Abstract
The nucleocytoplasmic exchange of macromolecules is mediated by receptors specialized in passage through the nuclear pore complex. The majority of these receptors belong to the importin β protein family, which has 14 members in Saccharomyces cerevisiae. Nine importins carry various cargos from the cytoplasm into the nucleus, whereas four exportins mediate nuclear export. Kap120 is the only receptor whose transport cargo has not been found previously. Here, we characterize Kap120 as an importin for the ribosome maturation factor Rpf1, which was identified in a two-hybrid screen. Kap120 binds directly to Rpf1 in vitro and is released by Ran-GTP. At least three parallel import pathways exist for Rpf1, since nuclear import is defective in strains with the importins Kap120, Kap114, and Nmd5 deleted. Both kap120 and rpf1 mutants accumulate large ribosomal subunits in the nucleus. The nuclear accumulation of 60S ribosomal subunits in kap120 mutants is abolished upon RPF1 overexpression, indicating that Kap120 does not function in the actual ribosomal export step but rather in import of ribosome maturation factors.
A characteristic feature of eukaryotic cells is that RNA synthesis and protein synthesis are separated by intracellular membranes. The compartmentalization entails transport processes between the cytoplasm and the nucleus, which take place through the nuclear pore complex (NPC). The NPCs are embedded in the double membrane of the nuclear envelope and are among the largest known protein complexes, with a size of 125 MDa in vertebrate cells and 60 MDa in the yeast Saccharomyces cerevisiae. They consist of multiple copies of at least 30 different proteins (nucleoporins) and allow the passage of molecules up to 40 to 60 kDa by passive diffusion (10, 48). However, macromolecules of a size even smaller than this diffusion limit are transported by facilitated translocation in a receptor-mediated and energy-dependent process (reviewed in references 15, 58, and 62).
Nucleocytoplasmic transport is primarily mediated by importin β-related receptors, which shuttle continuously between the cytoplasm and the nucleoplasm. These receptors have a molecular mass of 95 to 140 kDa but migrate rapidly through the NPC (46). The transport receptors contain a characteristic N-terminal Ran-GTP binding domain and various numbers of so-called HEAT repeats mediating the interaction with their cargo and the NPC (9). According to their function in import or export, the receptors can be divided into importins and exportins. The two receptor types respond in opposite ways to Ran-GTP binding.
The Ran GTPase is primarily bound to GDP in the cytoplasm and predominantly present as Ran-GTP within the nucleus (27). The asymmetric distribution of Ran-GTP is maintained by the localization of the Ran regulators, the cytoplasmically located GTPase-activating protein RanGAP (Rna1 in yeast), and the nuclear nucleotide exchange factor RCC1 (Prp20). Importins bind their transport substrate at low Ran-GTP levels in the cytoplasmic compartment. After NPC passage, importins release their cargo upon binding to Ran-GTP (17, 45). The cargo binding of exportins requires simultaneous association with the GTP-bound form of Ran in the nucleus. A trimeric cargo-exportin-Ran-GTP complex forms in the nucleus and dissociates in the cytoplasm when the Ran-bound GTP is hydrolyzed (14, 30, 54). Similarly, importins release Ran after export and GTP hydrolysis. Thus, a single GTP is consumed for one transport cycle of a given receptor. The Ran GTPase system provides a very efficient way to promote cargo binding/release cycles and to determine the transport direction (16, 40).
Whereas more than 20 members of the importin β family have been identified in vertebrates, the budding yeast Saccharomyces cerevisiae encodes 14 transport receptors. Remarkably, only four of these are essential proteins (Kap95, Pse1, Xpo1, and Cse1), which points to the existence of redundant transport pathways. Nine receptors were characterized as importins (Kap95, Pse1/Kap121, Yrb4/Kap123, Kap104, Sxm1, Kap114, Nmd5, Pdr6, and Mtr10) (reviewed in reference 15). The three yeast exportins Xpo1, Los1, and Cse1 carry the same cargo as their mammalian counterparts. Xpo1/Crm1 recognizes leucine-rich nuclear export signals (56), and Los1 is the export receptor for tRNAs (21). Cse1 is the specific exportin for importin α, which together with importin β mediates the nuclear uptake of proteins containing a classical nuclear localization signal (NLS) (22, 29, 54). Msn5 was found to be an exportin for various cargos (5, 6, 11, 26) but is also involved in the import of the replication protein A (64).
No transport cargo has been described for the yeast importin β-like protein Kap120. However, an export defect of the large ribosomal subunit was detected in cells with KAP120 deleted (57). Although the export pathways for the two ribosomal subunits are unknown, a number of factors necessary for ribosomal export have been found (32, 39, 57). The biogenesis of ribosomal subunits, which consist of 76 ribosomal proteins and 4 ribosomal RNAs, requires more than 70 additional factors, like snoRNAs, processing enzymes, and RNA helicases. These factors are involved in the temporally and spatially regulated assembly and maturation of the subunits in the nucleolus, the nucleoplasm, and the cytoplasm. The 35S pre-rRNA comprises the 18S rRNA of the small ribosomal subunit as well as the 25S and 5.8S rRNAs, which are found together with the 5S rRNA in the large ribosomal subunit. During ribosomal subunit assembly, the 35S precursor is extensively cleaved, processed, and modified (reviewed in references 28 and 60).
Here, we report the identification of Rpf1 as an import cargo for Kap120. Purified Kap120 binds to the GTP-bound form of Gsp1, the yeast homologue of Ran. Rpf1 also binds directly to Kap120 and is released by Gsp1-GTP binding to Kap120. Therefore, Kap120 has the characteristic properties of an importin. Rpf1 also associates with other importins in a Gsp1-dependent manner. At least three different importins, Kap120, Kap114, and Nmd5, can import Rpf1. The in vivo nuclear import is significantly inhibited in strains with all of these three importins deleted. We demonstrate that Rpf1 is a predominantly nucleolar protein which is required for 25S rRNA processing and for export of the large ribosomal subunit. Furthermore, we show that the export defect of large ribosomal subunits in kap120-null cells is reverted to a large extent when RPF1 is overexpressed, indicating that Kap120 is not a ribosomal export factor.
MATERIALS AND METHODS
Strains and plasmids.
Yeast strains and plasmids used in this study are listed in Table 1. The KAP114::LEU2 deletion strain (GSY503) was described before (18). The KAP120 knockout strains (KAP120::TRP1 MATα [GSY511] and MATa [GSY512]; KAP120::URA3 MATα [GSY513] and MATa [GSY514]) and NMD5 (MATα [GSY497] and MATa [GSY498]) were constructed by replacement of DNA fragments containing the respective coding sequence by selectable markers (25) using double crossover integration. Plasmid fragments containing 5′ and 3′ regions of the KAP120 or NMD5 gene were used to transform a wild-type strain. In the KAP120 deletion strains, TRP1 or URA3 substitutes for a HindIII fragment of the KAP120 open reading frame. Likewise, the NMD5-null strains contain a replacement within the NMD5 coding sequence by TRP1. Double or triple knockouts were isolated after crossing the KAP120-, KAP114-, and NMD5-null strains and subsequent tetrad dissection of the respective diploids. Carboxy-terminal green fluorescent protein (GFP) fusion proteins were generated by genomic integration as described previously (33). Appropriate oligonucleotides complementary to 3′ sequences of RPF1 or RPL11b and plasmid pFA6a-GFP(S65T)-His3MX6 (33) as the DNA template were used to amplify a PCR product, which was used for transformation. All genomic integrations were confirmed by control PCRs and immunoblot analysis.
TABLE 1.
Yeast strains and plasmids used in this study
| Strain or plasmid description | Genotype or encoded protein | Source or reference |
|---|---|---|
| Strains | ||
| Wild type | MATaura3-52 leu2Δ1 his3Δ200 trp1Δ63 (S288C, GSY155) | 51 |
| EGY48 | MATα ura3 leu2 his3 trp1 lexA op::LEU2 (GSY168) | OriGene |
| RPF1-GFP | MATaura3-52 leu2Δ1 his3Δ200 trp1Δ63 RPF1-GFP::HIS3 (GSY1044) | This study |
| prp20-1 RPF1-GFP | MATα ura3 leu2 trp1 prp20-1 RPF1-GFP::TRP1 (GSY1052) | This study |
| Δkap120 RPF1-GFP | MATα ura3 leu2 his3 trp1 KAP120::TRP1 RPF1-GFP::HIS3 (GSY1045) | This study |
| Δkap114 RPF1-GFP | MATα ura3 leu2 his3 trp1 KAP114::LEU2 RPF1-GFP::HIS3 (GSY1046) | This study |
| Δnmd5 RPF1-GFP | MATα ura3 leu2 his3 trp1 NMD5::TRP1 RPF1-GFP::HIS3 (GSY1050) | This study |
| Δkap114 Δkap120 RPF1-GFP | MATα ura3 leu2 his3 trp1 KAP114::LEU2 KAP120::TRP1 RPF1-GFP::HIS3 (GSY1047) | This study |
| Δnmd5 Δkap120 RPF1-GFP | MATα ura3 leu2 his3 trp1 NMD5::TRP1 KAP120::URA3 RPF1-GFP::HIS3 (GSY1051) | This study |
| Δnmd5 Δkap114 RPF1-GFP | MATα ura3 leu2 his3 trp1 NMD5::TRP1 KAP114::LEU2 RPF1-GFP::HIS3 (GSY1049) | This study |
| Δnmd5 Δkap114 Δkap120 RPF1-GFP | MATα ura3 leu2 his3 trp1 NMD5::TRP1 KAP114::LEU2 KAP120::URA3 RPF1-GFP::HIS3 (GSY1048) | This study |
| RPL11b-GFP | MATaura3 leu2 his3 trp1 RPL11b-GFP::HIS3 (GSY1036) | This study |
| Δnmd5 RPL11b-GFP | MATα ura3 leu2 his3 trp1 NMD5::TRP1 RPL11b-GFP::HIS3 (GSY1043) | This study |
| Δkap114 RPL11b-GFP | MATα ura3 leu2 his3 trp1 KAP114::LEU2 RPL11b-GFP::HIS3 (GSY1042) | This study |
| Δkap120 RPL11b-GFP | MATα ura3 leu2 his3 trp1 KAP120::TRP1 RPL11b-GFP::HIS3 (GSY1039) | This study |
| rpf1-1 | MATaura3 leu2 his3 trp1 ade2 RPF1::HIS3 (pRS315-rpf1-1) (GSY1076) | This study |
| rpf1-1 RPL11b-GFP | MATaura3 leu2 his3 trp1 ade2 RPF1::HIS3 RPL11b-GFP::TRP1 (pRS315-rpf1-1) (GSY1148) | This study |
| Plasmids | ||
| pSH18-34 (pGS479) 2μm URA3 lexA op::lacZ | β-Galactosidase | OriGene |
| pEG202 (pGS474) 2μm HIS3 | lexA DNA BD | OriGene |
| pEG-Bam (pGS898) 2μm HIS3 | lexA DNA BD | This study |
| pEG-KAP120 (pGS1020) | BD-Kap120 | This study |
| pJG4-5 (pGS476) 2μm TRP1 | B42 AD | OriGene |
| pJG-Bam (pGS898) 2μm TRP1 | B42 AD | This study |
| pJG-GSP1 (pGS893) | AD-Gsp1 | This study |
| pJG4-5-RPF1 47-295 (pGS1600) | AD-Rpf1 (47-295, library isolate) | This study |
| pJG-RPF1 (pGS1460) | AD-Rpf1 (1-295, full-length) | This study |
| pQE9-GSP1 Q71L (pGS468) | 6His-Gsp1 Q71L | 35 |
| pQE60-NMD5 (pGS1090) | Nmd5-6His | 18 |
| pQE60-KAP120 (pGS1441) | Kap120-6His | This study |
| pQE30-RPF1 (pGS1465) | 6His-Rpf1 | This study |
| pGEX-5G-KAP120 (pGS1235) | GST-Kap120 | This study |
| pGEX-4T-SXM1 (pGS1295) | GST-Sxm1 | This study |
| pGEX-4TEV-KAP104 (pGS1010) | GST-Kap104 | 35 |
| pGEX-4TEV-MTR10 (pGS882) | GST-Mtr10 | This study |
| pGEX-4T-KAP114 (pGS1188) | GST-Kap114 | This study |
| pGEX-4T-NMD5 (pGS1104) | GST-Nmd5 | This study |
| pGEX-4TEV-KAP95 (pGS962) | GST-Kap95 | 35 |
| pGEX-4T-PDR6 (pGS1124) | GST-Pdr6 | This study |
| pGEX-4T-MSN5 (pGS1095) | GST-Msn5 | This study |
| YEpGAL-GST-SXM1 (pGS1209) 2μm URA3 | GST-Sxm1 | 18 |
| YEpGAL-GST-PSE1 (pGS338) 2μm URA3 | GST-Pse1 | 52 |
| YEpGAL-GST-YRB4 (pGS334) 2μm URA3 | GST-Yrb4 | 52 |
| YEpGAL-GST-RPF1 (pGS1466) 2μm URA3 | GST-Rpf1 | This study |
| YCpGAL-GFP-KAP120 (pGS1233) CEN URA3 | GFP-Kap120 | This study |
| YCpGAL-KAP120 (pGS1231) CEN URA3 | Kap120 | This study |
| YCpGAL-KAP120ΔN CEN URA3 | Kap120ΔN (133-1,032) | This study |
| YCpGAL-SXM1 (pGS1206) CEN URA3 | Sxm1 | This study |
| YCpGAL-SXM1ΔN (pGS1210) CEN URA3 | Sxm1ΔN (125-944) | This study |
| pQE70-T7-RPF1 (pGS1298) | Rpf1 | This study |
| pRS316-RPF1 (pGS1454) CEN URA3 | Rpf1 | This study |
| pRS315-RPF1 (pGS1453) CEN LEU2 | Rpf1 | This study |
| pRS315-rpf1-1 (pGS1601) CEN LEU2 | Rpf1-1 | This study |
| pRS426-RPF1 (pGS1455) 2μm URA3 | Rpf1 | This study |
The RPF1 deletion strain was generated by genomic integration as described previously (33). Diploid wild-type cells were transformed with a PCR product generated with plasmid pFA6a-His3MX6 as the template. Tetrad dissection of the resulting heterozygous diploid strain (GSY1067) yielded only two viable His− spores, which confirms that RPF1 is an essential gene (63). The temperature-sensitive rpf1-1 strain (GSY1076) was constructed by transforming a haploid strain with RPF1 deleted but containing a RPF1 CEN URA3 plasmid (GSY921) with a gapped RPF1 CEN LEU2 plasmid digested with the restriction endonucleases BsaBI and SalI, which removes most of the RPF1 coding region (655 bp, codons 8 to 226) but leaves the 5′ and 3′ sequences of RPF1, and with an overlapping 915-bp RPF1 fragment generated by a mutagenic PCR (0.5 mM MnCl2 and excess of dGTP were added to the reaction) with Taq polymerase (MBI Fermentas). This approach leads to plasmid repair by the use of the PCR product via homologous recombination in yeast. The resulting colonies were incubated on plates containing 5-fluoroorotic acid to remove the URA3 plasmid and then screened for temperature sensitivity. The rpf1-1 LEU2 plasmid was isolated, amplified in Escherichia coli, and sequenced to determine the rpf1-1 mutations. Defining the RPF1 start codon as positions 1 to 3, the base exchanges on the DNA level were A164G, A259G, A376G, C505T, A571G, A575T, and A602G. This corresponds to the amino acid substitutions N55S, S87G, R126G, P169S, S192C, and N201D.
For expression in E. coli or yeast, the coding regions of the respective genes were amplified by PCR with the proofreading Pwo polymerase (Roche) and inserted as BamHI/BamHI or BamHI/XhoI fragments into the following vectors: pGEX-4T (Amersham Pharmacia), pGEX4-TEV, pGEX-5G (55), YEpGAL-GST (pGS139), YCpGAL-GFP (pGS372) (52), pQE30, pQE60 (QIAGEN), pQE70-T7 (pGS435) (52), pEG-Bam, and pJG-Bam (Table 1). The RPF1 gene, including its own promoter, was PCR amplified as a 1,539-bp XbaI/XhoI fragment and inserted into pRS315, pRS316 (53), and pRS426 (8). The multicloning sites of plasmids pEG202 and pJG4-5 provided by OriGene Technologies (Rockville, MD) were remodeled to obtain BamHI sites for subcloning with the suitable reading frame 5′-GGA.TTC-3′. Plasmid pEG202 was cleaved with EcoRI, treated with mung bean nuclease to digest the overhanging ends, and then ligated with T4 DNA ligase. The resulting plasmid pEG-Bam lacks an EcoRI site and contains a BamHI site with the desired reading frame. The BamHI site of pJG4-5 at position 2650 was removed by cleavage with BamHI, filling in of the overhangs with Klenow polymerase, and religation. This plasmid was then treated with EcoRI, Klenow polymerase, and T4 DNA ligase in the presence of annealed 5′-CGGATCCG-3′ linkers. Digestion with BamHI and religation gave rise to the plasmid pJG-Bam.
Two-hybrid screen.
The two-hybrid assays (7) were performed using the DupLEX-A system (OriGene Technologies). The strain EGY48, carrying an integrated lexA operon-LEU2 reporter, was transformed with the lexA operon-lacZ reporter plasmid pSH18-34 and with the bait plasmid pEG-KAP120 encoding an in-frame fusion of the lexA DNA binding domain (BD) with the complete coding sequence of KAP120. The synthesis of the BD-Kap120 fusion protein in these cells was confirmed by immunoblotting analysis with anti-LexA antibodies (Santa Cruz Biotechnology). The strain was transformed with a pJG4-5 library (OriGene Technologies), which contains fusions of the B42 activation domain (AD) with fragments of yeast genomic DNA under the control of the galactose-inducible GAL1 promoter. Two million transformants were plated onto agar plates with synthetic complete media lacking leucine and incubated for 5 days at 30°C. The Leu+ transformants were further screened for the formation of blue colonies due to β-galactosidase expression on plates containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) as a second test for interaction. After establishing that both interaction assays were galactose dependent, the library plasmids from candidate clones were isolated, amplified in E. coli KC8, and retransformed into EGY48/pSH18-34/pEG-KAP120 cells. The assays for galactose-dependent growth on Leu− plates and blue colony formation on X-Gal plates were repeated. The DNA of positive clones was sequenced to determine the interacting genes. The filter lift β-galactosidase assays with nitrocellulose and X-Gal were performed as described by the manufacturer (OriGene manual).
Protein and RNA analysis.
Cell lysate preparation and the purification of recombinant proteins were performed as described previously (35, 52). Glutathione S-transferase (GST) and GST fusion proteins were purified by affinity chromatography on glutathione Sepharose (Amersham Pharmacia). Fusion proteins to N-terminal or C-terminal six-histidine tags were purified with nickel-nitrilotriacetic acid agarose (QIAGEN). GST-Kap95, GST-Kap114, Kap120-6His, and Nmd5-6His were further purified by Mono Q (Amersham Pharmacia) chromatography. Kap120-6His and 6His-Rpf1 were used for the immunization of rabbits to obtain polyclonal antibodies. The affinity purification of these antibodies was performed as described previously (52). 6His-Gsp1Q71L loaded with GTP or GDP was purified on Mono S as described previously (35). Rpf1 was synthesized with the linked in vitro T7 transcription/translation kit (Roche) in the presence of [35S]methionine and linearized pQE70-T7-RPF1 plasmid. The reticulocyte lysates were diluted 1:100 into reaction mixtures containing GST fusion proteins bound to glutathione Sepharose in PBSKMT buffer (25 mM Na phosphate, 150 mM NaCl, 3 mM KCl, 1 mM MgCl2, 0.1% Tween 20, pH 7.3). For solution binding assays, GST fusion proteins were bound to glutathione Sepharose by incubation for 30 min at 4°C. After three washes with 1 ml PBSKMT buffer, six-histidine fusion proteins were added to a volume of 300 μl. The reaction mixtures were incubated for 30 or 60 min at 4°C. Bound proteins were washed three times with PBSKMT buffer and then eluted by incubation with sodium dodecyl sulfate (SDS) sample buffer for 4 min at 95°C. Direct immunofluorescence microscopy was used to visualize GFP-Kap120 in logarithmically grown cells with a Zeiss Axioscope at 1,000-fold magnification. GFP-Kap120 is functional because it rescues the Rpf1 mislocalization phenotype in a kap114 nmd5 strain background. Indirect immunofluorescence microscopy using affinity-purified polyclonal antibodies against GFP, Rpf1, and Kap120 or monoclonal antibodies against Nop1 (4) and staining of the cells with the DNA dye DAPI (4′,6′-diamidino-2-phenylindole) were described before (52, 54). Western blot analysis was carried out with the enhanced chemiluminescence kit (Amersham Pharmacia) using horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (Sigma) as secondary antibody.
Newly synthesized rRNA was analyzed by [methyl-3H]methionine pulse-chase labeling as described previously (57). Liquid cultures were grown in synthetic complete medium without methionine. At log phase (A600 = 1.5), the cells were pulsed with 100 μCi [methyl-3H]methionine (Amersham Pharmacia) for 3 min and then chased with 4 mM nonradioactive methionine. Samples corresponding to 15 optical density units were taken after 0, 3, and 10 min. After glass bead lysis of the yeast cells, total RNA was extracted with phenol and separated on 1.25% agarose gels containing 6.6% formaldehyde, as described previously (61). The RNA was blotted onto a Hybond-N membrane (Amersham Pharmacia), which was sprayed with En3Hance (Perkin-Elmer Life Sciences) and detected by exposition of X-ray films.
RESULTS
Kap120 is a Ran-binding protein primarily located in the nucleus.
In order to localize Kap120, we determined its intracellular distribution by indirect immunofluorescence microscopy. We generated polyclonal antibodies directed against recombinant Kap120. After affinity purification, these antibodies recognized in Western blots a protein of the expected size of 120 kDa in wild-type cells but not in cells with KAP120 deleted (data not shown). Standard immunofluorescence microscopy using formaldehyde-fixed cells showed that Kap120 is predominantly nuclear and also present in the cytoplasm to a lesser degree (Fig. 1A). Because formaldehyde fixation often causes loss of the NPC staining (52), we also localized Kap120 in living cells. A fusion of the GFP to Kap120 was again mostly nuclear but also found at the nuclear rim, indicating that a fraction of Kap120 resides at the nuclear envelope (Fig. 1A). This is in agreement with a previously reported partial punctate rim staining pattern of a Kap120-protein A fusion protein (49) and with a cytoplasmic and nuclear localization of a Kap120-GFP fusion protein (23). The presence of Kap120 in the cytoplasm, the nucleoplasm, and the nuclear envelope is typical for importin β-like proteins, which shuttle between the cytoplasm and the nucleus.
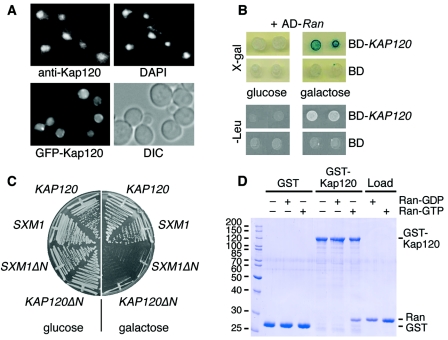
FIG. 1.
Kap120 is a mostly nuclear protein that interacts with Ran. (A) Kap120 is located predominantly in the nucleus. Wild-type yeast cells were cultured in liquid media at 30°C. The cells were prepared for immunofluorescence microscopy, probed with affinity-purified Kap120-specific antibodies and fluorescein isothiocyanate (FITC)-conjugated secondary antibodies to visualize Kap120, and treated with DAPI to stain the DNA (upper panel). Wild-type cells were transformed with plasmid pGS1233 encoding GFP-Kap120. The synthesis of the fusion protein was induced by addition of 2% galactose to liquid cultures. After 2 h, the cells were viewed by fluorescence microscopy to localize GFP-Kap120 in living cells and also viewed by Nomarski optics/differential interference contrast (DIC) (lower panel). (B) KAP120 interacts with GSP1 by two-hybrid analysis. The yeast strain EGY48 carrying the lexA operon-LEU2 reporter and the lexA operon-lacZ reporter was transformed with plasmid pJG-GSP1 containing a hybrid of the AD and the GSP1 open reading frame under control of a galactose-inducible promoter. This strain was transformed with plasmid pEG-KAP120 encoding a fusion of the lexA DNA BD with full-length Kap120 or with plasmid pEG202 encoding the BD only. Cells of two transformants were spotted onto X-Gal plates or Leu− plates containing either glucose (repression) or galactose (induction) and incubated for 3 days at 30°C. (C) Expression of KAP120ΔN causes a dominant-lethal phenotype. Wild-type cells containing plasmid YCpGAL-KAP120, YCpGAL-SXM1, YCpGAL-KAP120ΔN, or YCpGAL-SXM1ΔN as indicated were streaked on plates containing 2% glucose or 2% galactose and incubated for 2 days at 30°C. (D) Kap120 binds to Gsp1-GTP but not to Gsp1-GDP. Recombinant GST (8 μg per reaction) or a GST-Kap120 fusion protein (14 μg per reaction) purified from E. coli lysates was immobilized on glutathione Sepharose and incubated for 60 min at 4°C with buffer alone, 15 μg of Gsp1-GDP, or 15 μg of Gsp1-GTP as indicated. The bound material of the reactions was washed three times with binding buffer and aliquots were analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie blue staining together with 20% of the loads of Gsp1-GDP and Gsp1-GTP. Molecular mass markers are given in kDa.
A two-hybrid analysis demonstrated an in vivo interaction of Kap120 with Gsp1, the essential yeast homologue of Ran (Fig. 1B). The expression of a lexA DNA binding domain fusion with Kap120 and of an activation domain fusion with Gsp1 led to the transcription of two independent reporter genes, lacZ and LEU2, which was detected by blue color formation on X-Gal plates and by growth on leucine-deficient plates. The two-hybrid interaction was specific because it was not detected with the DNA binding domain alone or when the expression of the activation domain-GSP1 hybrid was repressed (Fig. 1B).
The Ran-binding domain of importin β-like receptors resides in their N-terminal domain. It was shown earlier that the synthesis of N-terminally truncated Yrb4/Kap123 or Los1 causes a dominant-negative phenotype with regard to nuclear transport mechanisms and cell growth. The inhibitory effect of receptors defective in Ran-GTP binding is probably caused by jamming of nuclear pores (21, 31, 52). We tested whether the expression of KAP120ΔN, a deletion mutant of KAP120 missing the amino-terminal 132 amino acid codons, is toxic for yeast cell growth. For comparison, we also analyzed Sxm1ΔN lacking 124 amino-terminal residues, a truncation mutant of the importin Sxm1 (47). The complete coding regions as well as the ΔN fragments of KAP120 and SXM1 were cloned into a yeast expression vector under the transcriptional control of the galactose-inducible GAL1 promoter. Wild-type cells carrying these plasmids were streaked onto plates containing glucose (repression) or galactose (induction). The expression of KAP120ΔN and SXM1ΔN led to a dominant-lethal phenotype, whereas the expression of KAP120 and SXM1 had no effect on growth (Fig. 1C). These results suggest that the nonessential proteins Kap120 and Sxm1 may play a role in nuclear transport and that their function depends on the interaction with Ran-GTP.
We next tested whether Kap120 binds to Gsp1 in vitro using pull-down assays. A fusion protein of GST to Kap120 was purified from a bacterial lysate, immobilized to glutathione Sepharose, and incubated with Gsp1 loaded with either GTP or GDP. Figure 1D shows that Kap120 bound specifically to Gsp1-GTP but not to Gsp1-GDP. Gsp1-GTP did not bind to GST alone in a control reaction. The direct binding to Gsp1-GTP in the absence of additional factors suggests that Kap120 acts as an importin.
Kap120 interacts with Rpf1.
We performed a two-hybrid screen with LexA-Kap120 as the bait to identify potential transport substrates for Kap120. One of the positive clones encoded a fusion protein of the activation domain with amino acid residues 47 to 295 of Rpf1 (Fig. 2). We observed a similarly strong two-hybrid interaction between Kap120 and either Rpf147-295 or full-length Rpf1 (not shown). Rpf1 is an essential protein of 295 amino acids with a molecular mass of 35 kDa that is required for ribosome biogenesis. It contains a conserved RNA binding motif of 17 amino acid residues near its C terminus. Rpf1 depletion and immunoprecipitation experiments suggested that it is required for early steps of large ribosomal subunit rRNA maturation (61).
FIG. 2.
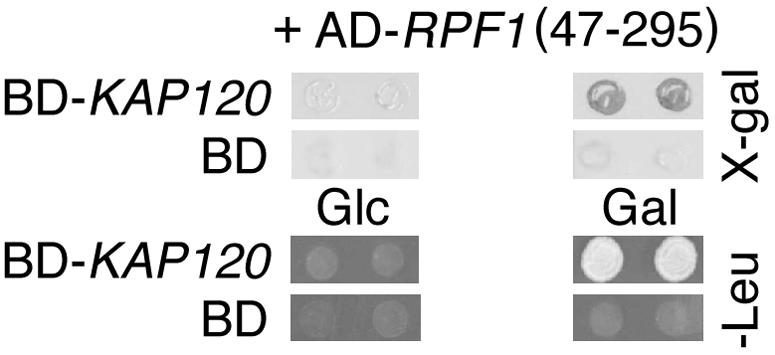
RPF1 interacts with KAP120 in two-hybrid assays. The strain EGY48 carrying LEU2 and lacZ reporters as well as plasmid pJG-RPF147-295 containing an AD fusion with codons 47 through 295 of RPF1, which was isolated in a two-hybrid screen using KAP120 as the bait, was transformed with plasmids coding for the BD-Kap120 hybrid protein or the BD only. Cells of two transformants were spotted onto Leu− plates containing glucose (Glc) or galactose (Gal) and analyzed by the X-Gal filter lift assay.
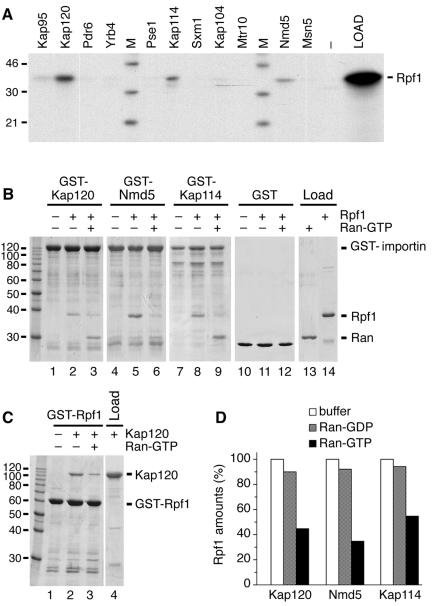
In order to examine which importin is able to bind to Rpf1 in vitro, we incubated [35S]methionine-labeled Rpf1 synthesized in a rabbit reticulocyte lysate with immobilized GST fusions to all yeast importins. We showed previously that these GST-importin fusion proteins are functional, since they all bind to Gsp1-GTP (18). Figure 3A demonstrates that Rpf1 bound to Kap120, Kap114, and Nmd5. It also showed a weak binding to Kap95 but not to the other importin fusions or GST alone. We investigated next whether Rpf1 can bind directly to Kap120, Kap114, and Nmd5 using exclusively recombinant proteins. GST-Kap120, GST-Nmd5, and GST-Kap114 were immobilized to glutathione Sepharose and incubated with a six-histidine fusion to Rpf1, which had been purified from a bacterial lysate. Rpf1 bound to these three importins (Fig. 3B, lanes 2, 5, and 8) but did not bind to GST (lane 11). Rpf1 also bound to Kap95 (data not shown). However, the binding to Kap95 (importin β) was not stimulated by Srp1 (importin α), indicating that Rpf1 does not contain a classical NLS. Because importins typically release their cargo by binding to Gsp1-GTP, we performed complexes of Rpf1 with Kap120, Nmd5, and Kap114 and then incubated with Gsp1-GTP (Fig. 3B, lanes 3, 6 and 9). Gsp1-GTP released the majority of Rpf1 and associated itself with the receptors. Similarly, Kap120 bound to immobilized GST-Rpf1 and was subsequently released by Gsp1-GTP (Fig. 3C). Thus, the association of Rpf1 with Kap120, Nmd5, and Kap114 in the absence of additional factors represents a specific cargo-importin binding which is inhibited by Gsp1-GTP. Remarkably, the dissociation of Rpf1 from the receptors by yeast Ran-GTP was not fully complete (Fig. 3D). Whereas Ran-GDP hardly affected the stability of the Rpf1-receptor complexes, most but not all Rpf1 was removed from the receptors by Ran-GTP (55 to 75% release). A fraction of these complexes were reproducibly resistant to Ran-GTP under various conditions. In analogy to previously reported similar observations (18, 41), this suggests that a so far unknown factor might assist in the process of import complex disassembly within the nucleus.
FIG. 3.
Rpf1 binds to Kap120, Kap114, and Nmd5 in a Ran-dependent manner. (A) GST fusion proteins to the indicated transport receptors (14 μg per reaction) or GST alone (−) were immobilized on glutathione Sepharose and incubated for 30 min at 4°C with [35S]methionine-labeled Rpf1 synthesized in a reticulocyte lysate. The resin was washed three times, and bound proteins as well as the Rpf1 load were analyzed by SDS-PAGE and autoradiography. Molecular mass markers (M) are in kDa. (B) Recombinant GST fusion proteins (14 μg per reaction) to Kap120 (lanes 1 to 3), Nmd5 (lanes 4 to 6), Kap114 (lanes 7 to 9), and GST alone (lanes 10 to 12) were immobilized on glutathione Sepharose and incubated for 30 min at 4°C without or with 10 μg of 6His-Rpf1 as indicated. After three washes, the samples were further incubated for 60 min with buffer alone or with 15 μg of Gsp1p-GTP (lanes 3, 6, 9, and 12). After washing, the bound material as well as 20% of the loads was analyzed by SDS-PAGE and Coomassie blue staining. (C) Immobilized GST-Rpf1 (10 μg per reaction) was incubated for 30 min at 4°C with 12 μg of a six-histidine fusion to Kap120 (lanes 2 and 3). After three washes, the bound proteins were further incubated for 30 min with buffer alone (lanes 1 and 2) or with 15 μg of Gsp1-GTP (lane 3). The reaction products were washed three times and examined by SDS-PAGE and Coomassie blue staining together with the load of Kap120. Molecular mass markers are given in kDa. (D) GST fusion proteins to Kap120, Nmd5, and Kap114 were immobilized on glutathione Sepharose and incubated with 6His-Rpf1 as described in the legend for panel B. The reaction mixtures were further incubated for 60 min with buffer alone, with 15 μg of Gsp1-GDP, or with 15 μg of Gsp1-GTP. After washing, the bound material was analyzed by SDS-PAGE. The amounts of Rpf1 bound to the receptors were quantified and compared to the buffer control.
Three different importins are required for nuclear import of Rpf1.
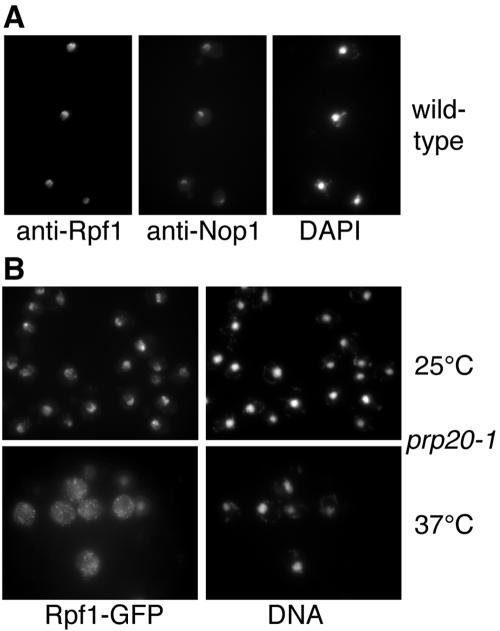
Preliminary localization data from a previous study indicated that Rpf1 might be a nucleolar protein (61). Using affinity-purified polyclonal antibodies against recombinant Rpf1, we localized Rpf1 in wild-type yeast cells (Fig. 4A). By immunofluorescence microscopy, Rpf1 colocalized with the nucleolar marker protein Nop1. Rpf1 is located in crescent-shaped structures that are typically observed for nucleoli, and it is present within the nucleus but does not colocalize with DNA. We conclude that Rpf1 resides predominantly in the nucleolus. We also analyzed strains whose chromosomal copy of RPF1 was replaced by an RPF1-GFP fusion. Rpf1-GFP was fully functional, because cells expressing the fusion protein show an identical growth behavior compared to isogenic wild-type cells (data not shown). Rpf1-GFP was again mostly nucleolar and also present in the nonnucleolar nucleoplasm to some extent (Fig. 4B and 5).
FIG. 4.
Rpf1 is a nucleolar protein that requires Ran for nuclear import. (A) Wild-type yeast cells were cultured in liquid-rich media at 30°C and prepared for immunofluorescence microscopy. The samples were probed with affinity-purified rabbit antibodies against Rpf1 and with mouse monoclonal antibodies against the nucleolar marker protein Nop1 and then incubated with FITC-conjugated secondary antirabbit antibodies and with Texas Red-conjugated secondary anti-mouse antibodies and stained with DAPI. (B) Mutant prp20-1 cells bearing a temperature-sensitive mutation in the Ran-specific guanine exchange factor and containing a chromosomal RPF1-GFP allele were cultured at 25°C, shifted for 1 h to 37°C, and analyzed by immunofluorescence microscopy. The samples were probed with anti-GFP antibodies and FITC-labeled secondary antibodies to visualize Rpf1 and treated with DAPI to stain the DNA.
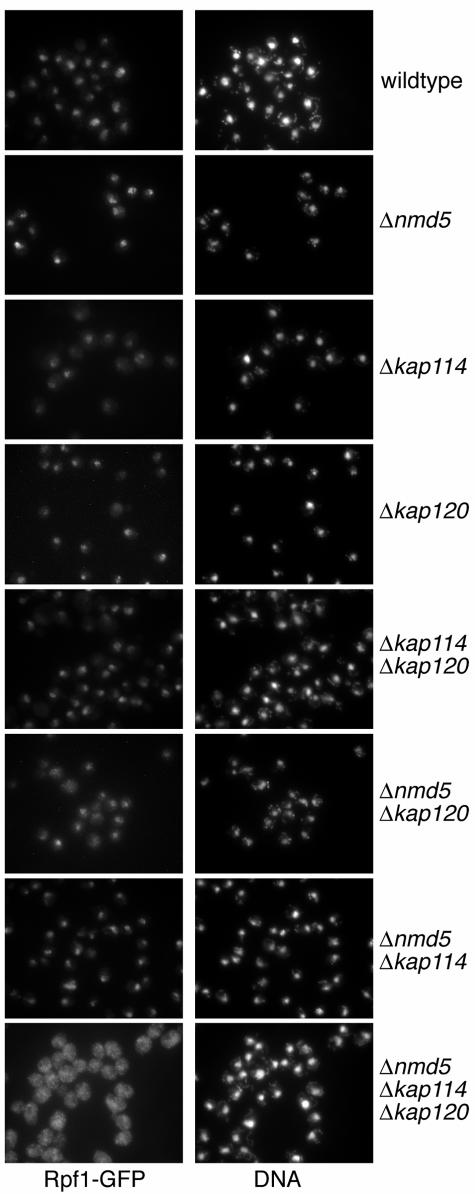
FIG. 5.
The nuclear import of Rpf1 is impaired in kap120 kap114 nmd5 triple mutants. Wild-type yeasts, cells with NMD5, KAP114, or KAP120 deleted, and strains carrying the indicated double or triple mutations were cultured in liquid-rich media at 30°C. The cells, which all contain a chromosomal integration of RPF1-GFP, were examined by immunofluorescence microscopy. The samples were probed with GFP-specific antibodies and with FITC-conjugated secondary antibodies and stained with DAPI.
To address whether Rpf1 requires Ran for nuclear uptake, we examined import in the temperature-sensitive prp20-1 mutant, which is defective in the Gsp1-specific guanine exchange factor at the nonpermissive temperature. The Ran GTPase cycle is severely perturbed after shifting these cells to 37°C. Whereas Rpf1-GFP was still mainly nucleolar at 25°C, the import of Rpf1-GFP was severely inhibited at 37°C, and it accumulated in the cytoplasm of these mutants (Fig. 4B). This indicates that nuclear import depends on the Ran GTPase.
We next analyzed Rpf1 import in vivo in various transport receptor mutants by immunofluorescence microscopy with GFP-specific antibodies (Fig. 5). Cells carrying the RPF1-GFP allele as well as a deletion of KAP120, KAP114, or NMD5 showed no cytoplasmic accumulation of Rpf1-GFP. Strains bearing all combinations of double knockouts of these importins were also not significantly inhibited in Rpf1 import, although some cytoplasmic Rpf1-GFP was detected in the kap120 nmd5 double mutant. However, the import of Rpf1 in the KAP120 KAP114 NMD5 triple-deletion mutant was dramatically defective (Fig. 5). This cytoplasmic accumulation was also observed in triple-null cells expressing untagged RPF1 (data not shown). The growth rate of the triple mutant is reduced to 65% in comparison to isogenic wild-type cells (not shown). However, other nuclear transport pathways are not affected by the triple gene deletion. The nuclear import of classical NLS proteins and the intracellular distribution of three proteins shuttling between the cytoplasm and the nucleus, Npl3 (13), importin α (54), and Yrb1 (35), were not affected by the triple mutation (not shown). We conclude that Kap120, Kap114, and Nmd5 all contribute to nuclear import of Rpf1. Remarkably, only the deletion of the three importins together reveals that they are required for import.
Rpf1 is necessary for 25S rRNA processing and export of large ribosomal subunits.
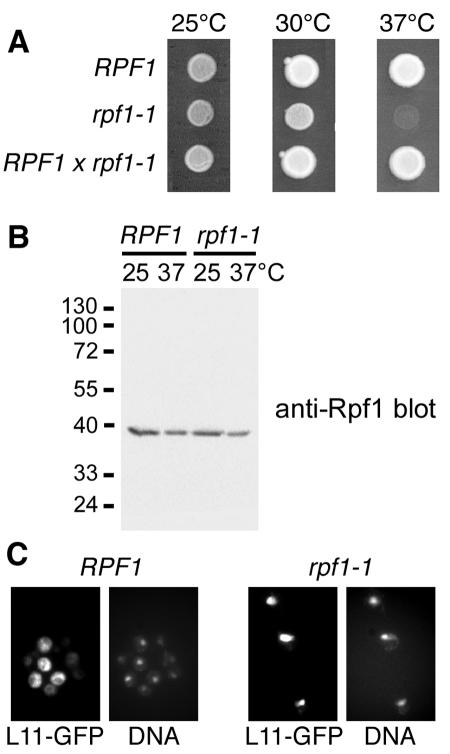
To examine the function of Rpf1, we used PCR-mediated mutagenesis to generate temperature-sensitive mutations in RPF1. The rpf1-1 mutant used in this study has five amino acid changes between residues 55 and 201 of Rpf1. The rpf1-1 mutations are recessive, since a heterozygous diploid that resulted from a cross to the wild type lost the temperature-sensitive phenotype (Fig. 6A). The mutant Rpf1-1 protein was still located in the nucleolus (data not shown) and was present in similar amounts to wild-type Rpf1 both at 25°C and 37°C (Fig. 6B).
FIG. 6.
Rpf1 is required for the export of large ribosomal subunits. (A) The rpf1-1 mutant is temperature sensitive for growth at 37°C. RPF1 wild-type cells, rpf1-1 mutants, and diploid cells generated by crossing the wild type with rpf1-1 cells were spotted onto plates containing rich yeast-peptone-dextrose media and incubated for 2 days at 25°C, 30°C, or 37°C. (B) The Rpf1-1 protein is stable at 37°C. Logarithmically grown RPF1 cells and rpf1-1 cells were cultured in liquid yeast-peptone-dextrose media at 25°C or cultured at 25°C and then shifted to 37°C for 2 h. The cells were analyzed by SDS-PAGE and Western blotting with affinity-purified Rpf1-specific antibodies. Molecular mass markers are in kDa. (C) rpf1-1 mutants are defective in export of large ribosomal subunits. RPF1 cells and rpf1-1 mutants containing a chromosomal integration of L11-GFP were grown in liquid media at 25°C and analyzed by immunofluorescence microscopy. The samples were probed with GFP-specific antibodies and with FITC-conjugated secondary antibodies and stained with the DNA dye DAPI.
To analyze the role of Rpf1 in 35S pre-rRNA processing, rpf1-1 cells were pulsed with [methyl-3H]methionine for 3 min at early log phase and then chased with an excess of nonradioactive methionine for 0, 3, or 10 min (see Fig. S1 in the supplemental material). This allows the specific labeling of newly synthesized rRNA which is highly methylated. In wild-type cells or rpf1-1 mutants transformed with an RPF1 plasmid, the processing of the 35S pre-rRNA to the mature 25S and 18S rRNAs was almost complete after 3 min at both 25°C and 37°C. The formation of the 18S rRNA was not affected in rpf1-1 mutants. However, a strong defect in processing of precursors to 25S rRNA was detected within the first 3 min of chase, which resulted in the accumulation of the 27S rRNA and 35S rRNA precursor species. The processing to 25S rRNA was especially delayed when rpf1-1 cells were shifted to 37°C. These results confirm the observation from RPF1 depletion experiments (61) that Rpf1 is required for early stages of large ribosomal subunit rRNA maturation.
Because mutations in rRNA processing factors can potentially cause an export defect of ribosomal subunits, we addressed the question of whether rpf1-1 cells are defective in the export of large ribosomal subunits. To this end, we analyzed a GFP fusion protein to Rpl11b (L11), a constituent of the large ribosomal subunit. The L11-GFP reporter protein is incorporated into 60S subunits and was previously used to detect export defects in various mutants (57). We genomically integrated the GFP coding sequence to generate the L11-GFP fusion protein, which was subsequently localized in wild-type or rpf1-1 cells (Fig. 6C). Large ribosomal subunits were efficiently exported to the cytoplasm in RPF1 cells. In contrast, rpf1-1 mutants strongly accumulated 60S subunits inside the nucleus even at the permissive temperature of 25°C, which shows that Rpf1 is required for the export of large ribosomal subunits.
RPF1 overexpression abolishes the 60S subunit export defect of kap120 mutants.
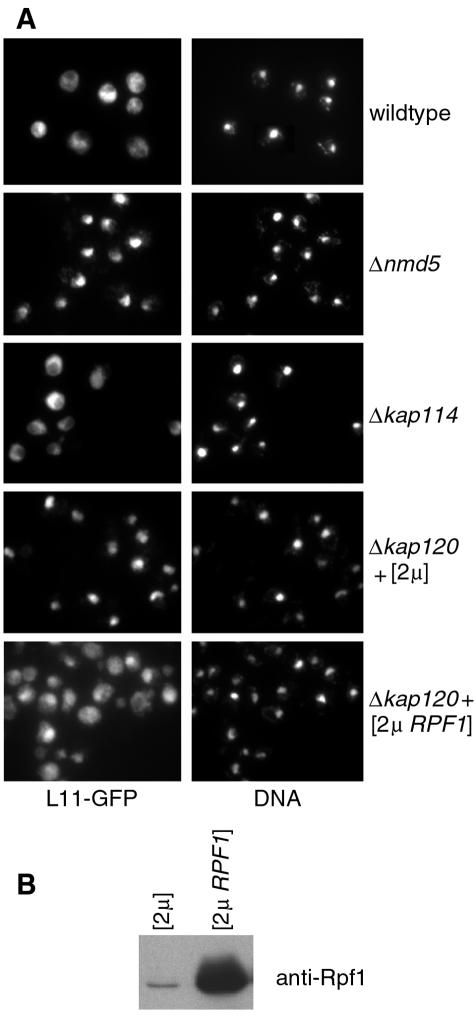
It was previously reported that KAP120 and NMD5 deletion mutants are defective in the export of 60S subunits (57). We confirmed these results with strains carrying a chromosomal allele of L11-GFP. Figure 7A shows that L11-GFP was cytoplasmic in wild-type cells and kap114 mutants, whereas it accumulated in the nuclei of kap120 and nmd5 mutants. Defective export could either be due to a direct effect, i.e., Kap120 and Nmd5 would be ribosomal export factors, or could be caused by an indirect effect. We tested the hypothesis that the export defect associated with KAP120 deletion mutants is caused by a diminished import of large ribosomal subunit maturation factors like Rpf1. In this case, excess amounts of Rpf1 would attenuate or prevent the kap120 mutant phenotype. Overexpression of RPF1 was achieved by transforming strains with a multicopy (2μm) plasmid carrying the RPF1 gene. This resulted in roughly 24-fold higher levels of Rpf1 as shown by immunoblot analysis (Fig. 7B). KAP120 deletion mutants transformed with an empty 2μm vector or with an unrelated 2μm plasmid (YEp-YRB1) still accumulated L11-GFP in the nucleus. In contrast, cells carrying the 2μm RPF1 plasmid showed a mostly even distribution of L11-GFP throughout the cell and a much less nuclear GFP signal (Fig. 7A). This effect of RPF1 overexpression was not detected in the NMD5 deletion mutant (not shown). We conclude that the large ribosomal subunit export defect of kap120 mutants is specifically abolished by RPF1 overexpression. This indicates that Kap120 is not a ribosomal export factor.
FIG. 7.
RPF1 overexpression in kap120 mutants relieves the export defect of large ribosomal subunits. (A) Wild-type cells, deletion mutants for NMD5 or KAP114, and KAP120-deletion mutants transformed with a multicopy plasmid (2μm) or with a 2μmRPF1 plasmid were grown in liquid media at 25°C. The cells, which all contain a chromosomal L11-GFP allele, were examined by immunofluorescence microscopy. The samples were probed with anti-GFP antibodies and with FITC-conjugated secondary antibodies, and the DNA was stained with DAPI. (B) The cells shown in the lower two sections of panel A were grown in liquid media and analyzed by SDS-PAGE and Western blotting with affinity-purified Rpf1-specific antibodies.
DISCUSSION
A number of properties characterize Kap120 as a nuclear transport receptor. By sequence homology, it belongs to the importin β superfamily. We showed by two-hybrid analysis that Kap120 interacts with Ran in vivo. Kap120 cofractionates with nucleoporins (3, 49) and is located in the nucleoplasm, at the nuclear envelope, and in the cytoplasm. This is a typical localization for importin β-like proteins, which shuttle between the nucleus and the cytoplasm. Furthermore, a Kap120 deletion mutant lacking the amino-terminal Ran binding domain leads to a dominant-negative phenotype. Amino-terminal truncations of the yeast transport receptors Yrb4/Kap123 and Los1 as well as of mammalian importin β were shown previously to block bidirectional transport processes through the NPC (21, 31, 52).
Purified Kap120 binds directly to the GTP-bound form of Ran in pull-down assays. This indicates that Kap120 belongs to the importins, which associate with Ran without the need for additional factors, whereas the binding of exportins to Ran requires the presence of their cognate cargo. We cannot exclude the possibility that Kap120 has a function as both an importin and an exportin, as was observed for yeast Msn5 (64) and mammalian importin 13 (36). Nevertheless, Rpf1 behaves like a typical import cargo. First, it directly binds to Kap120 and is subsequently released from Kap120 by Ran-GTP in vitro. Second, the nuclear import is inhibited in the absence of Kap120 in vivo.
These two criteria, Ran-dependent binding and an in vivo function in import of Rpf1, characterize Kap114 and Nmd5 as importins for Rpf1 as well. Remarkably, the inhibition of nuclear uptake is not detected until KAP120, KAP114, and NMD5 are deleted from the genome altogether. In the strains with single and double knockouts of the importins encoded by KAP120, KAP114, and NMD5, we observed no or only a slight cytoplasmic accumulation. Rpf1 completely mislocalizes to the cytoplasm solely in triple-knockout cells. This indicates the presence of at least three independent import pathways for Rpf1. Because the KAP120 KAP114 NMD5 deletion strain is still viable, yet another parallel import path has to exist which mediates import of the essential protein Rpf1. Because we observed a weak binding of Rpf1 to Kap95, import is likely to be performed also by Kap95, the essential yeast homologue of importin β. Since importin α does not stimulate the binding of Kap95, Rpf1 possesses no classical NLS typically consisting of one or two short clusters of basic amino acid residues. The nature of the Rpf1 nuclear targeting signal remains to be investigated. It is also not clear so far whether the different importins recognize the same motif within Rpf1. Nmd5 mediates the import of the mitogen-activated protein kinase Hog1 (12) as well as of the transcription factors TFIIS (2) and Crz1 (44). Kap114 was reported to perform the nuclear import of the TATA-binding protein Tbp1 (41) and of the core histones H2A and H2B (18, 38). With Rpf1 as an additional transport substrate, Nmd5 and Kap114 belong, in addition to Pse1 and Yrb4/Kap123, to those importins that recognize a wide range of cargos.
In this study, we describe Rpf1 as the first import cargo for Kap120. Importin 11, the mammalian ortholog of Kap120 (24% sequence identity), constitutes, like Kap120, a nuclear import receptor. Importin 11 is involved in nuclear import of the ubiquitin-conjugating enzyme UbcM2 (42) and the ribosomal protein L12 (43). Interestingly, like Rpf1, the two mammalian import substrates UbcM2 and L12 were also identified in two-hybrid screens with the importin as the bait. Rpf1 is highly conserved through evolution and shares a sequence identity of up to 50% with other eukaryotic homologues. It remains to be seen whether importin 11 also mediates the import of mammalian Rpf1. An interaction of Kap120 with Ubc4 and Ubc5, the yeast counterparts of UbcM2 (34), was not detectable by two-hybrid analysis (our unpublished observation). Further cargos for Kap120 besides Rpf1 are still to be discovered.
It was reported for a number of import pathways that an essential cargo is transported by a nonessential importin (1, 41, 59, 64). It must be postulated that all of these cargos are also imported on alternative routes. Thus, the nuclear import of Rpf1 by several parallel transport pathways is not unusual. Other examples for the import of one cargo by different importins are ribosomal proteins (50, 52), the core proteins of the signal recognition particle (19), and the histones (18, 37, 38). It seems to be a general phenomenon that one importin is the main receptor for a given cargo but other importins functionally overlap to support nuclear import. On the one hand, this redundancy offers the possibility of complex regulation processes of nucleocytoplasmic transport; on the other hand, it explains why only two out of ten importins are essential in yeast.
Rpf1 was identified as a component of the 66S preribosomal particle (20). In agreement with its function in ribosome biogenesis, we found that Rpf1 resides predominantly in the nucleolus. The Rpf1 localization depends on a functional Ran gradient, as demonstrated by the cytoplasmic mislocalization of Rpf1 in a yeast strain mutated for the Ran-specific nucleotide exchange factor. This observation is in accordance with the Ran-dependent interaction of Rpf1 with Kap120, Kap114, and Nmd5. A function of Rpf1 in the synthesis of large ribosomal subunit rRNA and in processing of the 27S precursor was previously shown by gene depletion experiments (61). We generated a temperature-sensitive mutant, rpf1-1, in which the formation of mature 25S rRNA from the 27S precursor is strongly delayed at the restrictive temperature. This supports the idea that Rpf1 is required for early steps of large ribosomal subunit RNA maturation. Rpf1 possesses a σ70-like helix-turn-helix RNA binding motif at the C terminus (residues 254 to 270). This motif is essential for the function of Rpf1 in RNA processing and is well conserved among the members of the Imp4 superfamily, which are all involved in ribosome biogenesis (61). None of the rpf1-1 point mutations is located within the σ70-like motif. Therefore, the binding to RNA is presumably unaffected. Since the expression level and the localization of Rpf1-1 are also unchanged, it seems likely that the function in rRNA maturation itself is impaired. The processing defect apparently causes an accumulation of large ribosomal subunits in the nucleus and leads to a severe inhibition of export of large subunits. This export defect was specific, because the rpf1-1 mutations had no effect on the localization of small ribosomal subunits (our unpublished result), which is in agreement with an unchanged efficiency of 20S precursor rRNA processing (see Fig. S1 in the supplemental material).
The nuclear import of ribosomal proteins is performed at least partially by the yeast importins Yrb4/Kap123 and Pse1 (50, 52), but other importins are most likely also involved (24). Moreover, several dozens of ribosomal assembly factors (28, 60) are expected to be imported into the nucleus separately by various importins. The accumulation of ribosomal subunits within the nucleus can be caused by an inhibition of ribosomal protein import, defective import of ribosome maturation factors, impaired ribosome assembly, or a failure in export. Nuclear accumulation of 60S subunits was detected in kap120 mutants (Fig. 7) (57). Which stage of ribosome biogenesis is affected in these mutants? Remarkably, the 35S pre-rRNA processing is delayed in kap120 mutants (57). Therefore, kap120 mutants and rpf1 mutants display similar phenotypes. Our results show that the nuclear accumulation of large ribosomal subunits in kap120 mutants is considerably released by RPF1 overexpression. Thus, Kap120 is dispensable for the ribosomal protein import step and the subunit export step. This strongly suggests that Kap120 is important for ribosome assembly. Interestingly, the kap120 mutant is affected in rRNA processing, and yet the nuclear import defect of Rpf1 is only obvious when, besides Kap120, Kap114 and Nmd5 also are deleted. This suggests that either the nuclear level of Rpf1 is highly critical for ribosomal maturation or that Rpf1 is not the primary factor that contributes to the maturation defect. One scenario is that a diminished import of Rpf1 in kap120 mutants causes a defect in large ribosomal subunit maturation, which in turn leads to an export block. Increasing amounts of Rpf1 are expected to compensate for compromised nuclear import. It is also possible that Kap120 is itself mechanistically involved in 27S rRNA processing and that RPF1 overexpression overcomes the absence of KAP120. Another explanation is that Rpf1 recruits export factors to the maturing large subunit and that RPF1 overexpression enhances the export velocity. The latter explanation is rather unlikely, because the nuclear accumulation of 60S subunits in the NMD5-null strain was not affected by RPF1 overexpression. Therefore, Nmd5 is likely to promote the import of another factor required for ribosome biogenesis. Moreover, since RPF1 overexpression did not reduce the 27S rRNA processing defect associated with the KAP120 deletion (our unpublished observation), we expect that yet another processing factor is imported by Kap120.
Supplementary Material
Acknowledgments
We thank Sandra Helfen, Karsten Mayr, and Silke Guthörl for expert technical assistance and Bernd Pohl for the construction of the pEG-Bam and pJG-Bam plasmids.
This work was supported by grants from the Deutsche Forschungsgemeinschaft.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Aitchison, J. D., G. Blobel, and M. P. Rout. 1996. Kap104p: a karyopherin involved in the nuclear transport of messenger RNA binding proteins. Science 274:624-627. [DOI] [PubMed] [Google Scholar]
- 2.Albertini, M., L. F. Pemberton, J. S. Rosenblum, and G. Blobel. 1998. A novel nuclear import pathway for the transcription factor TFIIS. J. Cell Biol. 143:1447-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen, N. P., L. Huang, A. Burlingame, and M. Rexach. 2001. Proteomic analysis of nucleoporin interacting proteins. J. Biol. Chem. 276:29268-29274. [DOI] [PubMed] [Google Scholar]
- 4.Aris, J., and G. Blobel. 1988. Identification and characterization of a yeast nucleolar protein that is similar to a rat liver nucleolar protein. J. Cell Biol. 107:17-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blondel, M., P. M. Alepuz, L. S. Huang, S. Shaham, G. Ammerer, and M. Peter. 1999. Nuclear export of Far1p in response to pheromones requires the export receptor Msn5p/Ste21p. Genes Dev. 13:2284-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boustany, L. M., and M. S. Cyert. 2002. Calcineurin-dependent regulation of Crz1p nuclear export requires Msn5p and a conserved calcineurin docking site. Genes Dev. 16:608-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brent, R., and R. L. Finley. 1997. Understanding gene and allele function with two-hybrid methods. Annu. Rev. Genet. 31:663-704. [DOI] [PubMed] [Google Scholar]
- 8.Christianson, T. W., R. S. Sikorski, M. Dante, J. H. Shero, and P. Hieter. 1992. Multi-functional yeast high-copy-number shuttle vectors. Gene 110:119-122. [DOI] [PubMed] [Google Scholar]
- 9.Conti, E., and E. Izaurralde. 2001. Nucleocytoplasmic transport enters the atomic age. Curr. Opin. Cell Biol. 13:310-319. [DOI] [PubMed] [Google Scholar]
- 10.Cronshaw, J. M., A. N. Krutchinsky, W. Zhang, B. T. Chait, and M. J. Matunis. 2002. Proteomic analysis of the mammalian nuclear pore complex. J. Cell Biol. 158:915-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeVit, M. J., and M. Johnston. 1999. The nuclear exportin Msn5 is required for nuclear export of the Mig1 glucose repressor of Saccharomyces cerevisiae. Curr. Biol. 9:1231-1241. [DOI] [PubMed] [Google Scholar]
- 12.Ferrigno, P., F. Posas, D. Koepp, H. Saito, and P. A. Silver. 1998. Regulated nucleo/cytoplasmic exchange of HOG1 MAPK requires the importin beta homologs NMD5 and XPO1. EMBO J. 17:5606-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flach, J., M. Bossie, J. Vogel, A. H. Corbett, T. Jinks, D. A. Willins, and P. A. Silver. 1994. A yeast RNA-binding protein shuttles between the nucleus and the cytoplasm. Mol. Cell. Biol. 14:8399-8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fornerod, M., M. Ohno, M. Yoshida, and I. W. Mattaj. 1997. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 90:1051-1060. [DOI] [PubMed] [Google Scholar]
- 15.Fried, H., and U. Kutay. 2003. Nucleocytoplasmic transport: taking an inventory. Cell. Mol. Life Sci. 60:1659-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Görlich, D., and U. Kutay. 1999. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 15:607-670. [DOI] [PubMed] [Google Scholar]
- 17.Görlich, D., N. Panté, U. Kutay, U. Aebi, and F. R. Bischoff. 1996. Identification of different roles for RanGDP and RanGTP in nuclear protein import. EMBO J. 15:5584-5594. [PMC free article] [PubMed] [Google Scholar]
- 18.Greiner, M., S. Caesar, and G. Schlenstedt. 2004. The histones H2A/H2B and H3/H4 are imported into the yeast nucleus by different mechanisms. Eur. J. Cell Biol. 83:511-520. [DOI] [PubMed] [Google Scholar]
- 19.Grosshans, H., K. Deinert, E. Hurt, and G. Simos. 2001. Biogenesis of the signal recognition particle (SRP) involves import of SRP proteins into the nucleolus, assembly with the SRP-RNA, and Xpo1p-mediated export. J. Cell Biol. 153:745-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harnpicharnchai, P., J. Jakovljevic, E. Horsey, T. Miles, J. Roman, M. P. Rout, D. Meagher, B. Imai, Y. Guo, C. J. Brame, J. Shabanowitz, D. F. Hunt, and J. L. Woolford. 2001. Composition and functional characterization of yeast 66S ribosome assembly intermediates. Mol. Cell 8:505-515. [DOI] [PubMed] [Google Scholar]
- 21.Hellmuth, K., D. M. Lau, F. R. Bischoff, M. Kunzler, E. Hurt, and G. Simos. 1998. Yeast Los1 has properties of an exportin-like nucleocytoplasmic transport factor for tRNA. Mol. Cell. Biol. 18:6374-6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hood, J. K., and P. A. Silver. 1998. Cse1p is required for export of Srp1p/importin-α from the nucleus in Saccharomyces cerevisiae. J. Biol. Chem. 273:35142-35146. [DOI] [PubMed] [Google Scholar]
- 23.Huh, W. K., J. V. Falvo, L. C. Gerke, A. S. Carroll, R. W. Howson, J. S. Weissman, and E. K. O'Shea. 2003. Global analysis of protein localization in budding yeast. Nature 425:686-691. [DOI] [PubMed] [Google Scholar]
- 24.Jäkel, S., and D. Görlich. 1998. Importin β, transportin, RanBP5 and RanBP7 mediate nuclear import of ribosomal proteins in mammalian cells. EMBO J. 17:4491-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones, J. S., and L. Prakash. 1990. Yeast Saccharomyces cerevisiae selectable markers in pUC18 polylinkers. Yeast 6:363-666. [DOI] [PubMed] [Google Scholar]
- 26.Kaffman, A., N. M. Rank, E. M. O'Neill, L. S. Huang, and E. K. O'Shea. 1998. The receptor Msn5 exports the phosphorylated transcription factor Pho4 out of the nucleus. Nature 396:482-486. [DOI] [PubMed] [Google Scholar]
- 27.Kalab, P., K. Weis, and R. Heald. 2002. Visualization of a Ran-GTP gradient in interphase and mitotic Xenopus egg extracts. Science 295:2452-2456. [DOI] [PubMed] [Google Scholar]
- 28.Kressler, D., P. Linder, and J. de la Cruz. 1999. Protein trans-acting factors involved in ribosome biogenesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:7897-7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Künzler, M., and E. C. Hurt. 1998. Cse1p functions as the nuclear export receptor for importin alpha in yeast. FEBS Lett. 433:185-190. [DOI] [PubMed] [Google Scholar]
- 30.Kutay, U., F. R. Bischoff, S. Kostka, R. Kraft, and D. Görlich. 1997. Export of importin α from the nucleus is mediated by a specific nuclear transport factor. Cell 90:1061-1071. [DOI] [PubMed] [Google Scholar]
- 31.Kutay, U., E. Izaurralde, F. R. Bischoff, I. W. Mattaj, and D. Görlich. 1997. Dominant-negative mutants of importin-β block multiple pathways of import and export through the nuclear pore complex. EMBO J. 16:1153-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lei, E. P., and P. A. Silver. 2002. Protein and RNA export from the nucleus. Dev. Cell 2:261-272. [DOI] [PubMed] [Google Scholar]
- 33.Longtine, M. S., A. McKenzie, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953-961. [DOI] [PubMed] [Google Scholar]
- 34.Matuschewski, K., H.-P. Hauser, M. Treier, and S. Jentsch. 1996. Identification of a novel family of ubiquitin-conjugating enzymes with distinct amino-terminal extensions. J. Biol. Chem. 271:2789-2794. [DOI] [PubMed] [Google Scholar]
- 35.Maurer, P., M. Redd, J. Solsbacher, F. R. Bischoff, M. Greiner, A. V. Podtelejnikov, M. Mann, K. Stade, K. Weis, and G. Schlenstedt. 2001. The nuclear export receptor Xpo1p forms distinct complexes with NES transport substrates and the yeast Ran binding protein 1 (Yrb1p). Mol. Biol. Cell 12:539-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mingot, J.-M., S. Kostka, R. Kraft, E. Hartmann, and D. Görlich. 2001. Importin 13: a novel mediator of nuclear import and export. EMBO J. 20:3685-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mosammaparast, N., Y. Guo, J. Shabanowitz, D. F. Hunt, and L. F. Pemberton. 2002. Pathways mediating the nuclear import of histones H3 and H4 in yeast. J. Biol. Chem. 277:862-868. [DOI] [PubMed] [Google Scholar]
- 38.Mosammaparast, N., K. R. Jackson, Y. Guo, C. J. Brame, J. Shabanowitz, D. F. Hunt, and L. F. Pemberton. 2001. Nuclear import of histone H2A and H2B is mediated by a network of karyopherins. J. Cell Biol. 153:251-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oeffinger, M., M. Dlakic, and D. Tollervey. 2004. A pre-ribosome-associated HEAT-repeat protein is required for export of both ribosomal subunits. Genes Dev. 18:196-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohno, M., M. Fornerod, and I. W. Mattaj. 1998. Nucleocytoplasmic transport: the last 200 nanometers. Cell 92:327-336. [DOI] [PubMed] [Google Scholar]
- 41.Pemberton, L. F., J. S. Rosenblum, and G. Blobel. 1999. Nuclear import of the TATA-binding protein: mediation by the karyopherin Kap114p and a possible mechanism for intranuclear targeting. J. Cell Biol. 145:1407-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plafker, S. M., and I. G. Macara. 2000. Importin-11, a nuclear import receptor for the ubiquitin-conjugating enzyme, UbcM2. EMBO J. 19:5502-5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plafker, S. M., and I. G. Macara. 2002. Ribosomal protein L12 uses a distinct nuclear import pathway mediated by importin 11. Mol. Cell. Biol. 22:1266-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Polizotto, R. S., and M. S. Cyert. 2001. Calcineurin-dependent nuclear import of the transcription factor Crz1p requires Nmd5p. J. Cell Biol. 154:951-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rexach, M., and G. Blobel. 1995. Protein import into nuclei: association and dissociation reactions involving transport substrate, transport factors, and nucleoporins. Cell 83:683-692. [DOI] [PubMed] [Google Scholar]
- 46.Ribbeck, K., and D. Görlich. 2001. Kinetic analysis of translocation through nuclear pore complexes. EMBO J. 20:1320-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenblum, J. S., L. F. Pemberton, and G. Blobel. 1997. A nuclear import pathway for a protein involved in tRNA maturation. J. Cell Biol. 139:1655-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rout, M. P., and J. D. Aitchison. 2001. The nuclear pore complex as a transport machine. J. Biol. Chem. 276:16593-16596. [DOI] [PubMed] [Google Scholar]
- 49.Rout, M. P., J. D. Aitchison, A. Suprapto, K. Hjertaas, Y. Zhao, and B. T. Chait. 2000. The yeast nuclear pore complex: composition, architecture, and transport mechanism. J. Cell Biol. 148:635-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rout, M. P., G. Blobel, and J. D. Aitchison. 1997. A distinct nuclear import pathway used by ribosomal proteins. Cell 89:715-725. [DOI] [PubMed] [Google Scholar]
- 51.Schlenstedt, G., C. Saavedra, J. D. J. Loeb, C. N. Cole, and P. A. Silver. 1995. The GTP-bound form of the yeast Ran/TC4 homologue blocks nuclear protein import and appearance of poly(A)+RNA in the cytoplasm. Proc. Natl. Acad. Sci. USA 92:225-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schlenstedt, G., E. Smirnova, R. Deane, J. Solsbacher, U. Kutay, D. Görlich, H. Ponstingl, and F. R. Bischoff. 1997. Yrb4p, a yeast Ran-GTP-binding protein involved in import of ribosomal protein L25 into the nucleus. EMBO J. 16:6237-6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Solsbacher, J., P. Maurer, F. R. Bischoff, and G. Schlenstedt. 1998. Cse1p is involved in export of yeast importin α from the nucleus. Mol. Cell. Biol. 18:6805-6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Solsbacher, J., P. Maurer, F. Vogel, and G. Schlenstedt. 2000. Nup2p, a yeast nucleoporin, functions in bidirectional transport of importin α. Mol. Cell. Biol. 20:8468-8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stade, K., C. S. Ford, C. Guthrie, and K. Weis. 1997. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell 90:1041-1050. [DOI] [PubMed] [Google Scholar]
- 57.Stage-Zimmermann, T., U. Schmidt, and P. A. Silver. 2000. Factors affecting nuclear export of the 60S ribosomal subunit in vivo. Mol. Biol. Cell 11:3777-3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suntharalingam, M., and S. R. Wente. 2003. Peering through the pore: nuclear pore complex structure, assembly, and function. Dev. Cell 4:775-789. [DOI] [PubMed] [Google Scholar]
- 59.Titov, A. A., and G. Blobel. 1999. The karyopherin Kap122p/Pdr6p imports both subunits of the transcription factor IIA into the nucleus. J. Cell Biol. 147:235-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Venema, J., and D. Tollervey. 1999. Ribosome synthesis in Saccharomyces cerevisiae. Annu. Rev. Genet. 33:261-311. [DOI] [PubMed] [Google Scholar]
- 61.Wehner, K. A., and S. J. Baserga. 2002. The sigma70-like motif: a eukaryotic RNA binding domain unique to a superfamily of proteins required for ribosome biogenesis. Mol. Cell 9:329-339. [DOI] [PubMed] [Google Scholar]
- 62.Weis, K. 2003. Regulating access to the genome: nucleocytoplasmic transport throughout the cell cycle. Cell 112:441-451. [DOI] [PubMed] [Google Scholar]
- 63.Winzeler, E. A., et al. 1999. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285:901-906. [DOI] [PubMed] [Google Scholar]
- 64.Yoshida, K., and G. Blobel. 2001. The karyopherin Kap142p/Msn5p mediates nuclear import and nuclear export of different cargo proteins. J. Cell Biol. 152:729-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.