Abstract
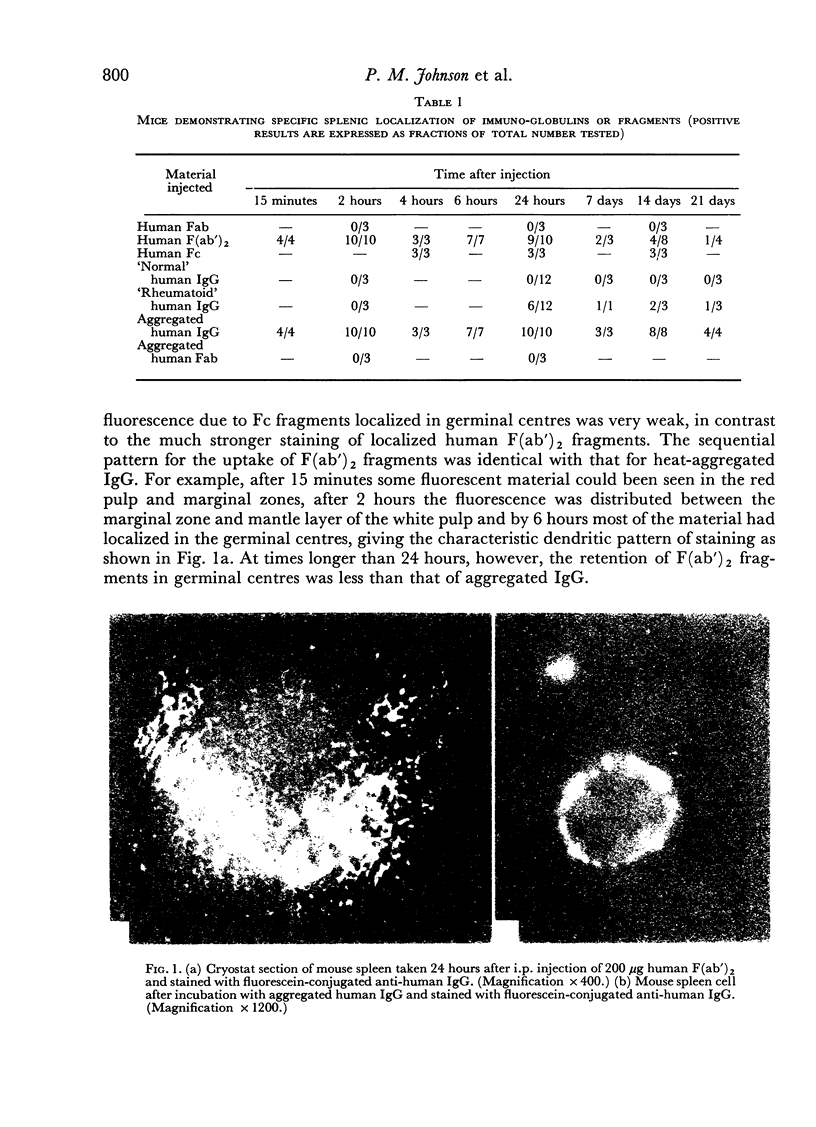
Evidence is presented which indicates the presence on murine lymphocytes of a membrane receptor for determinants on the hinge region of human IgG. These determinants are exposed following pepsin scission of IgG molecule, i.e. on the F(ab')2 fragment. The evidence for a hinge receptor derives, in vivo, from splenic localization of F(ab')2 in germinal centres and, in vitro, from immunofluorescent binding studies. The sequential immunofluorescent pattern for the uptake of human F(ab)2 fragments into murine spleen germinal centres was identical with that previously observed for heat-aggregated human IgG, but F(ab')2 fragments appeared to be retained in the germinal centres for a shorter time than aggregated IgG. Experiments with nude mice and T cell-deprived mice showed that the localization of F(ab')2 fragments does not require T cells. Competition experiments suggest that the receptor for F(ab')2 may bear little relation to the receptor for aggregated IgG. The relevance of such a lymphocyte membrane receptor to the immunopathology of rheumatoid arthritis is discussed in the light of previous findings that a proportion of the serum IgG of patients with rheumatoid arthritis has a structural anomaly compared with control IgG, characterized exposure of new determinants at the hinge region.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basten A., Miller J. F., Sprent J., Pye J. A receptor for antibody on B lymphocytes. I. Method of detection and functional significance. J Exp Med. 1972 Mar 1;135(3):610–626. doi: 10.1084/jem.135.3.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. C., Harris G., Papamichail M., Sljivić V. S., Holborow E. J. The localization of aggregated human -globulin in the spleens of normal mice. Immunology. 1973 Jun;24(6):955–968. [PMC free article] [PubMed] [Google Scholar]
- CRUICKSHANK B. Lesions of lymph nodes in rheumatoid disease and in disseminated lupus erythematosus. Scott Med J. 1958 Mar;3(3):110–119. doi: 10.1177/003693305800300302. [DOI] [PubMed] [Google Scholar]
- De Jesus D. G., Holborow E. J., Brown J. C. A defect of B-lymphocyte transport of aggregated HGG into germinal centres in NZB and NZB x NZW F 1 hybrid mice. Clin Exp Immunol. 1972 Aug;11(4):507–522. [PMC free article] [PubMed] [Google Scholar]
- Dickler H. B., Kunkel H. G. Interaction of aggregated -globulin with B lymphocytes. J Exp Med. 1972 Jul 1;136(1):191–196. doi: 10.1084/jem.136.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickler H. B. Studies of the human lymphocyte receptor for heat-aggregated or antigen-complexed immunoglobulin. J Exp Med. 1974 Aug 1;140(2):508–522. doi: 10.1084/jem.140.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froland S. S., Natvig J. B., Michaelsen T. E. Binding of aggregated IgG BY HUMAN B lymphocytes independent of Fc receptors. Scand J Immunol. 1974;3(3):375–380. doi: 10.1111/j.1365-3083.1974.tb01268.x. [DOI] [PubMed] [Google Scholar]
- Greenwood B. M., Brown J. C., De Jesus D. G., Holborow E. J. Immunosuppression in murine malaria. II. The effect on reticulo-endothelial and germinal centre function. Clin Exp Immunol. 1971 Sep;9(3):345–354. [PMC free article] [PubMed] [Google Scholar]
- Henney C. S., Stanworth D. R. The reactivity of rheumatoid factor with human gamma G globulin. Immunology. 1965 Aug;9(2):139–150. [PMC free article] [PubMed] [Google Scholar]
- Herd Z. L., Ada G. L. Distribution of 125I-immunoglobulins, IgG subunits and antigen-antibody complexes in rat lymph nodes. Aust J Exp Biol Med Sci. 1969 Feb;47(1):73–80. doi: 10.1038/icb.1969.6. [DOI] [PubMed] [Google Scholar]
- Johnson P. M., Watkins J., Scopes P. M., Tracey B. M. Differences in serum IgG structure in health and rheumatoid disease. Circular dichroism studies. Ann Rheum Dis. 1974 Jul;33(4):366–370. doi: 10.1136/ard.33.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Okafor G. O., Turner M. W., Hay F. C. Localisation of monocyte binding site of human immunoglobulin G. Nature. 1974 Mar 15;248(445):228–230. doi: 10.1038/248228a0. [DOI] [PubMed] [Google Scholar]
- Onyewotu I. I., Holborow E. J., Johnson G. D. Detection and radioassay of soluble circulating immune complexes using guinea pig peritoneal exudate cells. Nature. 1974 Mar 8;248(5444):156–159. doi: 10.1038/248156a0. [DOI] [PubMed] [Google Scholar]
- Paraskevas F., Lee S. T., Orr K. B., Israels L. G. A receptor for Fc on mouse B-lymphocytes. J Immunol. 1972 May;108(5):1319–1327. [PubMed] [Google Scholar]
- Smyth D. S., Utsumi S. Structure at the hinge region in rabbit immunoglobulin-G. Nature. 1967 Oct 28;216(5113):332–335. doi: 10.1038/216332a0. [DOI] [PubMed] [Google Scholar]
- Sordat B., Sordat M., Hess M. W., Stoner R. D., Cottier H. Specific antibody within lymphoid germinal center cells of mice after primary immunization with horseradish peroxidase: a light and electron microscopic study. J Exp Med. 1970 Jan 1;131(1):77–91. doi: 10.1084/jem.131.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M. W., Mårtensson L., Natvig J. B., Bennich H. Genetic (Gm) antigens associated with subfragments from the Fc fragment of human immunoglobulin G. Nature. 1969 Mar 22;221(5186):1166–1169. doi: 10.1038/2211166a0. [DOI] [PubMed] [Google Scholar]
- Watkins J., Roberts A., Johnson P. M. Catabolism of human IgG in mice sensitized to various IgG fragments. Similarities to the catabolism of rheumatoid IgG in mice. Immunology. 1975 Apr;28(4):755–759. [PMC free article] [PubMed] [Google Scholar]
- Watkins J., Swannell A. J. Enhanced catabolic rate and a structural anomaly in the serum IgG of RA patients. Ann Rheum Dis. 1972 May;31(3):218–219. doi: 10.1136/ard.31.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]