Abstract
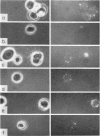
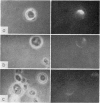
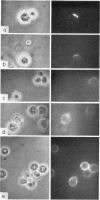
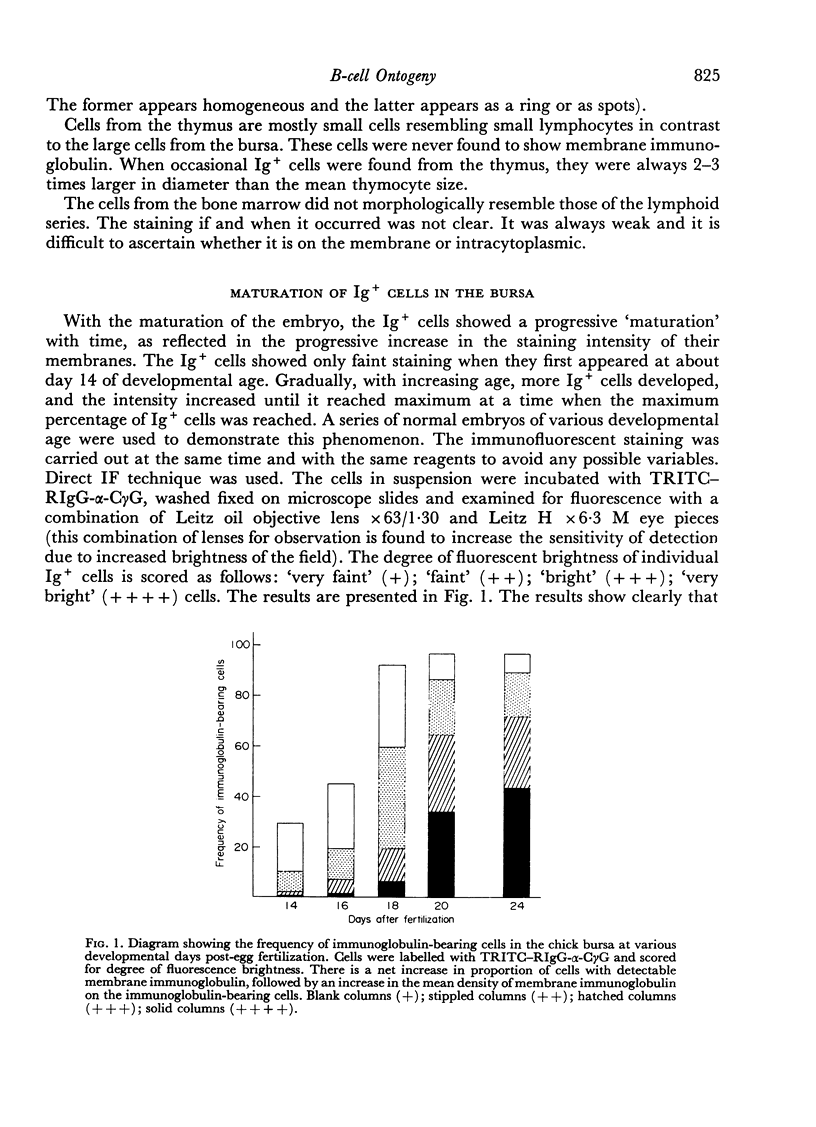
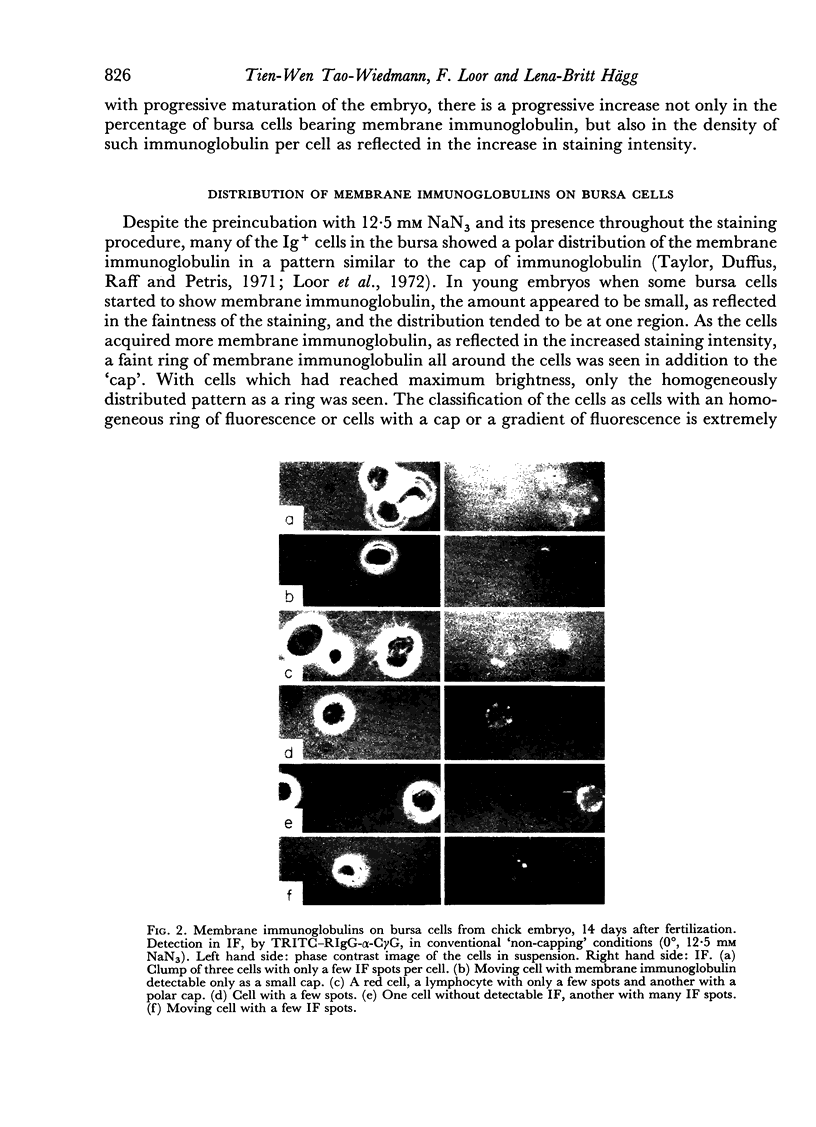
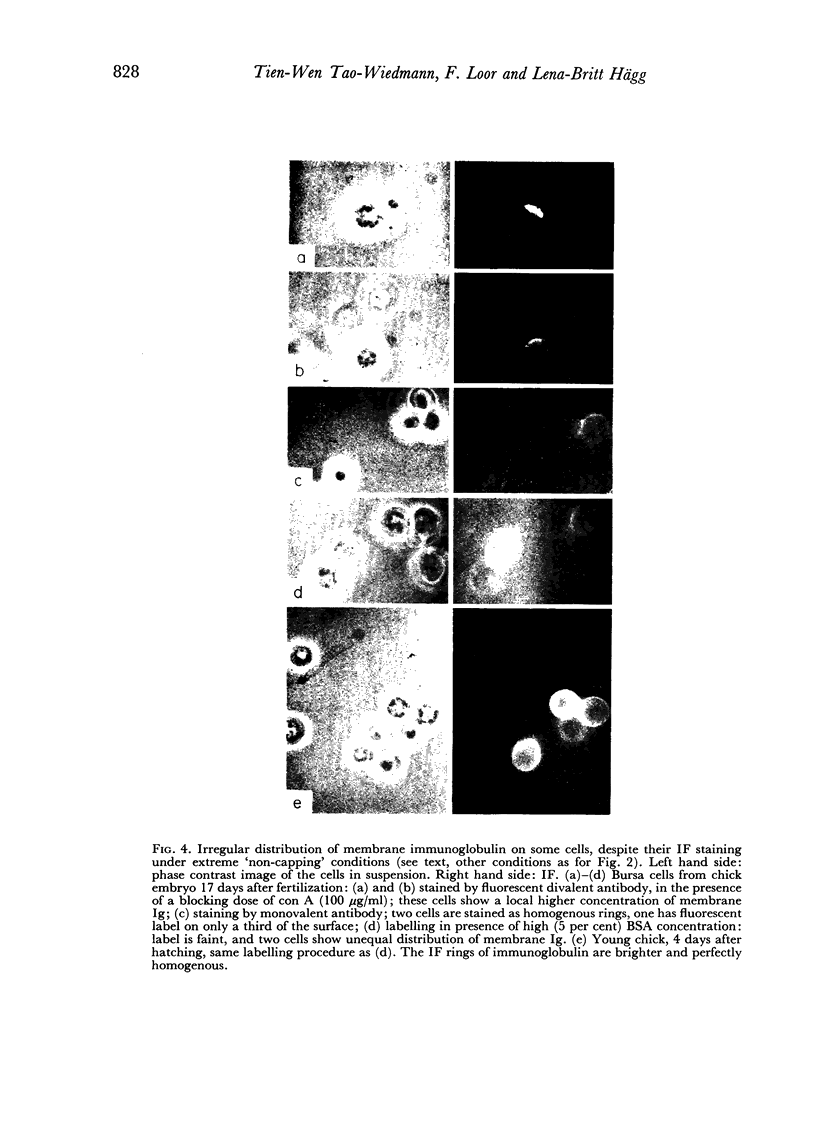
B-cell maturation in the chicken has been evaluated by the appearance of membrane immunoglobulins on cells in the spleen, the thymus, the bursa and the bone marrow during enbryonic development and shortly after hatching. The majority of the bursa cells acquire demonstrable membrane immunoglobulin between days 16 and 18 of incubational age and show significantly increased amounts of membrane immunoglobulin between days 18 and 20, even though immunoglobulin-bearing cells can be found in the bursa as early as day 14 of enbryonic age. The spleen shows cells possessing immunoglobulin receptors can their membranes (Ig+) only after the bursa cells have reached full membrane immunoglobulin maturation as reflected in the number of Ig+ cells and the amounts of membrane immunoglobuline. The thymus is practically devoid of Ig+ cells in the embry and it is not clear whether there any Ig-+ cells in the bone marrow. There are two phenomena which stand out in the observations. One is that there appears to be a gradual increase in the quantity of quality of the surface immunoglobulins on individual cells with advance in the embryonic development as reflected in the gradual increase in the staining intensity. The other is that there appears to be a polar distribution of membrane immunoglobulin in some cells especially in younger embryos. This polar distribution is seen under conditions where immunoglobulin capping is prevented by inhibitors and where immunoglobulin capping is impossible, such as with monomeric Fab. Immunoglobulin capping has been found to occur readily in embryonic cells and under conditions which would normally inhibit capping in adult cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albini B., Wick G. Immunoglobulin determinants on the surface of chicken lymphoid cells. Int Arch Allergy Appl Immunol. 1973;44(6):804–822. doi: 10.1159/000230984. [DOI] [PubMed] [Google Scholar]
- Hirano H., Parkhouse B., Nicolson G. L., Lennox E. S., Singer S. J. Distribution of saccharide residues on membrane fragments from a myeloma-cell homogenate: its implications for membrane biogenesis. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2945–2949. doi: 10.1073/pnas.69.10.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson L., Roitt I. M. Immunofluorescent detection of surface antigens specific to T and B lymphocytes in the chicken. Eur J Immunol. 1973 Feb;3(2):63–67. doi: 10.1002/eji.1830030202. [DOI] [PubMed] [Google Scholar]
- Kincade P. W., Self K. S., Cooper M. D. Survival and function of bursa-derived cells in bursectomized chickens. Cell Immunol. 1973 Jul;8(1):93–102. doi: 10.1016/0008-8749(73)90096-8. [DOI] [PubMed] [Google Scholar]
- Loor F. Binding and redistribution of lectins on lymphocyte membrane. Eur J Immunol. 1974 Mar;4(3):210–220. doi: 10.1002/eji.1830040311. [DOI] [PubMed] [Google Scholar]
- Loor F., Forni L., Pernis B. The dynamic state of the lymphocyte membrane. Factors affecting the distribution and turnover of surface immunoglobulins. Eur J Immunol. 1972 Jun;2(3):203–212. doi: 10.1002/eji.1830020304. [DOI] [PubMed] [Google Scholar]
- Warner N. L., Uhr J. W., Thorbecke G. J., Ovary Z. Immunoglobulins, antibodies and the bursa of Fabricus: induction of agammaglobulinemia and the loss of all antibody-forming capacity by hormonal bursectomy. J Immunol. 1969 Dec;103(6):1317–1330. [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. Restriction of the mobility of lymphocyte immunoglobulin receptors by concanavalin A. Proc Natl Acad Sci U S A. 1972 Mar;69(3):608–612. doi: 10.1073/pnas.69.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]