Abstract
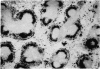
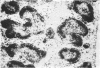
Paratyphoid vaccine injected between 4 days and 3 hours before injection of labelled immune complexes (125-I-labelled BGG-anti-BGG), inhibits follicular trapping of these complexes in the mouse spleen. Inhibition is maximal when paratyphoid vaccine is given 1 day before, almost no label being found in the spleen follicles. No inhibition of follicular trapping of the complexes occurred when paratyphoid vaccine was injected simultaneously with the labelled immune complexes. Competitive inhibition was found when unlabelled immune complexes were given together with labelled immune complexes. Simultaneous injection of mice with paratyphoid vaccine and labelled immune complexes resulted in an additonal form of localization of the labelled immune complexes in the white pulp, heavily labelled clumps also appearing in the periarteriolar lymphocyte sheaths and follicles. The results are discussed in relation to the mechanism of immune complex trapping in spleen follicles.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Ito T. Early events in the splenic lymphoid tissue and mesenteric lymph node after a single typhoid-paratyphoid vaccine injection in the mouse, with special reference to the topographic cellular changes in the early immune response. Arch Histol Jpn. 1972 Sep;34(5):471–489. doi: 10.1679/aohc1950.34.471. [DOI] [PubMed] [Google Scholar]
- Brown J. C., Harris G., Papamichail M., Sljivić V. S., Holborow E. J. The localization of aggregated human -globulin in the spleens of normal mice. Immunology. 1973 Jun;24(6):955–968. [PMC free article] [PubMed] [Google Scholar]
- Dukor P., Bianco C., Nussenzweig V. Tissue localization of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. Proc Natl Acad Sci U S A. 1970 Oct;67(2):991–997. doi: 10.1073/pnas.67.2.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajl-Peczalska K. J., Fish A. J., Meuwissen H. J., Frommel D., Good R. A. Localization of immunological complexes fixing beta1C (C3) in germinal centers of lymph nodes. J Exp Med. 1969 Dec 1;130(6):1367–1393. doi: 10.1084/jem.130.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna M. G., Jr, Szakal A. K. Localization of 125I-labeled antigen in germinal centers of mouse spleen: histologic and ultrastructural autoradiographic studies of the secondary immune reaction. J Immunol. 1968 Nov;101(5):949–962. [PubMed] [Google Scholar]
- Herd Z. L., Ada G. L. Distribution of 125I-immunoglobulins, IgG subunits and antigen-antibody complexes in rat lymph nodes. Aust J Exp Biol Med Sci. 1969 Feb;47(1):73–80. doi: 10.1038/icb.1969.6. [DOI] [PubMed] [Google Scholar]
- Humphrey J. H., Frank M. M. The localization of non-microbial antigens in the draining lymph nodes of tolerant, normal and primed rabbits. Immunology. 1967 Jul;13(1):87–100. [PMC free article] [PubMed] [Google Scholar]
- Nettesheim P., Hammons A. S. Effect of immunosuppressive agents on retention of antigen in the mouse spleen. Proc Soc Exp Biol Med. 1970 Feb;133(2):696–701. doi: 10.3181/00379727-133-34547. [DOI] [PubMed] [Google Scholar]
- Nossal G. J., Abbot A., Mitchell J., Lummus Z. Antigens in immunity. XV. Ultrastructural features of antigen capture in primary and secondary lymphoid follicles. J Exp Med. 1968 Feb 1;127(2):277–290. doi: 10.1084/jem.127.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen J. C., Borgen D. F., Graupner K. C. A morphological and histochemical study of the primary and secondary immune responses in the rat spleen. Am J Anat. 1967 Sep;121(2):305–317. doi: 10.1002/aja.1001210209. [DOI] [PubMed] [Google Scholar]
- STAVITSKY A. B. Micromethods for the study of proteins and antibodies. I. Procedure and general applications of hemagglutination and hemagglutination-inhibition reactions with tannic acid and protein-treated red blood cells. J Immunol. 1954 May;72(5):360–367. [PubMed] [Google Scholar]
- Williams G. M. Antigen localization in lymphopenic states. I. Localization pattern following chronic thoracic duct drainage. Immunology. 1966 Nov;11(5):467–474. [PMC free article] [PubMed] [Google Scholar]
- Williams G. M. Antigen localization in lymphopenic states. II. Further studies on whole body x-irradiation. Immunology. 1966 Nov;11(5):475–488. [PMC free article] [PubMed] [Google Scholar]
- van Rooijen N. Antigens in the spleen. The non-specificity of the follicles in the process of antigen trapping and the role of antibody. Immunology. 1972 May;22(5):757–765. [PMC free article] [PubMed] [Google Scholar]
- van Rooijen N. Mechanism of follicular antigen trapping. Evidence for a two cell mechanism using double isotope autoradiography. Immunology. 1974 Oct;27(4):617–622. [PMC free article] [PubMed] [Google Scholar]
- van Rooijen N. Mechanism of follicular antigen trapping. Migration of antigen-antibody complexes from marginal zone towards follicle centres. Immunology. 1973 Nov;25(5):847–852. [PMC free article] [PubMed] [Google Scholar]
- van Rooijen N. The vascular pathways in the white pulp of the rabbit spleen. Acta Morphol Neerl Scand. 1972 Dec;10(4):351–357. [PubMed] [Google Scholar]