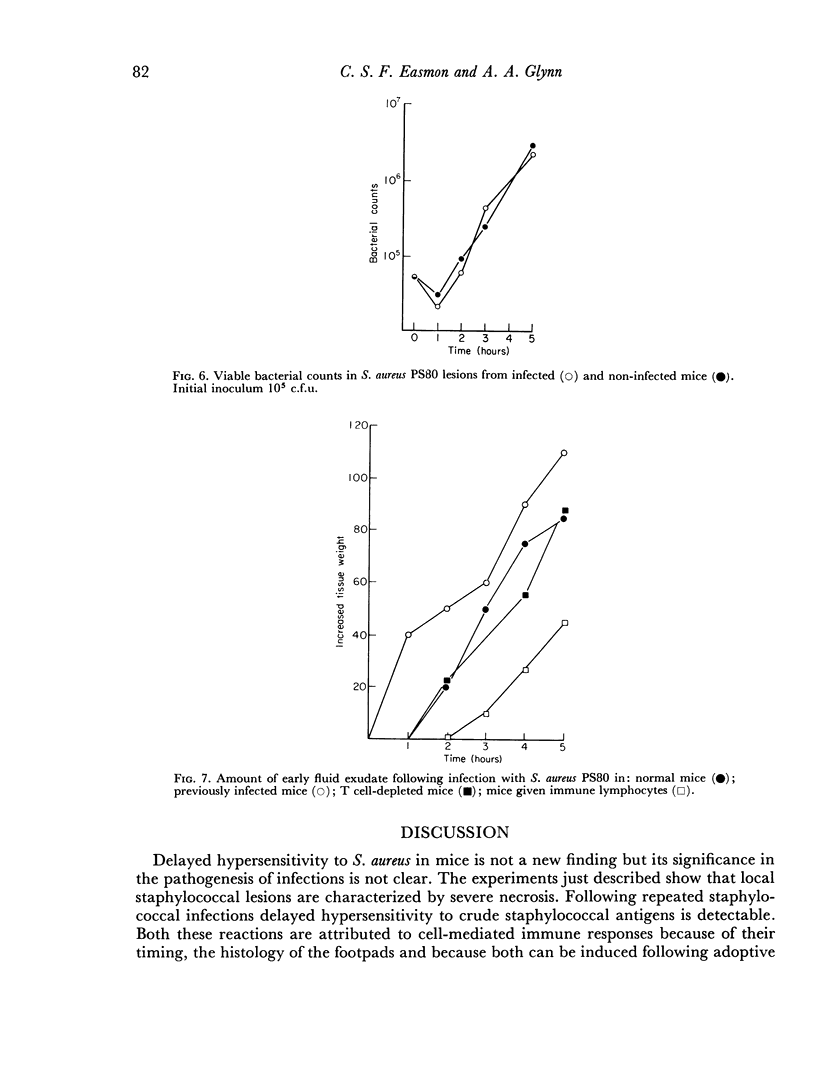
Delayed hypersensitivity to staphylococcal antigens was shown in mice repeatedly infected with Staphylococcus aureus. It was characterized by footpad swelling at 48 hours with a mononuclear cell infiltrate and could be transferred to non-infected recipients by T lymphocytes from infected animals, but not by serum. Recipients of immune T cells produced very severe necrotic lesions when challenged with staphylococci. This was in contrast to the protection against necrosis in recipients afforded by serum from infected donors. When both serum and cells were transferred into the same mouse the humoral effects overshadowed or perhaps inhibited those mediated by cells with resultant protection against staphylococcal dermonecrosis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal D. S. Subcutaneous staphylococcal infection in mice. I. The role of cotton-dust in enhancing infection. Br J Exp Pathol. 1967 Aug;48(4):436–449. [PMC free article] [PubMed] [Google Scholar]
- Allen E. G., Mudd S. Protection of mice against vaccinia virus by bacterial infection and sustained stimulation with specific bacterial antigens. Infect Immun. 1973 Jan;7(1):62–67. doi: 10.1128/iai.7.1.62-67.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Schlesinger M. Absorption of guinea pig serum with agar. A method for elimination of itscytotoxicity for murine thymus cells. Transplantation. 1970 Jul;10(1):130–132. doi: 10.1097/00007890-197007000-00027. [DOI] [PubMed] [Google Scholar]
- Dumont F. Destruction and regeneration of lymphocyte populations in the mouse spleen after cyclophosphamide treatment. Int Arch Allergy Appl Immunol. 1974;47(1):110–123. doi: 10.1159/000231206. [DOI] [PubMed] [Google Scholar]
- Easmon C. S., Glynn A. A. The role of humoral immunity and acute inflammation in protection against staphyloccocal dermonecrosis. Immunology. 1975 Jul;29(1):67–74. [PMC free article] [PubMed] [Google Scholar]
- Easmon C. S., Hamilton I., Glynn A. A. Mode of action of a staphylococcal anti-inflammatory factor. Br J Exp Pathol. 1973 Dec;54(6):638–645. [PMC free article] [PubMed] [Google Scholar]
- JOHANOVSKY J. Role of hypersensitivity in experimental staphylococcal infection. Nature. 1958 Nov 22;182(4647):1454–1454. doi: 10.1038/1821454a0. [DOI] [PubMed] [Google Scholar]
- Jokipii A. M., Jokipii L. Suppression of cell-mediated immunity by cyclophosphamide: its independence of concomitant B cell response. Cell Immunol. 1973 Dec;9(3):477–481. doi: 10.1016/0008-8749(73)90063-4. [DOI] [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B., Miller T. E. Potentiation of T-cell-mediated immunity by selective suppression of antibody formation with cyclophosphamide. J Exp Med. 1974 Jun 1;139(6):1529–1539. doi: 10.1084/jem.139.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhart N., Mudd S. Staphylococcidal capability of rabbit peritoneal macrophages in relation to infection and elicitation: induction and elicitation of activated macrophages. Infect Immun. 1972 May;5(5):763–768. doi: 10.1128/iai.5.5.763-768.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman S. P., Weidanz W. P. The effect of cyclophosphamide on the ontogeny of the humoral immune response in chickens. J Immunol. 1970 Sep;105(3):614–619. [PubMed] [Google Scholar]
- Mackaness G. B., Blanden R. V. Cellular immunity. Prog Allergy. 1967;11:89–140. [PubMed] [Google Scholar]
- North R. J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973 Apr;7(1):166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- Shayegani M., De Courcy S. J., Jr, Mudd S. Cell-mediated immunity in mice infected with S. aureus and elicited with specific bacterial antigens. J Reticuloendothel Soc. 1973 Jul;14(1):44–51. [PubMed] [Google Scholar]
- Stockman G. D., Heim L. R., South M. A., Trentin J. J. Differential effects of cyclophosphamide on the B and T cell compartments of adult mice. J Immunol. 1973 Jan;110(1):277–282. [PubMed] [Google Scholar]
- Taubler J. H., Mudd S. Staphylococcal delayed hypersensitivity in mice. II. In vitro demonstration and specificity of delayed hypersensitivity. J Immunol. 1968 Sep;101(3):550–555. [PubMed] [Google Scholar]
- Taubler J. H. Staphylococcal delayed hypersensitivity in mice. I. Induction and in vivo demonstration of delayed hypersensitivity. J Immunol. 1968 Sep;101(3):546–549. [PubMed] [Google Scholar]
- Turk J. L., Poulter L. W. Selective depletion of lymphoid tissue by cyclophosphamide. Clin Exp Immunol. 1972 Feb;10(2):285–296. [PMC free article] [PubMed] [Google Scholar]
- Voisin G. A. Immunological facilitation, a broadening of the concept of the enhancement phenomenon. Prog Allergy. 1971;15:328–485. [PubMed] [Google Scholar]
- Winkelstein A. Differential effects of immunosuppressants on lymphocyte function. J Clin Invest. 1973 Sep;52(9):2293–2299. doi: 10.1172/JCI107417. [DOI] [PMC free article] [PubMed] [Google Scholar]




