Abstract
Interspecific comparative molecular analyses of transposed genes and their flanking regions can help to elucidate the time, direction, and mechanism of gene transposition. In the Drosophila melanogaster genome, three Larval serum protein 1 (Lsp1) genes (α, β and γ) are present and each of them is located on a different chromosome, suggesting multiple transposition events. We have characterized the molecular organization of Lsp1 genes in D. buzzatii, a species of the Drosophila subgenus and in D. pseudoobscura, a species of the Sophophora subgenus. Our results show that only two Lsp1 genes (β and γ) exist in these two species. The same chromosomal localization and genomic organization, different from that of D. melanogaster, is found in both species for the Lsp1β and Lsp1γ genes. Overall, at least two duplicative and two conservative transpositions are necessary to explain the present chromosomal distribution of Lsp1 genes in the three Drosophila species. Clear evidence for implication of snRNA genes in the transposition of Lsp1β in Drosophila has been found. We suggest that an ectopic exchange between highly similar snRNA sequences was responsible for the transposition of this gene. We have also identified the putative cis-acting regulatory regions of these genes, which seemingly transposed along with the coding sequences.
SEQUENCE analysis of genomes has revealed that gene transposition contributes significantly to the reorganization of eukaryotic genomes. Gene transposition refers to the movement of relatively small genomic segments, containing one or a few genes, from one chromosomal position to another. This movement may be accompanied or not by the duplication of the genomic segment, two processes that may be denoted as duplicative and conservative transposition, respectively. In nematodes, gene transposition seems to be the most frequent kind of genome rearrangement (Coghlan and Wolfe 2002) whereas duplications of chromosomal segments encompassing a few genes followed by differential gene loss is a common cause of gene order changes in yeasts (Llorente et al. 2000; Fischer et al. 2001). In plants, repeated rounds of large-scale genome duplication followed by selective gene loss are the main factors in genome evolution. Chromosomal rearrangements were thought to be only a minor factor in the divergence of plant genomes (Ku et al. 2000). However, when more detailed comparisons were performed, many chromosomal rearrangements were found. For example, the comparison of genome sequences of rice to orthologous regions from other grass species revealed that numerous local rearrangements, including transpositions of single genes to different chromosomes, have occurred (Bennetzen and Ma 2003). Transposition has also played a significant role in the evolution of the mammalian genome. Segmental duplications originated from the duplicative transposition of small portions of chromosomal material represent ∼5% of the human genome (Eichler 2001; Lander et al. 2001; Bailey et al. 2002) and at least 1.2% of the mouse genome (Cheung et al. 2003). Some of the duplicated segments in the human genome are associated with rapid gene innovation and chromosomal rearrangement in the genomes of man and the great apes (Samonte and Eichler 2001; Armengol et al. 2003; Locke et al. 2003).
In Drosophila, segmental duplications seem to be rare in comparison to the number found in mammalian genomes (Lander et al. 2001; Celniker et al. 2002). Also, detailed analyses by in situ hybridization show that the gene content of chromosomal elements is generally conserved and suggest that gene transpositions are relatively scarce (González et al. 2002; Ranz et al. 2003) in relation to paracentric inversions, which have been traditionally considered as the chief type of chromosomal change (Krimbas and Powell 1992; Powell 1997). However, recent sequence analyses comparing five different Drosophila species point to similar numbers of inversions and gene transpositions (Bergman et al. 2002) and a number of new genes originated by retroposition has been unveiled (Betrán et al. 2002).
Diverse molecular mechanisms (sometimes poorly understood) may be responsible for gene transposition events. A common mechanism for gene transposition is retroposition, which implies reverse transcription of RNA and insertion of the resulting cDNA into a different genome site. In humans, the long interspersed element (LINE) L1 often associates 3′ flanking DNA as a read-through transcript and carries the non-L1 sequence to a new genomic location, a process termed L1-mediated transduction (Moran et al. 1999; Lander et al. 2001). Seemingly the LINE machinery can also act in trans to cellular RNA substrates giving rise to the trans-mobilization of genomic DNA, processed pseudogenes, and occasionally new functional genes (Esnault et al. 2000; Betrán et al. 2002; Ejima and Yang 2003; Long et al. 2003). Another mechanism of transposition is transposon-mediated excision and insertion of genomic segments. For instance, in D. melanogaster, Folback elements flanking relatively large genomic segments are able to transport these segments to sites far away in the genome, forming the so-called “giant transposons” (Chia et al. 1985; Lovering et al. 1991). Excision and insertion of these giant transposons is mediated by homologous recombination involving the Foldback sequences at the transposon termini. Transposable elements seem to be implicated also in the origin of segmental duplications in humans. Duplication junctions have been found to be enriched for Alu short interspersed element sequences with a significant proportion of all segmental duplications ending within Alu sequences (Bailey et al. 2003). This observation suggests Alu-Alu homologous recombination as the most likely mechanism for these rearrangements. A similar mechanism has previously been shown to generate small duplications, deletions, and inversions in diverse organisms.
Larval serum protein 1 (Lsp1) genes provide one of the few examples of gene transposition in the genus Drosophila. In D. melanogaster, each of the three Lsp1 genes is located on a different chromosome: Lsp1α in chromosome X, Lsp1β in chromosomal arm 2L, and Lsp1γ in chromosomal arm 3L (Roberts and Evans-Roberts 1979; Smith et al. 1981). These chromosomal arms correspond to Muller's elements A, B, and D, respectively (Table 1). Brock and Roberts (1983) mapped Lsp1 genes by in situ hybridization in nine other species of the Sophophora subgenus (including D. pseudoobscura) and five species of the Drosophila subgenus (including D. hydei as a representative species of the repleta group). In the melanogaster subgroup species they were able to localize the three Lsp1 genes: Lsp1α on element A, Lsp1β on element B, and Lsp1γ on element D. In all the other species, both Lsp1α and Lsp1β hybridized to the same polytene band of Muller's element E, suggesting a gene exchange between elements. No hybridization of the Lsp1γ gene was observed although γ-like proteins were detected with specific antibodies. To determine Lsp1 gene number, Brock and Roberts (1983) performed Southern analyses and concluded that at least two genes, one α-like and one β-like, were present in all the species analyzed. Their data also suggested that the ancestor of the genus Drosophila probably had its Lsp1 genes on element E. Recently, we localized by in situ hybridization Lsp1α in chromosome 2 (Muller's element E) of D. repleta and D. buzzatii (González et al. 2002; Ranz et al. 2003), corroborating their results.
TABLE 1.
Chromosomal homologies betweenD. melanogaster,D. pseudoobscura, andD. buzzatii, the three Drosophila species analyzed in this study (from Powell 1997)
| Muller's element
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Species | Subgenus | Species group | A | B | C | D | E | F |
| D. melanogaster | Sophophora | melanogaster | X | 2L | 2R | 3L | 3R | 4 |
| D. pseudoobscura | Sophophora | obscura | XL | 4 | 3 | XR | 2 | 5 |
| D. buzzatii | Drosophila | repleta | X | 3 | 5 | 4 | 2 | 6 |
In this work, Lsp1 genes and their flanking sequences have been cloned and sequenced in D. buzzatii, a species belonging to the repleta group of the Drosophila subgenus, which diverged from D. melanogaster 40–62 MYA (Beverly and Wilson 1984; Russo et al. 1995). In addition, the genome sequence of D. pseudoobscura (available at http://www.hgsc.bcm.tmc.edu) has been searched and Lsp1 genes have been annotated in this species, which diverged from D. melanogaster ∼30 MYA (Throckmorton 1975) and belongs to the same subgenus. The aims of this study are: (i) to determine beyond doubt the number and localization of Lsp1 genes in these two Drosophila species because Southern analyses and in situ hybridization results for members of gene families may be misleading (Bachtrog and Charlesworth 2003); (ii) to ascertain the number and type of transposition events undergone by Lsp1 genes during the evolution of the genus Drosophila; (iii) to uncover the molecular mechanism of transposition, in particular to test the hypothesis of an involvement of transposable elements; and (iv) to identify putative regulatory sequences of Lsp1 genes and determine whether these regulatory sequences transposed along with the coding sequences or were recruited ex novo at the new chromosomal location.
MATERIALS AND METHODS
Drosophila stocks:
Two lines of D. buzzatii were used: line j-19 from Ticucho (Argentina) is fixed for chromosomal arrangement 2j and line jq7-4 from Otamendi (Argentina) is fixed for arrangement 2jq7. The genome sequence of D. pseudoobscura comes from inbred line MV2-25 (http://www.hgsc.bcm.tmc.edu).
Screening of genomic libraries:
Two different lambda genomic libraries were screened by plaque hybridization: the j-19 library (Cáceres et al. 2001) and the jq7-4 library (Casals et al. 2003). Both libraries were previously amplified as described in Sambrook et al. (1989). DNA from positive phages was digested and the resulting fragments were subcloned into Bluescript II SK vector (Stratagene, La Jolla, CA) after gel purification. The j-19 library was screened with a 0.7-kb BamHI fragment of the D. melanogaster Lsp1α gene (Brock and Roberts 1983). Six positive phages were recovered and one of them, λj-19/8, was partially sequenced and found to contain the 5′ region of Lsp1β (Figure 1a). Another lambda phage, λj-19/25, containing the 3′ region of this gene had been previously isolated in our laboratory (Casals et al. 2003). None of the six positive phages contained Lsp1γ. To clone this gene, the j-19 library was screened with a 0.6-kb HindIII-SalI fragment of D. buzzatii Lsp1β (Figure 1a). Nine positive phages were identified but again none of them included Lsp1γ. To clone D. buzzatii Lsp1γ, a different library (jq7-4) was screened with the same fragment of D. melanogaster Lsp1α previously used to screen the j-19 library. Five positive phages were identified and one of them, λjq7-4/12, contained the 3′ end of the Lsp1γ gene. To clone the other end of the gene, a 0.75-kb SalI-ClaI fragment of D. buzzatii Lsp1γ was used to screen the same library (Figure 2a). Seventeen positive phages were recovered and one of them, λjq7-4/27, included almost the entire Lsp1γ gene.
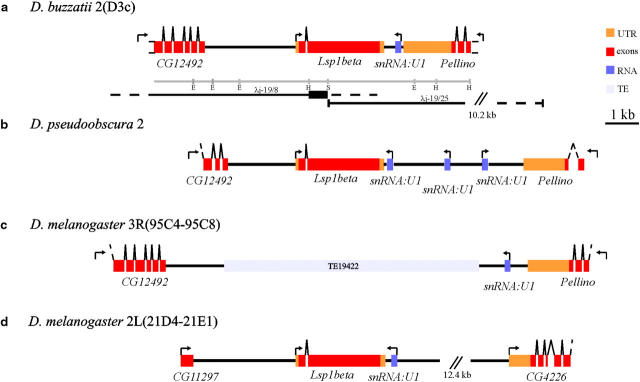
Figure 1.—
Genome organization of the Lsp1β region in D. buzzatii, D. pseudoobscura, and D. melanogaster. Arrows indicate the start point and direction of transcription. (a) Annotation and chromosomal localization of the 9464 bp sequenced in D. buzzatii. Restriction map includes EcoRI (E), HindIII (H), and SalI (S) sites. The λ-phages used to sequence this region are represented as lines: solid lines are sequenced regions and broken lines are regions cloned but not sequenced. The short thick segment represents the probe used to screen the D. buzzatii libraries. (b) Annotation of the homologous region in D. pseudoobscura. (c) Fragment of the AE003745 genomic clone of D. melanogaster where those genes flanking Lsp1β in D. buzzatii and D. pseudoobscura are located. The chromosomal localization of these genes is also given. Note that in this species Lsp1β is not present between CG12492 and Pellino. (d) Genomic organization and chromosomal localization of the Lsp1β region in D. melanogaster. This region is included in the genomic clone AE003588 of this species.
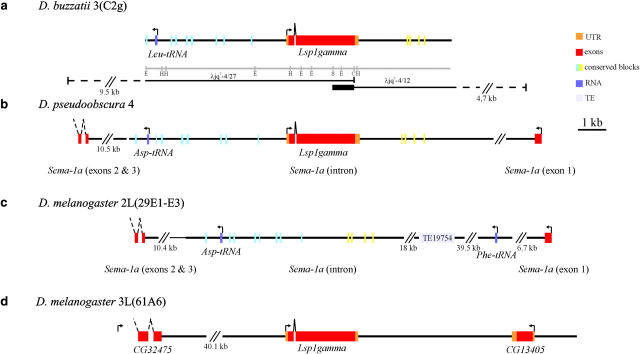
Figure 2.—
Genome organization of the Lsp1γ region in D. buzzatii, D. pseudoobscura, and D. melanogaster (for symbols see legend for Figure 1). (a) Annotation and chromosomal localization of the 10,817 bp sequenced in D. buzzatii. Restriction map includes EcoRI (E), HindIII (H), SalI (S), and ClaI (C) sites. (b) Annotation of the homologous region in D. pseudoobscura. Note that Lsp1γ is nested within Sema-1a gene. (c) Fragment of the AE003621 genomic clone of D. melanogaster containing Sema-1a gene. Note that Lsp1γ is not present within the first intron. (d) Fragment of D. melanogaster AE003467 genomic clone that contains Lsp1γ.
Southern analysis:
Southern hybridization was performed as described in Sambrook et al. (1989). Probes were labeled by random primer with digoxygenin-11-dUTP and hybridization was carried out overnight in standard buffer with 50% formamide at 42° for homologous probes and at 37° for heterologous probes. Stringency washes were performed with 2× SSC 0.1% SDS and 0.1× SSC 0.1% SDS solutions at 68° and 50° for homologous and heterologous hybridizations, respectively.
DNA sequencing and sequence analysis:
Sequences were obtained with an ABI 373 A (Perkin Elmer, Norwalk, CT) automated DNA sequencer using M13 universal forward and reverse primers. A few internal primers were also designed when necessary. Nucleotide sequences were assembled using GeneToolLite software (BioTools). Similarity searches in the GenBank/EMBL and in the Drosophila pseudoobscura genome project (available at http://www.hgsc.bcm.tmc.edu) databases were performed using blastn and fasta3 programs. Multiple sequence alignments were obtained with ClustalW (Thompson et al. 1994) and DiAlign (Morgenstern 1999). PAML software (Yang 1997) was used to estimate the number of synonymous (dS) and nonsynonymous (dN) substitutions per site. This software avoids using reconstructed ancestral sequences to estimate dS and dN for lineages in a phylogeny by using a maximum likelihood approach. Different codon-based likelihood models that allow for different dN/dS ratios among evolutionary lineages can be devised. The models can then be compared to test the neutral prediction that the dN/dS ratio is identical among lineages (Yang and Bielawski 2000).
In situ hybridization:
In situ hybridization of DNA probes was carried out as described in Montgomery et al. (1987). Hybridization temperature was 37°. Probes were labeled with biotin-16-dUTP by nick translation and detection was done using the ABC-Elite kit from Vector Laboratories (Burlingame, CA). Hybridization signals were localized using the cytological maps of D. buzzatii (Ruiz and Wasserman 1993) and photographs were taken with a phase contrast Nikon Optiphot-2 microscope at ×600 magnification.
RESULTS
Lsp1 gene number:
Screening of two genomic libraries of D. buzzatii with different probes allowed us to isolate clones containing the Lsp1β and Lsp1γ genes (see materials and methods). To determine the number of Lsp1 genes present in the D. buzzatii genome, a Southern analysis was performed (see Figure S1 available as online supplementary material at http://www.genetics.org/supplemental/). Genomic DNA from D. buzzatii and D. melanogaster (as control) was digested with EcoRI and HindIII and hybridized with the same three probes used to screen the D. buzzatii libraries (see materials and methods). The D. melanogaster Lsp1α probe hybridized to 6- and 4.1-kb fragments of D. melanogaster genomic DNA corresponding to Lsp1α and Lsp1β, respectively (Brock and Roberts 1983). In D. buzzatii this probe hybridized to a single fragment of 3.1 kb corresponding to Lsp1β. The D. buzzatii Lsp1β probe hybridized to 6- and 4.1-kb fragments of D. melanogaster corresponding to Lsp1α and Lsp1β, respectively (Brock and Roberts 1983), and a 3.1-kb fragment of D. buzzatii genomic DNA corresponding to Lsp1β. Finally, the D. buzzatii Lsp1γ probe hybridized to the 4.1-kb fragment corresponding to Lsp1β and to 1.8- and 1.4-kb fragments both corresponding to Lsp1γ of D. melanogaster (Brock and Roberts 1983) and also with a 3.1-kb fragment corresponding to Lsp1β and a 1-kb fragment corresponding to Lsp1γ of D. buzzatii. Overall, the results of Southern analyses indicated that only two genes, Lsp1β and Lsp1γ, are present in the D. buzzatii genome. No Lsp1α gene was detected by Southern analysis in the D. buzzatii genome.
Similarity searches against the D. pseudoobscura genome sequence database were carried out (Table 2). Two sequences with significant similarity were found with Lsp1β. The most similar sequence, that included in contig 815_contig 5737, was considered to correspond to the D. pseudoobscura Lsp1β ortholog. When the database was searched with the coding sequence of Lsp1γ as query the same two hits were recovered. This time the most similar sequence included in contig 1500_contig 3546 was considered to correspond to the D. pseudoobscura ortholog of Lsp1γ. No new sequences were identified when D. melanogaster Lsp1α sequence was used as query, the only significant hits being those previously identified as Lsp1β and Lsp1γ. We conclude that only Lsp1β and Lsp1γ are present in the D. pseudoobscura genome.
TABLE 2.
Results of the blast search against theD. pseudoobscura genome database as of August 28, 2003, using as queries the coding sequences ofLsp1α,Lsp1β, andLsp1γ
| Query | Blast hits | Score (bits) | E-value |
|---|---|---|---|
| Lsp1β | Contig 815_contig 5737 | 2044 | 0.0 |
| Contig 1500_contig 3546 | 222 | 3e−56 | |
| Lsp1γ | Contig 1500_contig 3546 | 1237 | 0.0 |
| Contig 815_contig 5737 | 121 | 9e−26 | |
| Lsp1α | Contig 815_contig 5737 | 1269 | 0.0 |
| Contig 1500_contig 3546 | 111 | 9e−23 |
Organization of the Lsp1β and Lsp1γ genomic regions in D. buzzatii and D. pseudoobscura:
In D. buzzatii, the Lsp1β gene was localized by in situ hybridization, using the λj-19/25 phage as probe to polytene band D3c of chromosome 2 (see Figure S2 at http://www.genetics.org/supplemental/). This chromosome is homologous to chromosomal arm 3R of D. melanogaster (Muller's element E; Table 1). Overall, 9464 bp including the entire D. buzzatii Lsp1β gene and its flanking regions were sequenced (accession no. AY561258). In this species, Lsp1β is flanked by CG12492 and Pellino (Figure 1a). Between Lsp1β and Pellino there is a snRNA:U1 gene, orthologous to snRNA:U1:95Ca of D. melanogaster.
In D. pseudoobscura, the Lsp1β gene is included within contig 815_contig 5737, which has been putatively assigned to chromosome XR, homologous to D. melanogaster chromosomal arm 3L (Muller's element D; Table 1). As in D. buzzatii, Lsp1β is flanked by CG12492 and Pellino but in D. pseudoobscura there are three snRNA:U1 genes between Lsp1β and Pellino (Figure 1b). In D. melanogaster, CG12492, snRNA:U1:95Ca, and Pellino are localized in chromosomal arm 3R (Figure 1c) while Lsp1β is located at 21D-E in chromosomal arm 2L (Figure 1d). The alignments of D. pseudoobscura and D. melanogaster genome sequences, available at http://pipeline.lbl.gov/pseudo/, show that Lsp1β and its flanking genes are in fact included in contig 7891_contig 7492 assigned to chromosome 2 (Muller's element E; Table 1). We concluded that this must be the correct localization on the basis of the fact that CG12492 and Pellino are found on element E in both D. melanogaster and D. buzzatii.
In situ hybridization of the λjq7-4/27 phage, containing the entire Lsp1γ coding sequence, to the polytene chromosomes of D. buzzatti allowed us to map this gene to band C2g of chromosome 3 (see Figure S2 at http://www.genetics.org/supplemental/). This chromosome is homologous to chromosomal arm 2L of D. melanogaster (Muller's element B; Table 1). In total, 10,817 nucleotides including the entire Lsp1γ gene and its flanking regions have been sequenced in D. buzzatii (accession no. AY561259). Upstream of Lsp1γ, 4.5 kb from the ATG codon, there is a Leucyl transfer RNA (Leu-tRNA) gene (Figure 2a).
In D. pseudoobscura, Lsp1γ is found within contig 1500_contig 3546, which belongs to chromosome 4, homologous to chromosomal arm 2L of D. melanogaster (Muller's element B; Table 1). Analysis of the D. pseudoobscura genomic sequence revealed that 4.9 kb upstream from Lsp1γ there is an Aspartic acid transfer RNA (Asp-tRNA). Both Lsp1γ and Asp-tRNA are nested inside the first intron of the gene Sema-1a (Figure 2b). In both D. pseudoobscura and D. buzzatii, we detected eight short sequences, 28 to 59 nucleotides long, scattered along the 5-kb region upstream of the Lsp1γ coding sequence, that are highly conserved in D. melanogaster (86–97% nucleotide identity). Another four short sequences, 41–55 nucleotides long, also highly conserved between the three species (91–98% of nucleotide identity), were found downstream of Lsp1γ in D. pseudoobscura and D. buzzatii (Figure 2). The conservation in number and relative position of these 12 highly conserved noncoding sequences flanking Lsp1γ in both D. buzzatii and D. pseudoobscura led us to conclude that the molecular organization of this gene region is the same in the two species; i.e., Lsp1γ is nested within an intron of Sema-1a in both cases. On the other hand, in D. melanogaster (Figure 2, c and d), the Sema-1a gene is located in band 29E1-3 on chromosomal arm 2L (Muller's element B; Table 1) and inside its first intron there is also an Asp-tRNA gene (Figure 2c) but not the Lsp1γ gene, which is located on chromosomal arm 3L (Muller's element D; Table 1).
Molecular structure of Lsp1β and Lsp1γ genes in D. buzzatii and D. pseudoobscura:
In the two species, both Lsp1β and Lsp1γ are made up of two exons separated by a small intron (Table 3). The alignment of D. buzzatii, D. pseudoobscura, and D. melanogaster nucleotide sequences shows a 79.4% nucleotide identity for Lsp1β and 75.7% nucleotide identity for Lsp1γ. For both genes, the intron is placed in the same precise site in the three species and has a similar length (63–71 nucleotides for Lsp1β and 57–65 nucleotides for Lsp1γ) but shows little nucleotide conservation other than the donor (G·T·A/G·A·G·T/C) and acceptor (C·A·G) splice sites (Delaney et al. 1986).
TABLE 3.
Molecular structure ofLsp1 genes inD. buzzatii andD. pseudoobscura compared to those ofD. melanogaster
|
D. buzzatiia
|
D. pseudoobscurab
|
D. melanogasterc
|
|||||
|---|---|---|---|---|---|---|---|
| Gene region | Lsp1β | Lsp1γ | Lsp1β | Lsp1α | Lsp1α | Lsp1β | Lsp1γ |
| 5′-UTR | 89 | 68 | 82 | 88 | 88 | 85 | 82 |
| Exon 1 | 210 | 210 | 210 | 207 | 210 | 210 | 207 |
| Intron 1 | 63 | 57 | 71 | 64 | 67 | 67 | 65 |
| Exon 2 | 2157 | 2112 | 2154 | 2115 | 2241 | 2160 | 2112 |
| 3′-UTR | 108 | 127 | 117 | 139 | 69 | 151 | 105 |
Data from this work.
Our annotation of the D. pseudoobscura genome (contig 7891_contig 7492 for Lsp1β and contig 1500_contig 3546 for Lsp1γ of the D. pseudoobscura database).
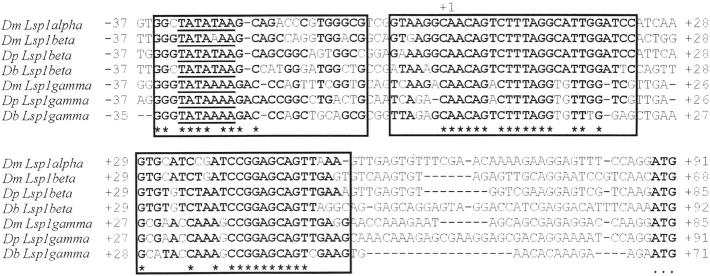
Both Lsp1β and Lsp1γ possess a TATA box, which is localized in D. buzzatii and D. pseudoobscura at the same nucleotide position as in D. melanogaster (−32 to −26). Sequence similarity extends for several nucleotides on either side of the TATA box (Figure 3). The 5′-UTR of D. buzzatii Lsp1β has 89 bp, the first 21 nucleotides being identical to those in D. melanogaster and D. pseudoobscura. The 5′-UTR of D. buzzatii Lsp1γ is 68 bp long and 17 of the first 21 nucleotides are identical to those in D. melanogaster and D. pseudoobscura. In both cases, another block with significant sequence similarity is found further downstream (Figure 3). A polyadenylation signal (AATAAA) is located ∼100 bp downstream from the stop codon in Lsp1β and Lsp1γ. In Lsp1β, but not in Lsp1γ, the sequence around this signal is highly conserved in the three species (Table 4a). Apart from this, no other conserved sequences have been found in the 3′ region of Lsp1 genes.
Figure 3.—
ClustalW alignment of 5′-UTR and close upstream sequences of Lsp1α in D. melanogaster (Dm) and Lsp1β and Lsp1γ in D. melanogaster, D. pseudoobscura (Dp), and D. buzzatii (Db). Conserved regions are included in rectangles. Asterisks show nucleotides identical in the seven sequences and boldface type indicates the most common nucleotide in each position. TATA box is underlined; +1 shows the first nucleotide of the 5′-UTR. The first AUG codon is in boldface type and is indicated by dots below the sequence.
TABLE 4.
Conserved sequences betweenD. melanogaster,D. pseudoobscura, andD. buzzatii in the 3′-UTR (a) and 5′ region (b, c, and d) ofLsp1β and in the 5′ region (e, f, and g) ofLsp1γ
| Lsp1β | |
|---|---|
| a. | |
| Dm +2638 | GCAAAAAGTCTAATAAACTTTCGAAAA +2664 |
| ********** **************** | |
| Dp +2618 | GCAAAAAGTCAAATAAACTTTCGAAAA +2644 |
| ********* ***************** | |
| Db +2611 | GCAAAAAGTGAAATAAACTTTCGAAAA +2637 |
| b. | |
| Dm −216 | AATAGAAGTCTGGCT--TTGATAAG −194 |
| * * ** **** * ******* | |
| Dp −191 | ACGACAAC-CTGGATGGCTGATAAG −168 |
| * * *** **** * ******* | |
| Db −189 | ATGGCAA--CTGGGCGATTGATAAG −167 |
| c. | |
| Dm −130 | AGCACCTGAGATACACCC −113 |
| * *********** ** | |
| Dp −111 | ACCACCTGAGATAGACTT −94 |
| * ****** **** *** | |
| Db −126 | AGCACCTGCGATACACTC −109 |
| d. | |
| Dm −411 | TATCTACATTTTTGAGGA −394 |
| ** * ******** * * | |
| Dp −512 | TAGCAACATTTTTCTGCA −495 |
| ****************** | |
| Db −683 | TAGCAACATTTTTCTGCA −666 |
| Lsp1γ | |
| e. | |
| Dm −380 | AATTAAACCTGAAC--TGATATG −360 |
| ** ***** ***** ** | |
| Dp −494 | AAATAAACTTGAACTTTGCATAA −472 |
| * **** **** ** ** | |
| Db −379 | ATGTAAA-TTGACGATT--ATCG −360 |
| f. | |
| Dm −180 | ACCACCTGAATTGAGGC −168 |
| ******* ** | |
| Dp −299 | CGCACCTGACGTGCACT −178 |
| ** * ** * | |
| Db −181 | GCCAGGGTAGTTGAGCC −165 |
| g. | |
| Dm −74 | GTCTGCCGCTGATATGGTGCA −54 |
| ****** ************* | |
| Dp −126 | GTCTGCGACTGATATGGTGCA −106 |
| ***** ************* | |
| Db −71 | GTCTGATGCTGATATGGTGCA −51 |
Blocks inside the sequences that fulfill the requirements of Bergman and Kreitman (2001) to identify noncoding conserved sequences are shown in boldface type. Dm, D. melanogaster; Dp, D. pseudoobscura; and Db, D. buzzatii. Asterisks denote nucleotides between two consecutive sequences. Polyadenylation signal is underlined.
We searched for putative regulatory sequences in the 5′ regions of Lsp1 genes in D. buzzatii and D. pseudoobscura following the criteria devised by Bergman and Kreitman (2001). Comparison of the 5′ ends of Lsp1β led to the identification of three conserved sequences starting at sites −189, −126, and −683 of D. buzzatii (Table 4, b, c, and d). Another three conserved sequences starting at sites −379, −181, and −71 of D. buzzatii were found in the 5′ region of Lsp1γ (Table 4, e, f, and g).
Molecular evolution of the Lsp1 genes:
The coding sequence of Lsp1β and Lsp1γ from D. buzzatii, D. melanogaster, and D. pseudoobscura and that of Lsp1α from D. melanogaster were aligned using ClustalW. The number of synonymous and nonsynonymous substitutions per site for pairwise comparisons between the three species were estimated using maximum likelihood methods (Yang 1997). The dN/dS ratios for Lsp1β (0.0226–0.0449) are similar to those for Lsp1γ (0.0209–0.0512). Comparisons between Lsp1α of D. melanogaster with Lsp1β (0.0470–0.0545) and Lsp1γ (0.0367–0.0386) of the other two species also yielded similar results (Table 5). Overall, the dN/dS ratios were low, suggesting a relatively high degree of functional constraint of these genes in the three species analyzed.
TABLE 5.
Maximum likelihood estimates of the number of synonymous and nonsynonymous substitutions per site in the coding regions ofLsp1 genes for pairwise comparisons amongD. melanogaster,D. pseudoobscura, andD. buzzatii
| Comparison | t | k | S | N | dS | dN | dN/dS |
|---|---|---|---|---|---|---|---|
| Lsp1β | |||||||
| D. melanogaster-D. buzzatii | 1.4840 | 1.9491 | 268.1 | 1708.9 | 3.1884 | 0.0720 | 0.0226 |
| D. melanogaster-D. pseudoobscura | 0.4923 | 1.6360 | 179.9 | 1797.1 | 1.2450 | 0.0559 | 0.0449 |
| D. buzzatii-D. pseudoobscura | 0.9964 | 1.8803 | 286.8 | 1690.2 | 1.9216 | 0.0624 | 0.0325 |
| Lsp1γ | |||||||
| D. melanogaster-D. buzzatii | 1.5423 | 1.7122 | 336.8 | 1640.2 | 2.5579 | 0.0944 | 0.0369 |
| D. melanogaster-D. pseudoobscura | 1.1835 | 1.4705 | 252.3 | 1724.7 | 2.7054 | 0.0565 | 0.0209 |
| D. buzzatii-D. pseudoobscura | 0.8167 | 1.4804 | 261.9 | 1715.1 | 1.5395 | 0.0787 | 0.0512 |
| Lsp1α-Lsp1β | |||||||
| D. melanogaster-D. melanogaster | 1.0643 | 1.5939 | 247.8 | 1729.2 | 2.2380 | 0.0849 | 0.0379 |
| D. melanogaster-D. buzzatii | 1.2360 | 1.1993 | 309.2 | 1667.8 | 2.0358 | 0.1109 | 0.0545 |
| D. melanogaster- D. pseudoobscura | 1.0806 | 1.6403 | 269.3 | 1707.7 | 2.0375 | 0.0957 | 0.0470 |
| Lsp1α-Lsp1γ | |||||||
| D. melanogaster-D. melanogaster | 5.5285 | 1.4217 | 316.1 | 1660.9 | 10.1718 | 0.2578 | 0.0253 |
| D. melanogaster-D. buzzatii | 4.4263 | 1.3910 | 321.9 | 1655.1 | 7.6226 | 0.2800 | 0.0367 |
| D. melanogaster-D. pseudoobscura | 3.0028 | 1.2382 | 247.5 | 1729.5 | 6.2989 | 0.2429 | 0.0386 |
t, branch length; k, transition/transversion rate ratio; S, synonymous positions; N, nonsynonymous positions; dN/dS, ratio of nonsynonymous/synonymous substitution rate.
Two different methods, neighbor joining and the unweighted pair group method using arithmetic averages (UPGMA), were used to construct phylogenetic trees using PHYLIP software (Felsenstein 1989). In addition to the seven above-mentioned sequences, we included in the trees those of arylphorin, an Lsp1-like gene of Calliphora vicina (Naumann and Scheller 1991), and Lsp2 of D. melanogaster (Adams et al. 2000). Alternative models for the evolution of the Lsp genes were then tested using maximum likelihood methods (Yang 1997; Bielawski and Yang 2003). First we tested for the constancy of evolution rates by comparing both trees: that produced with the UPGMA method, which assumes a molecular clock, and that built with the neighbor-joining method, assuming no clock. In both cases a single dN/dS ratio for all lineages was considered. The difference between the likelihood of both trees was significant (2Δl = 32.64; 7 d.f.; P < 0.005) indicating that the model assuming no clock provides a significantly better fit to the data (Figure 4). We then tested for homogeneity in the dN/dS ratio between lineages by comparing the model assuming no clock and a single dN/dS ratio with a free-ratio model, which assumes an independent dN/dS ratio for each lineage. The result was significant (2Δl = 83.18; 14 d.f.; P < 0.0001) indicating that the dN/dS ratios are heterogeneous among lineages. Finally we compared a model assuming several dN/dS ratios (one background ratio and one ratio for each of the lineages leading to the Lsp1 genes in D. melanogaster) with the single-ratio model. Again the difference was statistically significant (2Δl = 21.86; 3 d.f.; P < 0.005) indicating a better fit to the data for the model assuming several dN/dS ratios.
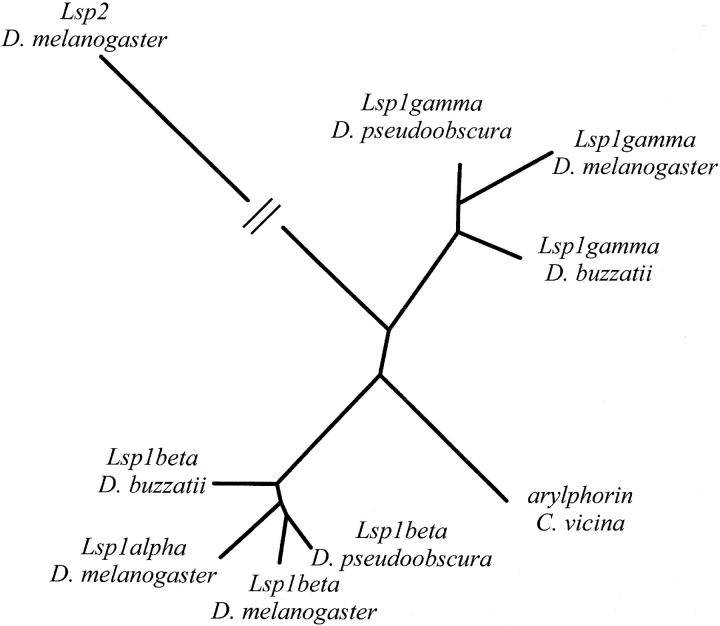
Figure 4.—
Neighbor-joining tree of Lsp1 genes from D. melanogaster, D. pseudoobscura, and D. buzzatii species. Arylphorin from C. vicina and Lsp2 from D. melanogaster are also included. The tree was constructed with an alignment of the coding sequence of the genes.
DISCUSSION
Lsp1 gene number:
In D. melanogaster the LSP-1 protein is made up of three subunits encoded by three Lsp1 genes: Lsp1α, Lsp1β and Lsp1γ (Roberts and Evans-Roberts 1979). However, in D. buzzatii, a species of the Drosophila subgenus, and in D. pseudoobscura, a species of the Sophophora subgenus, only two Lsp1 genes seem to be present: Lsp1β and Lsp1γ. Two different genomic libraries of D. buzzatii have been screened using as probes a fragment of D. melanogaster Lsp1α and a fragment of D. buzzatii Lsp1β or Lsp1γ. Overall, four different library screenings have been carried out and in every case all positive clones contained either Lsp1β or Lsp1γ. No Lsp1α gene was found. To corroborate this result, genomic DNA was digested with restriction enzymes and hybridized with the same three probes used to screen the libraries and again the results were in agreement with the existence of only two genes, Lsp1β and Lsp1γ, in the D. buzzatii genome. Also, the D. pseudoobscura genome has been recently sequenced to approximately sevenfold coverage and is available at http://www.hgsc.bcm.tmc.edu. Similarity searches against this database have been performed, allowing us to annotate Lsp1β and Lsp1γ but not Lsp1α. Thus, our results indicate that Lsp1α is not present in D. buzzatii or D. pseudoobscura. The most parsimonious explanation for this observation is that the duplicative transposition that gave rise to Lsp1α took place in the lineage leading to D. melanogaster after the divergence of the D. pseudoobscura lineage ∼30 MYA (but see below). The fact that D. melanogaster Lsp1α is not dosage compensated although it is X linked is in agreement with a recent duplicative transposition onto this chromosome (Roberts and Evans-Roberts 1979).
Brock and Roberts (1983) reported that in eight species of the Drosophila and Sophophora subgenera, Lsp1α and Lsp1β map to the same polytene band in chromosomal element E except in D. pseudoobscura, where they observed an extra signal in element B (see Table 1). They concluded that there were at least two genes, one α-like and one β-like, given that Lsp1γ could not be localized by in situ hybridization. Contrasting results have been obtained in this work. We have unambiguously localized Lsp1β to chromosomal element E and Lsp1γ gene to element B in both D. buzzatii and D. pseudoobscura. According to our results the extra signal in D. pseudoobscura element B likely corresponds to Lsp1γ. A plausible explanation for their results is that Lsp1α and Lsp1β probes were cross-hybridizing to Lsp1β as is suggested by the fact that the signals obtained with the Lsp1β probe were stronger than those obtained with the Lsp1α probes (Brock and Roberts 1983). Likewise, our previous in situ hybridization of D. melanogaster Lsp1α to D. buzzatii (and D. repleta) chromosomes (González et al. 2002; Ranz et al. 2003) must be reinterpreted as due to cross-hybridization with Lsp1β (see Figure S2 at http://www.genetics.org/supplemental/).
Larval serum proteins belong to the hemocyanin superfamily and are thought to act as storage proteins that provide amino acids and energy during nonfeeding periods of immature or adult development (Burmester et al. 1998). In D. melanogaster LSP-1 is a heterohexamer of randomly associated subunits encoded by Lsp1α, Lsp1β, and Lsp1γ genes. The lack of one subunit in D. buzzatii and D. pseudoobscura does not imply that the LSP-1 protein will not be functional. In fact, it has been reported that an inbred stock of D. melanogaster lacking the γ-chain is viable under laboratory conditions, suggesting that a subunit specific function for the LSP-1 monomers does not exist (Brock and Roberts 1980). The polypeptides encoded by Lsp1β and Lsp1γ of D. melanogaster, D. pseudoobscura, and D. buzzatii and by Lsp1α of D. melanogaster were aligned with the ClustalW algorithm (see Figure S3 at http://www.genetics.org/supplemental/). These seven sequences have a similar length (772–789 aa), 49% of the amino acids are identical, and 33% of the amino acid substitutions are conservative or semiconservative. However, different subunits can accumulate different specific amino acids and this could allow the organism to modulate the availability of these amino acids in different developmental processes (Massey et al. 1997). The three subunits of the LSP-1 protein of D. melanogaster, as well as similar proteins in other Diptera, are enriched in aromatic amino acids (Burmester et al. 1998). Aromatic residues are thought to serve as precursors for quinones, which play a role in cuticle hardening during metamorphosis (Burmester et al. 1998). The polypeptides coded by Lsp1α and Lsp1β are also enriched in methionine but not those encoded by Lsp1γ. The same pattern is observed in D. pseudoobscura and D. buzzatii. The role of methionine in Drosophila development is not clear (Massey et al. 1997). Gene duplication is considered a major force in gene family expansion and gene innovation. After duplication, one copy may become silenced (nonfunctionalization) or assume a novel function (neofunctionalization) or both copies may split the multiple functions of the ancestral gene (subfunctionalization; Lynch and Conery 2000). Gene amplification of highly expressed functions often lead to highly conserved paralogs in microbial genomes (Hooper and Berg 2003). The latter seems to be the case of the Lsp1 genes in Drosophila. The protein coded by these genes is accumulated to high levels by feeding larvae (Massey et al. 1997) and gene amplification has not resulted in different discernible functions.
Genome organization and number of transposition events:
In both D. buzzatii and D. pseudoobscura, Lsp1β is flanked by CG12492 and Pellino genes on chromosomal element E (Figure 1, a and b). The homologous region in D. melanogaster, where CG12492 and Pellino are found, is also located on element E. This region shows no sequence similarity to any Lsp1 gene whatsoever (Figure 1c). In D. melanogaster, Lsp1β is located on element B (Figure 1d). In the three species, an snRNA:U1 is closely linked to the Lsp1β gene. Given that D. buzzatii and D. pseudoobscura, which belong to two different subgenera separated by 80–124 MYR (Beverly and Wilson 1984; Spicer 1988), show the same genomic organization for Lsp1β, this is likely to be the ancestral organization of the genus and therefore Lsp1β would have conservatively transposed from element E to element B in the lineage leading to D. melanogaster. The maximum size of the transposed region is 5.7 kb and the only protein-coding gene included is Lsp1β.
In D. buzzatii and D. pseudoobscura, Lsp1γ is flanked by a tRNA and both genes are located inside the first intron of Sema-1a on element B (Figure 2, a and b). In D. melanogaster, Sema-1a is located on element B while Lsp1γ is located on element D (Figure 2, c and d). Since the genomic organization of Lsp1γ is the same in D. buzzatii as in D. pseudoobscura, this is likely to be the ancestral organization of the genus and thus the Lsp1γ gene would have conservatively transposed from element B to element D in the lineage leading to D. melanogaster. The maximum size of the transposed region is 5.9 kb and includes the putative regulatory regions located upstream of the gene. Another transposition event where the nested organization was the ancestral one had been previously reported in Drosophila (Neufeld et al. 1991).
Given that Lsp1β and Lsp1γ are present in the three species studied in different chromosomes, the duplicative transposition originating these two genes must have occurred before the divergence of these species (40–62 MYA). The chromosomal localization of the ancestral Lsp1 gene in the genus Drosophila is unknown. There is no reason to believe that this chromosome was element E as suggested by Brock and Roberts (1983) because in both D. buzzatii and D. pseudoobscura Lsp1β is located on element E and Lsp1γ is located on element B. Therefore, the localization of the ancestral Lsp1 gene could be any of these two elements. In any case, to explain the current localization of these two genes in D. melanogaster at least two conservative transpositions are needed (see above). Another duplicative transposition gave rise to D. melanogaster Lsp1α. The absence of this gene in D. pseudoobscura indicates that this transposition likely occurred after the divergence between D. melanogaster and D. pseudoobscura. However, the neighbor-joining tree of Lsp1 sequences (Figure 4) suggests that this transposition occurred before the divergence between D. melanogaster and D. pseudoobscura. If this were the case, this gene would have been lost in the D. pseudoobscura lineage. Overall, at least two duplicative and two conservative transpositions are needed to explain the present localization of Lsp1 genes (Figure 5).
Figure 5.—
Phylogenetic relationships and karyotypic organization of D. melanogaster and D. buzzatii. The present chromosomal localization of Lsp1 genes in D. melanogaster and D. buzzatii is shown. At least two duplicative and two conservative transpositions are required to explain the evolution of these genes from an ancestral Lsp1 gene.
Mechanism of transposition:
As stated before, the position of the unique intron of Lsp1β and Lsp1γ and the 5′ putative regulatory sequences are conserved in the three species analyzed. This allows us to rule out retroposition (Betrán et al. 2002) as the mechanism of transposition. The analysis of the flanking regions of both genes provides no evidence that transposition had been mediated by transposable elements (TEs). A single TE copy was found in the original position of Lsp1β in D. melanogaster (Figure 1c) but there are no indications of its involvement in the transposition. However, taking into account the divergence time between the species analyzed, mobile elements could have played a role in the origin of the transposition and then be lost by deletion or excision. The most striking feature of Lsp1 genes in the three species analyzed is that they are very close to snRNA or tRNA genes. Both are repetitive genes that are scattered in the genome. However, the probability that Lsp1 genes are close to snRNA or tRNA genes just by chance seems very low. A recent review of D. melanogaster genome sequence-Release 3 has found 290 tRNA genes and only 28 snRNAs in the euchromatin (Misra et al. 2002), i.e., ∼1 tRNA gene/0.4 Mb and 1 snRNA gene/4.3 Mb of euchromatic DNA. Repeated genes, e.g., tRNA and ribosomal protein genes, have been previously implied in the origin of chromosomal rearrangements in yeasts (Szankasi et al. 1986; Kellis et al. 2003).
We propose that an ectopic exchange between snRNA:U1 sequences mediated the Lsp1β transposition in Drosophila. Three observations indicate that snRNA:U1 genes are implied in this transposition. First, they are present in the original location of chromosomal element E as well as in the destination site of element B. Second, snRNA:U1 genes present a high level of nucleotide identity among the three species (98%) suggesting that they might act as substrates for ectopic exchanges. Finally, the snRNA:U1 gene represents the downstream boundary of the transposed chromosomal segment (Figure 1), which would be expected if ectopic recombination actually took place. To our knowledge, snRNA genes have not been previously implicated in the generation of rearrangements. Nevertheless, there is no reason why they could not act as substrates for ectopic recombination in a manner similar to that of tRNAs in yeasts or Alu sequences in humans. As a matter of fact, the mechanism for gene transposition in Drosophila could be quite similar to that originating segmental duplications in humans (see Figure 6 in Bailey et al. 2003).
The evidence for the implication of tRNA genes in the transposition of Lsp1γ is weak. The tRNA genes found near Lsp1γ in D. buzzatii and D. pseudoobscura belong to a different isoacceptor type and no tRNA gene was found near Lsp1γ in D. melanogaster. In addition these tRNAs lie outside the transposed chromosomal segment. Therefore, an involvement of tRNA genes in the transposition of Lsp1γ is unlikely and in this case the mechanism remains uncertain.
5′ noncoding conserved sequences:
Transposition implies the localization of the transposed gene in a new genomic environment. The probability of success of a transposition should be higher when it includes the regulatory regions than when it does not. To be functional, in the latter case the transposed gene would need to recruit new regulatory regions (Betrán et al. 2002). Comparative sequence analysis allows the identification of conserved DNA sequences in noncoding regions that are considered putative cis-acting regulatory elements (Bergman and Kreitman 2001). Comparison of the 5′ ends of Lsp1α, Lsp1β and Lsp1γ genes of D. melanogaster led to the identification of two such conserved sequences (Delaney et al. 1986). These sequences are also conserved in Lsp1β (Table 4, b and c) and Lsp1γ (Table 4, e and f) in the three species analyzed. These two regions are more conserved between Lsp1α and Lsp1β than between Lsp1β and Lsp1γ, which is in agreement with the origin of Lsp1α from a duplication of Lsp1β (Smith et al. 1981). Two conserved sequences not previously described in D. melanogaster have been also identified. One is exclusive of Lsp1β, the other is exclusive of Lsp1γ (Table 4, d and g), and both are highly conserved in the three species analyzed. All conserved sequences except that located at −181 of D. buzzatii Lsp1γ fulfill the requirements used by Bergman and Kreitman (2001) to identify noncoding conserved blocks. The mean size of these five blocks is 13 bp, similar to the modal size of 11 bp reported by these authors, and three of them are included in the conserved blocks that they have described. They follow the pattern described for cis-regulatory elements in Drosophila, i.e., highly conserved sequences separated by unalignable gaps (Bergman and Kreitman 2001).
The 5′-untranslated regions of the RNA show two highly conserved sequences common to the three Lsp1 genes in the three species analyzed (Figure 3). Although the significance of these homologies in the 5′-UTR is not clear, it has been suggested that the synthesis of LSP-1 protein may be under translational control (Powell et al. 1984). All the signals necessary for the correct tissue and temporal specificity expression of Lsp1α and Lsp1β in D. melanogaster lie 1650 and 2250 bp upstream from these two genes, respectively (Jowett 1985; Davies et al. 1986). In this work we have analyzed more than 2 kb upstream from Lsp1β and Lsp1γ genes and we are quite confident that the majority if not all the cis-acting regulatory sequences have been identified. The fact that the transposition of Lsp1 genes included not only the genes but also the regulatory regions has probably played a role in the success of these transpositions.
Molecular evolution:
Maximum likelihood methods of phylogenetic inference were used to test alternative models for the evolution of Lsp1 genes (Yang 1997). Figure 4 shows the phylogenetic tree that provides a significantly better fit to the data. The tree is in agreement with the appearance of Lsp1α from a duplication of Lsp1β in the Sophophora subgenus.
The ratio of synonymous (silent, dS) to nonsynonymous (amino acid changing, dN) substitution rates is a measure of selective pressure on a protein: if amino acid changes are neutral dN/dS = 1, if they are mostly deleterious dN/dS < 1, and if they offer a selective advantage dN/dS > 1 (Yang and Bielawski 2000; Bielawski and Yang 2003). A model assuming free dN/dS ratios for different lineages provided a significantly better fit to the data than a model with a single ratio for all lineages. This led us to test if a model assuming four different dN/dS ratios, one background ratio and one ratio for each of the three lineages leading to D. melanogaster Lsp1 genes, was significantly different from the single-ratio model. According to our observations, the Lsp1 genes have transposed in these three lineages, and we wanted to test if the change of chromosomal location has affected the molecular evolution of these genes. Again the results were significant, indicating that the model assuming four different dN/dS ratios fits better to the data. The estimates obtained for the dN/dS ratios in this model were 0.0518 for the background ratio and 0.0769, 0.0598, and 0.0231 for the lineages leading to Lsp1α, Lsp1β, and Lsp1γ of D. melanogaster, respectively. These results suggest that changes in the selection regime (degree of functional constraint) are associated with Lsp1 transpositions. However dN/dS ratios do not show a consistent pattern, being higher than the background ratio for Lsp1α and lower than the background ratio for Lsp1γ. In addition, the background ratio is itself heterogeneous, indicating that other factors besides transposition have an effect on the molecular evolution of Lsp1 genes.
Acknowledgments
We thank L. Sánchez (Centro de Investigaciones Biológicas, Consejo Superior de Invesigaciones Cientifícas, Madrid) for providing the D. melanogaster Lsp1α clone and E. Hasson (Universidad de Buenos Aires) for D. buzzatii stocks. We also thank B. Negre for technical advice and helpful discussion. This work was supported by grant BMC2002-01708 from the Dirección General de Investigación (Ministerio de Ciencia y Tecnología, Spain) awarded to A.R.
References
- Adams, M. D., S. E. Celniker, R. A. Holt, C. A. Evans, J. D. Gocayne et al., 2000. The genome sequence of Drosophila melanogaster. Science 287: 2185–2195. [DOI] [PubMed] [Google Scholar]
- Armengol, L., M. A. Pujana, J. Cheung, S. W. Scherer and X. Estivill, 2003. Enrichment of segmental duplications in regions of breaks of synteny between the human and mouse genomes suggests their involvement in evolutionary rearrangements. Hum. Mol. Genet. 12: 2201–2208. [DOI] [PubMed] [Google Scholar]
- Bachtrog, D., and B. Charlesworth, 2003. On the genomic location of the exuperantia1 gene in Drosophila miranda: the limits of in situ hybridization experiments. Genetics 164: 1237–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, J. A., Z. Gu, R. A. Clark, K. Reinert, R. V. Samonte et al., 2002. Recent segmental duplications in the human genome. Science 297: 1003–1007. [DOI] [PubMed] [Google Scholar]
- Bailey, J. A., G. Liu and E. E. Eichler, 2003. An Alu transposition model for the origin and expansion of human segmental duplications. Am. J. Hum. Genet. 73: 823–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetzen, J. L., and J. Ma, 2003. The genetic colinearity of rice and other cereals on the basis of genomic sequence analysis. Curr. Opin. Plant Biol. 6: 128–133. [DOI] [PubMed] [Google Scholar]
- Bergman, C. M., and M. Kreitman, 2001. Analysis of conserved noncoding DNA in Drosophila reveals similar constraints in intergenic and intronic sequences. Genome Res. 11: 1335–1345. [DOI] [PubMed] [Google Scholar]
- Bergman, C. M., B. D. Pfeiffer, D. E. Rincon-Limas, R. A. Hoskins, A. Gnirke et al., 2002 Assessing the impact of comparative genomic sequence data on the functional annotation of the Drosophila genome. Genome Biol. 3: research0086–0086.1–0086.20. [DOI] [PMC free article] [PubMed]
- Betrán, E., K. Thornton and M. Long, 2002. Retroposed new genes out of the X in Drosophila. Genome Res. 12: 1854–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley, S. M., and A. C. Wilson, 1984. Molecular evolution in Drosophila and higher Dipterans. II. A time scale for fly evolution. J. Mol. Evol. 21: 1–12. [DOI] [PubMed] [Google Scholar]
- Bielawski, J. P., and Z. Yang, 2003. Maximum likelihood methods for detecting adaptive evolution after gene duplication. J. Struct. Funct. Genomics 3: 201–212. [PubMed] [Google Scholar]
- Brock, H. W., and D. B. Roberts, 1980. Comparison of the Larval serum proteins of Drosophila melanogaster using one and two-dimensional peptide mapping. Eur. J. Biochem. 106: 129–135. [DOI] [PubMed] [Google Scholar]
- Brock, H. W., and D. B. Roberts, 1983. Location of the LSP-1 genes in Drosophila species by in situ hybridization. Genetics 103: 75–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester, T., H. C. Massey, Jr., S. O. Zakharkin and H. Benes, 1998. The evolution of hexamerins and the phylogeny of insects. J. Mol. Evol. 47: 93–108. [DOI] [PubMed] [Google Scholar]
- Cáceres, M., M. Puig and A. Ruiz, 2001. Molecular characterization of two natural hotspots in the Drosophila buzzatii genome induced by transposon insertions. Genome Res. 11: 1353–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casals, F., M. Caceres and A. Ruiz, 2003. The Foldback-like transposon Galileo is involved in the generation of two different natural chromosomal inversions of Drosophila buzzatii. Mol. Biol. Evol. 20: 674–685. [DOI] [PubMed] [Google Scholar]
- Celniker, S. E., D. A. Wheeler, B. Kronmiller, J. W. Carlson, A. Halpern et al., 2002 Finishing a whole-genome shotgun: release 3 of the Drosophila melanogaster euchromatin genome sequence. Genome Biol. 3: research0079.1–0079.14. [DOI] [PMC free article] [PubMed]
- Cheung, J., M. D. Wilson, J. Zhang, R. Khaja, J. R. Macdonald et al., 2003. Recent segmental duplications in the mouse genome. Genome Biol. 4: R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia, W., S. McGill, R. Karp, D. Gubb and M. Ashburner, 1985. Spontaneous excision of a large composite transposable element of Drosophila melanogaster. Nature 316: 81–83. [DOI] [PubMed] [Google Scholar]
- Coghlan, A., and K. H. Wolfe, 2002. Fourfold faster rate of genome rearrangement in nematodes than in Drosophila. Genome Res. 12: 857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, J. A., C. F. Addison, S. J. Delaney, C. Sunkel and D. M. Glover, 1986. Expression of the prokaryotic gene for chloramphenicol acetyl transferase in Drosophila under the control of Larval serum protein 1 gene promoters. J. Mol. Biol. 189: 13–24. [DOI] [PubMed] [Google Scholar]
- Delaney, S. J., D. F. Smith, A. McClelland, C. Sunkel and D. M. Glover, 1986. Sequence conservation around the 5′ ends of the Larval serum protein 1 genes of Drosophila melanogaster. J. Mol. Biol. 189: 1–11. [DOI] [PubMed] [Google Scholar]
- Eichler, E. E., 2001. Recent duplication, domain accretion and the dynamic mutation of the human genome. Trends Genet. 17: 661–669. [DOI] [PubMed] [Google Scholar]
- Ejima, Y., and L. Yang, 2003. Trans mobilization of genomic DNA as a mechanism for retrotransposon-mediated exon shuffling. Hum. Mol. Genet. 12: 1321–1328. [DOI] [PubMed] [Google Scholar]
- Esnault, C., J. Maestre and T. Heidmann, 2000. Human LINE retrotransposons generate processed pseudogenes. Nat. Genet. 24: 363–367. [DOI] [PubMed] [Google Scholar]
- Felsenstein, J., 1989 PHYLIP: phylogeny inference package (version 3.2). Cladistics, 164–166.
- Fischer, G., C. Neuvéglise, P. Durrens, C. Gaillardin and B. Dujon, 2001. Evolution of gene order in the genomes of two related yeast species. Genome Res. 11: 2009–2019. [DOI] [PubMed] [Google Scholar]
- González, J., J. M. Ranz and A. Ruiz, 2002. Chromosomal elements evolve at different rates in the Drosophila genome. Genetics 161: 1137–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper, S. D., and O. G. Berg, 2003. On the nature of gene innovation: duplication patterns in microbial genomes. Mol. Biol. Evol. 20: 945–954. [DOI] [PubMed] [Google Scholar]
- Jowett, T., 1985. The regulatory domain of a larval serum protein gene in Drosophila melanogaster. EMBO J. 4: 3789–3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellis, M., N. Patterson, M. Endrizzi, B. Birren and E. S. Lander, 2003. Sequencing and comparison of yeast species to identify genes and regulatory elements. Nature 423: 241–254. [DOI] [PubMed] [Google Scholar]
- Krimbas, C. B., and J. R. Powell, 1992 Drosophila Inversion Polymorphism. CRC Press, Boca Raton, FL.
- Ku, H., T. Vision, J. Liu and S. D. Tanksley, 2000. Comparing sequenced segments of the tomato and Arabidopsis genomes: large-scale duplication followed by selective gene loss creates a network of synteny. Proc. Natl. Acad. Sci. USA 97: 9121–9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander, E. S., L. M. Linton, B. Birren, C. Nusbaum, M. C. Zody et al., 2001. Initial sequencing and analysis of the human genome. Nature 409: 860–921. [DOI] [PubMed] [Google Scholar]
- Llorente, B., A. Malpertuy, C. Neuvéglise, J. De Montigny, M. Aigle et al., 2000. Genomic exploration of the Hemiascomycetous yeasts: 18. Comparative analysis of chromosome maps and synteny with Saccharomyces cerevisiae. FEBS Lett. 47: 101–112. [DOI] [PubMed] [Google Scholar]
- Locke, D. P., N. Archidiacono, D. Misceo, M. F. Cardone, S. Deschamps et al., 2003. Refinement of a chimpanzee pericentric inversion breakpoint to a segmental duplication cluster. Genome Biol. 4: R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, M., E. Betrán, K. Thornton and W. Wang, 2003. The origin of new genes: glimpses from the young and old. Nat. Rev. Genet. 4: 865–875. [DOI] [PubMed] [Google Scholar]
- Lovering, R., N. Harden and M. Ashburner, 1991. The molecular structure of TE146 and its derivatives in Drosophila melanogaster. Genetics 128: 357–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, M., and J. S. Conery, 2000. The evolutionary fate and consequences of duplicate genes. Science 290: 1151–1155. [DOI] [PubMed] [Google Scholar]
- Massey, H. C., Jr., J. Kejzlarova-Lepesant, R. L. Willis, A. B. Castleberry and H. Benes, 1997. The Drosophila Lsp-1 beta gene. A structural and phylogenetic analysis. Eur. J. Biochem. 245: 199–207. [DOI] [PubMed] [Google Scholar]
- Misra, S., M. A. Crosby, C. J. Mungall, B. B. Matthews, K. S. Campbell et al., 2002 Annotation of the Drosophila melanogaster euchromatic genome: a systematic review. Genome Biol. 3: research0083.1–0083.22. [DOI] [PMC free article] [PubMed]
- Montgomery, E., B. Charlesworth and C. H. Langley, 1987. A test for the role of natural selection in the stabilization of transposable element copy number in a population of Drosophila melanogaster. Genet. Res. 49: 31–41. [DOI] [PubMed] [Google Scholar]
- Moran, J. V., R. J. DeBerardinis, H. H. Kazazian, Jr., 1999. Exon shuffling by L1 retrotransposition. Science 283: 1465–1467. [DOI] [PubMed] [Google Scholar]
- Morgenstern, B., 1999. DIALIGN 2: improvement of the segment-to-segment approach to multiple sequence alignment. Bioinformatics 15: 211–218. [DOI] [PubMed] [Google Scholar]
- Naumann, U., and K. Scheller, 1991. Complete cDNA and gene sequence of the developmentally regulated arylphorin of Calliphora vicina and its homology to insect hemolymph proteins and arthropod hemocyanins. Biochem. Biophys. Res. Commun. 177: 963–972. [DOI] [PubMed] [Google Scholar]
- Neufeld, T. P., R. W. Carthew and G. M. Rubin, 1991. Evolution of gene position: chromosomal arrangement and sequence comparison of the Drosophila melanogaster and Drosophila virilis sina and Rh4 genes. Proc. Natl. Acad. Sci. USA 88: 10203–10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell, D., J. D. Sato, H. W. Brock and D. B. Roberts, 1984. Regulation and synthesis of the Larval serum proteins of Drosophila melanogaster. Dev. Biol. 102: 206–215. [DOI] [PubMed] [Google Scholar]
- Powell, J. R., 1997 Progress and Prospects in Evolutionary Biology: The Drosophila Model. Oxford University Press, New York.
- Ranz, J. M., J. González, F. Casals and A. Ruiz, 2003. Low occurrence of gene transposition events during the evolution of the genus Drosophila. Evolution 57: 1325–1335. [DOI] [PubMed] [Google Scholar]
- Roberts, D. B., and S. Evans-Roberts, 1979. The X-linked alpha-chain gene of Drosophila LSP-1 does not show dosage compensation. Nature 280: 691–692. [DOI] [PubMed] [Google Scholar]
- Ruiz, A., and M. Wasserman, 1993. Evolutionary cytogenetics of the Drosophila buzzatii species complex. Heredity 70: 582–596. [DOI] [PubMed] [Google Scholar]
- Russo, C. A. M., N. Takezaki and M. Nei, 1995. Molecular phylogeny and divergence times of Drosophilid species. Mol. Biol. Evol. 12: 391–404. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., E. F. Fritsch and T. Maniatis, 1989 Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Samonte, R. V., and E. E. Eichler, 2001. Segmental duplications and the evolution of the primate genome. Nat. Rev. Genet. 3: 65–72. [DOI] [PubMed] [Google Scholar]
- Smith, D. F., A. McClelland, B. N. White, C. F. Addison and D. M. Glover, 1981. The molecular cloning of a dispersed set of developmentally regulated genes which encode the major larval serum protein of D. melanogaster. Cell 23: 441–449. [DOI] [PubMed] [Google Scholar]
- Spicer, G. S., 1988. Molecular evolution among some Drosophila species groups as indicated by two-dimensional electrophoresis. J. Mol. Evol. 27: 250–260. [DOI] [PubMed] [Google Scholar]
- Szankasi, P., C. Gysler, U. Zehntner, U. Leupold, J. Kohli et al., 1986. Mitotic recombination between dispersed but related tRNA genes of Schizosaccharomyces pombe generates a reciprocal translocation. Mol. Gen. Genet. 202: 394–402. [Google Scholar]
- Thompson, J. D., D. G. Higgins and T. J. Gibson, 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Throckmorton, L. H., 1975 The phylogeny, ecology and geography of Drosophila, pp. 421–469 in Handbook of Genetics, edited by R. C. King. Plenum Press, New York.
- Yang, Z., 1997. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput. Appl. Biosci. 13: 555–556. [DOI] [PubMed] [Google Scholar]
- Yang, Z., and J. P. Bielawski, 2000. Statistical methods for detecting molecular adaptation. Trends Ecol. Evol. 15: 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]