Abstract
The molecular mechanisms whereby volatile general anesthetics (VAs) disrupt behavior remain undefined. In Caenorhabditis elegans mutations in the gene unc-64, which encodes the presynaptic protein syntaxin 1A, produce large allele-specific differences in VA sensitivity. UNC-64 syntaxin normally functions to mediate fusion of neurotransmitter vesicles with the presynaptic membrane. The precise role of syntaxin in the VA mechanism is as yet unclear, but a variety of results suggests that a protein interacting with syntaxin to regulate neurotransmitter release is essential for VA action in C. elegans. To identify additional proteins that function with syntaxin to control neurotransmitter release and VA action, we screened for suppressors of the phenotypes produced by unc-64 reduction of function. Loss-of-function mutations in slo-1, which encodes a Ca2+-activated K+ channel, and in unc-43, which encodes CaM-kinase II, and a gain-of-function mutation in egl-30, which encodes Gqα, were isolated as syntaxin suppressors. The slo-1 and egl-30 mutations conferred resistance to VAs, but unc-43 mutations did not. The effects of slo-1 and egl-30 on VA sensitivity can be explained by their actions upstream or parallel to syntaxin to increase the level of excitatory neurotransmitter release. These results strengthen the link between transmitter release and VA action.
DESPITE longstanding efforts, volatile anesthetic (VA) mechanisms remain undefined. At a cellular level, the synapse is generally accepted as the site of action (Pocock and Richards 1991; Franks and Lieb 1994). In vertebrate nervous systems, both presynaptic (Zorychta and Capek 1978; Takenoshita and Takahashi 1987; Kullmann et al. 1989; Miao et al. 1995; Perouansky et al. 1995; Schlame and Hemmings 1995; MacIver et al. 1996; Nishikawa and MacIver 2000) and postsynaptic anesthetic effects (Jones et al. 1992; Mihic et al. 1997; Patel et al. 1999; Yamakura et al. 2001) have been observed that might contribute to a state of general anesthesia. However, establishing a causal link between VA effects in vitro and in vivo has been problematic. Genetics is one approach for making such a link. For example, a point mutation in the β3 subunit of the GABAA receptor eliminates the action of the intravenously administered general anesthetics etomidate and propofol against response to a painful stimulus in a mouse knockin strain (Jurd et al. 2003). Thus, potentiation of GABAA receptors is the major relevant action for these anesthetics. However, for VAs, no mutations of a vertebrate gene have resulted in a highly resistant animal. Screens in Drosophila have isolated mutant stains that are modestly VA resistant to some anesthetic endpoints (Krishnan and Nash 1990; Tinklenberg et al. 1991; Leibovitch et al. 1995; Gamo et al. 2003); however, highly resistant mutants have not been uncovered.
In Caenorhabditis elegans, two sorts of approaches have been taken. Using supraclinical concentrations of VAs to produce immobilization as an anesthetic endpoint, Morgan, Sedensky, and co-workers have isolated novel mutants that are VA hypersensitive or that are suppressors of the VA-hypersensitive phenotype (Morgan and Cascorbi 1985; Sedensky and Meneely 1987; Morgan et al. 1988; Morgan et al. 1990; Morgan and Sedensky 1994). One of the mutations producing VA hypersensitivity was found to lie in the gene gas-1, which encodes the 49-kD subunit of mitochondrial NADH:ubiquinone–oxidoreductase (complex I of the respiratory chain). Suppressors of the hypersensitivity were found among mutations in the unc-1, unc-7, unc-8, and unc-9 genes. unc-1 encodes a stomatin homolog (Rajaram et al. 1998); unc-8 encodes an epithelial sodium channel (Tavernarakis et al. 1997); unc-7 and unc-9 encode innexins, which are thought to be structural components of invertebrate gap junctions (Starich et al. 1996; Barnes and Hekimi 1997; Phelan et al. 1998; Phelan and Starich 2001). The suppression of VA hypersensitivity by loss-of-function mutations in innexins suggests that mutations in gas-1 and the other genes producing VA hypersensitivity may directly or indirectly sensitize to VA immobilization through a mechanism requiring gap junctions. Allele-specific interactions between unc-1 and unc-8 also suggest the possibility that these gene products interact and form a complex that regulates the binding or activity of VAs at supraclinical concentrations (Rajaram et al. 1999).
At lower concentrations similar to those used for human anesthesia, VAs do not immobilize C. elegans; rather their locomotion becomes uncoordinated and sluggish (Crowder et al. 1996). Mutations in genes that alter neurotransmitter release in C. elegans have been found to control these VA behavioral effects (van Swinderen et al. 1999, 2001, 2002). These mutations do not alter sensitivity to supraclinical VA concentrations in the immobilization assay (Humphrey et al. 2002). Thus, the mechanisms underlying the effects of VAs at clinical and supraclinical concentrations are distinct. In the C. elegans, Drosophila, and vertebrate nervous systems (Zorychta and Capek 1978; Takenoshita and Takahashi 1987; Kullmann et al. 1989; Miao et al. 1995; Perouansky et al. 1995; Schlame and Hemmings 1995; MacIver et al. 1996; Nishikawa and Kidokoro 1999; van Swinderen et al. 1999; Nishikawa and MacIver 2000), VAs have been shown to reduce excitatory neurotransmitter release, an action that could be responsible for at least some behavioral effects of anesthetics. In support of this hypothesis, some VA-resistant mutants in C. elegans and Drosophila antagonize the effect of VAs on acetylcholine and glutamate release, respectively (Nishikawa and Kidokoro 1999; van Swinderen et al. 1999).
The presumptive presynaptic VA target(s) in C. elegans is unknown. Direct screens for high-level VA resistance have been difficult and thus far unsuccessful. However, testing of existing C. elegans mutants found that mutations in unc-64, which encodes the presynaptic protein syntaxin 1A, produced large allele-specific differences in VA sensitivity. In particular, an unusual neomorphic syntaxin allele, unc-64(md130), was able to dominantly antagonize VA action, producing complete resistance to VAs at clinical concentrations (van Swinderen et al. 1999). Syntaxin 1A is an integral membrane protein that interacts with SNAP-25 and synaptobrevin (VAMP), forming a ternary complex (the SNARE complex) that mediates fusion of the synaptic vesicle with the presynaptic membrane (Chen and Scheller 2001). Multiple other proteins function along with syntaxin and the SNARE complex to regulate the fusion process (Jahn and Sudhof 1999; Mochida 2000; Rizo and Sudhof 2002). Our genetic data are consistent with the hypothesis that a protein physically interacting with syntaxin is the VA target responsible for effects of VAs on transmitter release. Alternatively, a syntaxin-interacting protein is not the target but is essential for the action of VAs on their target. In either case, identification of this hypothesized protein and more generally proteins that regulate VA action on transmitter release is required to define the VA mechanism. Reduction-of-function mutations exist in some of these syntaxin interactors; however, they generally produce severe behavioral deficits that preclude testing of their VA sensitivity. To circumvent this problem, here we test the VA sensitivity of mutations isolated in a screen for enhancers of transmitter release. The screen was performed in the background of a syntaxin reduction-of-function mutation. The syntaxin mutant background facilitated screening and potentially enriched for mutations that directly enhance syntaxin function. Analysis of the synaptic but not the anesthetic phenotypes of mutations in one of the genes identified in this screen was reported in Wang et al. (2001).
MATERIALS AND METHODS
Strains and strain constructions:
C. elegans strains were obtained from multiple laboratories referenced herein, from the Caenorhabditis Genetics Center funded by the National Institutes of Health National Center for Research Resources, and from our own mutagenesis screens as described below. All strains were grown on nematode growth media (NGM) agar seeded with OP50 bacteria (Brenner 1974); well-fed young adults were used for all assays. Strains used in this work include the wild-type strain N2 (var. Bristol; Brenner 1974) and mutant strains by linkage group as follows:
LGI: egl-30(js126, tg26, n686), dpy-5(e61).
LGII: bli-2(e768).
LGIII: dpy-19(e1259), unc-64(e246, md130, js21).
LGIV: bli-6(sc16), unc-43(js125, js383, e755).
PS2284: dpy-20(e1282);syEx125[pMH86+pLB2+pBS] (Brundage et al. 1996). PS2284 is referred to in the text as Ex[Gq(+)]. pMH86 contains a full-length genomic clone of dpy-20(+), which rescues dpy-20(e1282), inserted into pBluescript SK+ and as such was used as a transformation marker; pLB2 contains a full-length egl-30(+) genomic clone inserted into pBluescript SK+ (pBS; Brundage et al. 1996). PS2554 is referred to in the text as GqHS(Q205L) = dpy-20(e1282);syIs38[pMH86+pLB24+pBS]; pLB24 contains a mutant egl-30 genomic fragment (GqQ205L), predicted to produce a constitutively active EGL-30 protein, inserted into pPD49.78, which has a heat-shock-inducible promoter from the hsp16-2 gene (Stringham et al. 1992; Mello and Fire 1995). pLB24 was injected at 50 ng/μl along with pMH86 and pBS, and the resultant array was integrated with a 38-Gy X-ray source and outcrossed eight times (C. Bastiani, L. Brundage and P. Sternberg, personal communication). PS2284 and PS2554 were kindly provided by Carol Bastiani and Paul Sternberg.
LGV: slo-1(js100, js118, js379, js380, js381, js382, eg24, eg161, eg73) dpy-11(e224), egl-8(n488), unc-34(e566), unc-60(e677), dpy-11(e224).
LGX: lon-2(e678), dgk-1(nu199). Note that dgk-1(nu199) is also known as sag-1(nu199).
TY1657: dpy-5(e61) I; rol-6(e187) II, lon-1(e185) III, bli-6(sc16) IV; unc-23(e324) V.
Construction of double mutants:
Four double mutant strains were constructed: egl-30(js126);unc-64(md130), egl-30(js126);unc-64(js21), egl-30(js126);dgk-1(nu199), and egl-30(js126);egl-8(n488). To build egl-30(js126);unc-64(md130), egl-30(js126);unc-64(js21), and egl-30(js126);dgk-1(nu199), dpy-5(e61) was used as a visible marker in cis with egl-30(js126). egl-30(js126) dpy-5(e61)/+ males were crossed into unc-64(md130), unc-64(js21), and dgk-1(nu199). In the F2 generation, non-Dpy animals displaying the Unc (md130 and js21) or loopy (nu199) phenotype were selected and Dpy F3 progeny were chosen as presumptive double mutants. egl-30(js126);egl-8(n488) was built by crossing egl-30(js126)/+ males into unc-34(e566) unc-60(e677) dpy-11(e224), a balancer for egl-8(n488). The male progeny were mated with egl-8(n488). Animals in the F1 progeny that displayed the js126 semidominant weak loopy phenotype were picked singly to plates and strong loopy F2 animals were chosen from plates that segregated the balancer phenotypes. F3 animals not segregating the balancer were presumed to be the double mutant. Genotypes of all double mutants were confirmed by outcrossing and segregation of both mutant phenotypes and by sequencing egl-30 to confirm homozygosity of the js126 mutation.
Mutagenesis screen for suppressors of unc-64(rf):
unc-64(e246rf) L4 larvae were exposed to 50 mm EMS in M9 buffer for 4 hr with agitation and then washed three times with M9. The F2 progeny were screened visually for improved locomotion compared to unc-64(e246). Progeny of putative suppressor mutants were similarly screened and one animal that clearly moved better than e246 was kept to establish each strain. Overall, 24,000 genomes were screened and 14 confirmed suppressors were isolated. Each suppressor strain was outcrossed against N2 at least three times and outcrossed strains with and without e246 in the background were kept for further characterization. Suppressor mutations crossed out of the e246 background were examined for behavioral, morphological, anesthetic-sensitivity, and aldicarb-sensitivity phenotypes (see below). Any additional phenotypes of the suppressor mutations after outcrossing were confirmed to cosegregate with suppression of e246.
Genetic characterization and mapping:
Prior to mapping, mutants were analyzed for dominance of their phenotypes and, if recessive, were placed into complementation groups on the basis of their suppression of unc-64(e246). Of the mutations presented here, only js126 exhibited dominant phenotypes. The mutations fell into at least five complementation groups; representatives from three complementation groups are analyzed in this report. For placement of mutant loci onto chromosomes, the following visible marker mutations were used: dpy-5(e61) I; bli-2(e768) and rol-6(e187) II; unc-64(e246) and lon-1(e185) III; dpy-19(e1259) and bli-6(sc16) IV, dpy-11(e224) and unc-23(e324) V, and lon-2(e678) X. The general method was to cross homozygous or heterozygous test mutant males into the mapping strain and then pick F2 progeny expressing the phenotype(s) of the test mutant [e.g., loopy, aldicarb hypersensitivity, or suppression of unc-64(246)]. The F3 progeny were scored for the marker phenotype and homozygosity of the test mutation.
slo-1 alleles:
Genetic characterization, mapping, and identification of the six slo-1 mutants isolated in this screen (js100, js118, js379, js380, js381, and js382) were described previously (Wang et al. 2001).
egl-30(js126):
For mapping of e246 suppression by js126, males with the genotype e246/+;marker/+ were crossed with js126;e246 and the brood of suppressor;e246 animals was scored for frequency of marker segregation. Twelve of 51 Unc Sup animals produced Dpy progeny, indicating loose linkage of js126 to dpy-5 on chromosome I. szT1 balances the left arm of chromosome I and was found to balance the js126 dpy-5 interval. Candidate genes within this interval consistent with the map distance from dpy-5 included egl-30. Overexpression of egl-30 had been shown to exhibit loopy locomotion similar to js126 (Brundage et al. 1996). The entire egl-30 coding region in js126 genomic DNA including exon/intron boundaries was sequenced and was found to have a single mutation, a G-to-A transition, resulting in a valine to methionine at position 180. For examination of cosegregation of js126's various phenotypes, egl-30(js126) dpy-5(e61) was outcrossed, and homozygous loopy non-Dpy F2 hermaphrodites with the presumed genotype of js126 +/js126 dpy-5 were allowed to self. The brood of loopy non-Dpy F3's that segregated all loopies and no Dpy's were tested for their VA and aldicarb sensitivities. In 53 of 53 independent recombinants, the loopy locomotion, the aldicarb-hypersensitive, and the VA-resistance phenotypes cosegregated.
unc-43 alleles:
js125 and js383 failed to complement for suppression of the aldicarb-resistance phenotype of unc-64(e246). The Sup phenotype of js125 was placed onto chromosome IV on the basis of limited recombination with dpy-13. Only 5/49 animals of the genotype unc-64(e246); dpy-13 +/+ js125 segregated Dpy Unc Sup progeny placing js125 near dpy-13. Both j125 and js383 were noted to exhibit a shrinker phenotype, the simultaneous contraction of all four body-wall muscle quadrants in response to anterior touch, which is characteristic of reduced GABAergic neurotransmission (McIntire et al. 1993). Two genes that when mutated produced the shrinker phenotype were within the mapped interval, so complementation was performed with both. js125 fully complemented the shrinker phenotype of unc-30(e191) but failed to complement unc-43(e408). The unc-43 coding region along with exon/intron boundaries was sequenced in js383 and G-to-A transition in the slice acceptor site before exon 15 was found. In js125, a 17-kb region extending from 10 kb upstream of the transcription initiation site through exon 10 was refractory to PCR amplification, indicative of a deletion of this region.
Behavioral assays:
All assays were performed at room temperature (22°–23°) on young adults. Two assays were used to quantify locomotion.
Velocity assays:
The speed of spontaneous and stimulated movement was measured by digital video imaging. One-day post-L4 young adults were picked onto plates seeded with OP50 Escherichia coli. Movies (60 sec) of single animals were taken immediately before and immediately after dropping a weight from a fixed height onto the lid of the Petri dish. The average forward movement of the midbody in each 1-sec frame was measured. For each strain, >20 animals were assayed to calculate the speed (micrometers per second) for that strain.
Body bend assays:
The body bend assay was used to measure the VA sensitivity of strains. Without transferring bacteria (either by transferring first to an unseeded plate or by picking animals that were off of the bacterial lawn), young adults were transferred via platinum wire to an unseeded NGM plate. After a 29-min incubation period, the plate was vigorously shaken (inside a clear glass chamber containing volatile anesthetic; see below) for ∼5 sec. After one more minute, body bends were counted for 2 min. One body bend consisted of a complete period of the waveform in the nematode's forward sinusoidal motion. Backward body bends were not counted. The body bends of at least 10 animals were counted per strain and condition.
Heat-shock experiments:
Young adult animals on agar plates were placed at 30° for 4 hr. Then, the body bend assay was performed as described above. Non-heat-shock controls were kept at 20° during the heat-shock incubation.
Drug assays:
Volatile anesthetics:
After transfer of animals to assay plates, the plates were placed immediately into small glass chambers as previously described (Morgan and Cascorbi 1985; Crowder et al. 1996). The chambers were sealed except for a small sideport and a measured volume of anesthetic in its liquid phase was deposited through the sideport onto the roof of each chamber using a Hamilton syringe and the sideport was quickly sealed. The anesthetic completely volatilizes within 30 sec and produces a steady-state behavioral effect within 10 min. The volume of anesthetic placed in the chamber was calculated to produce a desired gas phase concentration according to the ideal gas equation given the volume of the chamber and the density and molecular weight of the liquid anesthetic. At the end of each assay, the actual VA concentrations in the chambers were measured by gas chromatography and expressed as volume % (vol%). The predicted and actual concentrations typically differed by <10%. The measured VA concentrations were plotted against the body bends per minute using the sigmoidal equation y = min + (max − min)/(1 + (x/x50)−k) where y is the body bends per minute, x is the [VA] concentration in vol%, max is the body bends per minute with no VA added, min is the body bends per minute where it is at the lowest (i.e., at the highest VA concentrations), x50 is the EC50 (VA concentration at which the body bends per minute is half maximal), and k is the slope of the curve. The EC50's were used as the measure of VA sensitivity. A minimum of 10 points was used to generate each concentration/response curve. The VA sensitivities of strains were statistically compared by simultaneous curve fitting (Waud 1972; DeLean et al. 1978).
Aldicarb assays:
Two methods (kinetic and steady state) were used to determine aldicarb sensitivity. Kinetic assays were used for mapping of egl-30(js126) and for the determination of the native aldicarb sensitivity. A total of 20–25 animals were transferred onto a plate containing aldicarb and the fraction of animals paralyzed at multiple time points was measured. Animals were counted as paralyzed when they failed to move spontaneously or upon stimulation with a platinum wire. The steady-state assay (van Swinderen et al. 1999, 2001) was used for all aldicarb experiments performed with volatile anesthetics. Aldicarb sensitivities were measured after a 4-hr incubation on an aldicarb plate so that a concentration of drug inside the animal approximating that at steady state could be achieved. After this 4-hr preincubation, ∼30 animals were moved into a 0.5-cm-diameter circle delineated on the back of the aldicarb plate. After one additional hour, the fraction of animals that were outside the circle was scored as the movement index (MI). Because this assay did not require touching the animals and the aldicarb effect was not varying significantly with time, the steady-state assay was better suited for measuring effects of anesthetics (where the animals are enclosed in a gas tight chamber) on aldicarb sensitivity (van Swinderen et al. 1999, 2001).
Phorbol ester assays:
Phorbol 12-myristate 13-acetate dissolved in 100% ethanol was added to liquid NGM agar cooled to 60° to produce a 0.5 μg/ml final concentration. Plates containing an identical volume of ethanol were prepared as a control. A total of 40–50 healthy wild-type adults were transferred to and incubated on seeded phorbol ester and control plates for 2 hr and 20 min. Animals were then transferred onto unseeded phorbol ester and control plates, and a body bends per minute assay was performed as described above.
Arecoline assay:
The VA sensitivity of animals was measured on plates containing the muscarinic agonist arecoline hydrobromide (Avery 1993; Lackner et al. 1999). A sterile 142 mg/ml arecoline stock solution was made and stored at 4°; 167 μl of a sterile 142 mg/ml arecoline solution in water was added to the surface of freshly poured unseeded 10-ml NGM plates to produce a concentration of 2.4 mg/ml. Then, a fraction of the plates were seeded with OP50. Test animals were incubated on the seeded arecoline plates for 30 min and then transferred to an unseeded arecoline plate and a body bend assay in the presence and absence of VA was performed as described above.
RESULTS
Isolation and characterization of suppressors of syntaxin reduction-of-function mutants:
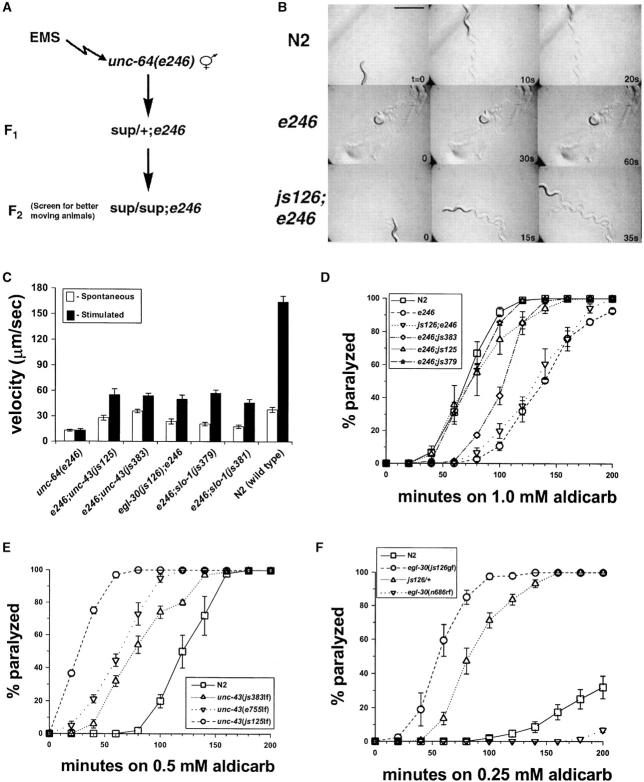
We performed a mutagenesis screen in C. elegans for mutations suppressing the aldicarb-resistance and lethargic locomotion phenotype of the syntaxin reduction-of-function (rf) mutant, unc-64(e246rf) (Figure 1). Aldicarb is an acetylcholinesterase inhibitor that is widely used as an indirect measure of the level cholinergic neurotransmission in C. elegans (Rand and Nonet 1997). Mutations that reduce cholinergic neurotransmission, such as unc-64(e246rf), are resistant to aldicarb, and mutations that increase cholinergic neurotransmission are hypersensitive. A total of 24,000 haploid genomes were screened in the F2 generation, and 14 suppressor mutations were isolated that fell into at least five complementation groups. Representatives of three of the complementation groups were chosen for characterization. The other mutants were set aside for later analysis. Consistent with a role of the suppressor mutations in regulation of transmitter release, the mutations suppressed both the sluggish locomotion and the aldicarb resistance of unc-64(e246rf) (Figure 1, B–D). Outcrossing of suppressor mutations away from unc-64(e246rf) revealed that all suppressor mutations conferred hypersensitivity to aldicarb (Figure 1, E and F; Wang et al. 2001). Thus, the suppressor mutations are capable of enhancing cholinergic neurotransmission of both wild-type and reduction-of-function syntaxin alleles; that is, the effects on neurotransmission are not syntaxin allele specific.
Figure 1.—
Isolation of mutations suppressing syntaxin reduction-of-function phenotypes. (A) Strategy for isolation of suppressors of syntaxin reduction of function. (B) Example of suppression of lethargy of unc-64(e246) by egl-30(js126). Shown in each row are three frames taken at the times noted from 60-sec movies of a representative N2, unc-64(e246), or egl-30(js126);unc-64(e246) animals. The animals were allowed to move freely on agar pads with a freshly seeded lawn of bacteria. Note different times for each strain. Bar, 1 mm. (C) Quantification of suppression of unc-64(e246) lethargy. Velocities were calculated from serial images of at least 20 animals moving on agar plates seeded with E. coli. Velocities just prior to (open bars) and immediately after stimulation (solid bars) by dropping a weight from a fixed height onto the lid of the Petri dish are shown. (D) Suppression of unc-64(e246) aldicarb resistance. The percentage paralyzed was plotted vs. incubation time on agar plates containing 1.0 mm aldicarb. Animals were considered paralyzed if no spontaneous or touch-evoked movement was observed. (E) Aldicarb sensitivity of unc-43 single mutants. Assay was exactly as in D except on plates containing 0.5 mm aldicarb. unc-43(e755) is a loss-of-function mutant described previously (Reiner et al. 1999). (F) Aldicarb sensitivity of egl-30 single mutants. Assay was exactly as in D except on plates containing 0.25 mm aldicarb. egl-30(n686) is a partial reduction-of-function mutant (Brundage et al. 1996). All values are expressed as mean ±SEM.
Loss of slo-1 function confers VA resistance:
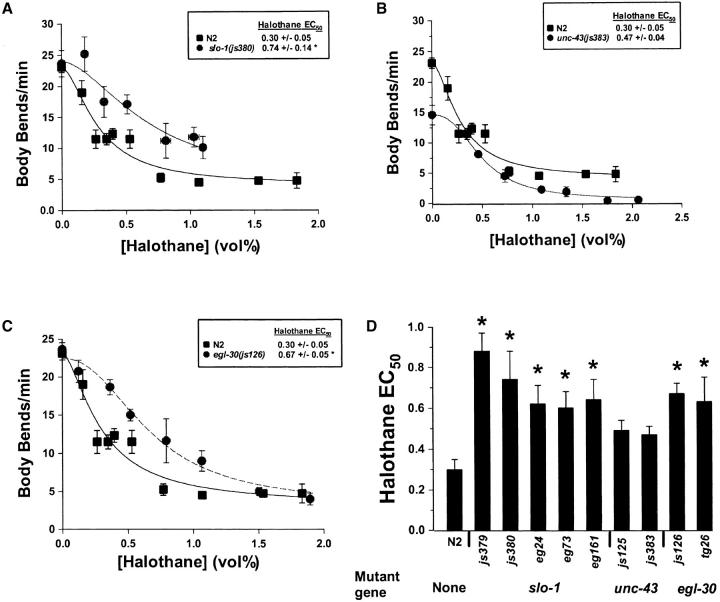
As previously reported, the largest complementation group isolated in our screen consisted of loss-of-function mutations in the slo-1 locus, which codes for a calcium-activated potassium channel (BK channel) negatively regulating the duration of transmitter release in C. elegans (Wang et al. 2001). After outcrossing the suppressor mutations away from unc-64(e246), all slo-1(lf) alleles tested were found to be resistant to the VA halothane (Figure 2, A and D). slo-1(lf) mutations have also been isolated in a screen for resistance to ethanol (Davies et al. 2003). Three slo-1 alleles isolated in this screen (eg24, eg161, and eg73) were tested for VA sensitivity. All three were resistant to halothane (Figure 2D). Loss of slo-1 function has been shown to enhance cholinergic neurotransmission through a prolongation of acetylcholine transmitter release at C. elegans motor neuron synaptic terminals (Wang et al. 2001). Although SLO-1 could be a direct target of VAs, the VA resistance of slo-1(lf) mutants can be explained by an indirect antagonism of the reduction in excitatory neurotransmitter release produced by VAs. Resistance to ethanol, however, does not appear to be the indirect consequence of an alteration in the level of transmitter release; rather, ethanol was shown to directly potentiate the SLO-1 channel (Davies et al. 2003). While supraclinical concentrations of VAs have been shown to inhibit vertebrate SLO-1 orthologs, clinical concentrations of VAs have little effect (Pancrazio et al. 1993; Hong et al. 1994). Moreover, to inhibit transmitter release, VAs would be predicted to augment the SLO-1 channel as ethanol does.
Figure 2.—
Sensitivity of suppressor mutants to the volatile anesthetic halothane. (A) Concentration/response curve of halothane against the locomotion rate of slo-1(js380) and N2. The rate of body bends was measured for each animal over a 2-min period. Each data point represents the mean ±SEM of at least 10 animals. (B) Concentration/response curve of halothane against the locomotion rate of unc-43(js383) and N2. (C) Concentration/response curve of halothane against the locomotion rate of egl-30(js126) and N2. (D) Halothane sensitivities of all suppressor mutants compared to N2. Bars represent EC50's plus or minus the standard error of the estimate. *P < 0.05 compared to N2 by simultaneous nonlinear regression (DeLean et al. 1978; Waud 1972).
Loss of unc-43 function suppresses syntaxin reduction of function without producing VA resistance:
js125 and js383 formed a second complementation group of unc-64(rf) suppressors. When outcrossed from unc-64(e246), both mutants were found to have VA sensitivities not significantly different from those of wild type (Figure 2, B and D). js125 and js383 were also behaviorally quite distinct from the other suppressor mutants. While the other suppressors outcrossed from e246 moved in a hyperactive manner, both js125 and js383 moved sluggishly and exhibited a shrinker phenotype, the simultaneous contraction of all four body-wall muscle quadrants in response to touch. Shrinking is a relatively uncommon mutant phenotype, found in strains with reduced GABAergic neurotransmission (McIntire et al. 1993) and in mutants in the unc-43 gene, which lie within the genetic interval to which js125 mapped. unc-43 encodes calcium/calmodulin-dependent protein kinase II (CaM-kinase II; Reiner et al. 1999; Rongo and Kaplan 1999). Sequencing of unc-43 revealed mutations in both js125 and j383. js125 deletes the first 10 exons of unc-43; js383 had a G-to-A transition in the splice acceptor prior to exon 15 (see materials and methods). Thus, js125 likely produces a complete loss of function of unc-43. Gain-of-function and loss-of-function unc-43 alleles have been isolated previously (Reiner et al. 1999). As expected given that the mutation deletes a large portion of the coding sequence, js125 resembled unc-43(e755), a previously isolated severe loss-of-function mutant. js125 and e755 as well as js383 are sluggish, shrinkers, and aldicarb hypersensitive (Reiner et al. 1999; Robatzek and Thomas 2000; Figures 1E and 2B). unc-43(gf) alleles do not shrink and are aldicarb resistant (Robatzek and Thomas 2000). unc-43(e755) but not unc-43(gf) alleles suppressed the phenotypes of unc-64(rf) (data not shown). Thus, we conclude that both js125 and js383 are loss-of-function alleles of unc-43 and that unc-43 negatively regulates syntaxin function but not VA action.
Gqα signaling antagonizes VA action:
Unlike the slo-1 and unc-43 mutants, the js126 mutation produced dominant phenotypes, including loopy hyperactive locomotion, aldicarb hypersensitivity, and VA resistance (Figures 1F and 2, C and D). The syntaxin suppressor phenotype of js126 mapped to a region containing the egl-30 gene (see materials and methods), which encodes the α-subunit of the G protein Gq shown to positively regulate locomotion, feeding, egg laying, and cholinergic neurotransmission (Brundage et al. 1996). Sequencing of js126 identified a mutation in egl-30, a G-to-A transition, resulting in a valine-to-methionine substitution at position 180 of EGL-30. While valine 180 has not itself been directly implicated in Gqα function, this region in vertebrate orthologs has been shown to bind to the γ-phosphate of GTP and ADP-ribosylation of the adjacent arginine 179 inhibits GTPase activity (Noel et al. 1993). Thus, a reasonable hypothesis to explain the phenotype of js126 is alteration of GTPase activity. Indeed, expression of a GTPase-defective EGL-30 results in animals with loopy hyperactive locomotion, constitutive egg laying, and aldicarb hypersensitivity similar to the phenotypes of js126 (Brundage et al. 1996; Lackner et al. 1999). On the other hand, egl-30 reduction-of-function mutants such as n686 (Figure 1F) are sluggish and aldicarb resistant (Brundage et al. 1996; Lackner et al. 1999; Miller et al. 1999). Thus, js126 behaves as an egl-30(gf) allele and not as an egl-30(lf) allele. Testing of egl-30(tg26), a behaviorally similar egl-30 allele thought to produce a gain of function (Doi and Iwasaki 2002), showed that this allele also suppressed unc-64(e246), increasing its mean velocity nearly fourfold (data not shown), and was VA resistant (Figure 2D). egl-30(n686), a weak reduction-of-function allele, was halothane hypersensitive (EC50 = 0.178 ± 0.047 vol%). On the basis of these data, we conclude that js126 produces a gain of function in egl-30, which antagonizes VA action.
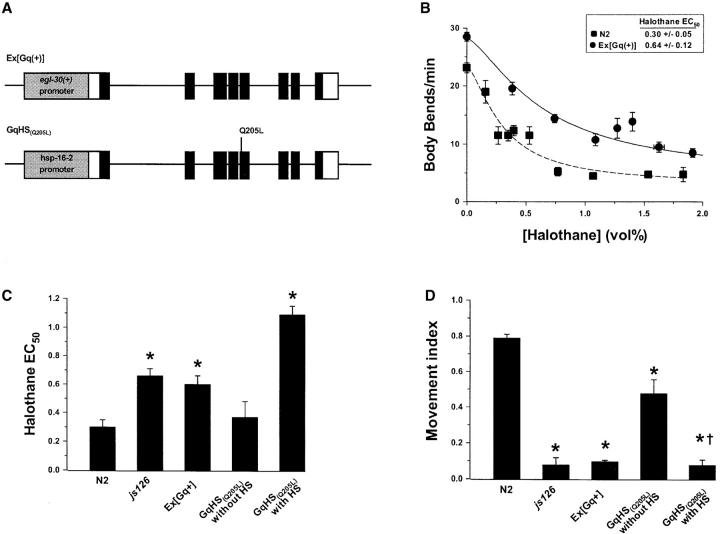
Both js126 and tg26 could produce a novel activity not normally present in the wild-type egl-30 product. To test whether wild-type egl-30 antagonizes VA activity, we measured the VA sensitivity of a strain overexpressing wild-type egl-30 (Brundage et al. 1996; Lackner et al. 1999). Animals transformed with extra copies of egl-30(+) were significantly halothane resistant with EC50's greater than twofold that of wild-type animals (Figure 3B). Similarly, heat shock of animals transformed with a GTPase-defective egl-30 transgene fused to a heat-shock-inducible promoter (Lackner et al. 1999) resulted in an even higher level of halothane resistance (Figure 3C). To confirm that the transformed genes were conferring a gain of egl-30 function, aldicarb sensitivity was measured, and as expected both strains were aldicarb hypersensitive (Figure 3D). These data are consistent with antagonism of VAs by the wild-type activity of EGL-30 and argue against the hypothesis that the js126 mutation produces a novel function not normally present in egl-30.
Figure 3.—
Gain of function in egl-30 confers resistance to halothane. (A) Schematic of the constructs used for transformation to produce egl-30 gain-of-function strains. The Ex[Gq(+)] is a genomic egl-30 fragment including the native promoter, the entire coding sequence, and 3′ noncoding sequence (Brundage et al. 1996). In GqHS(Q205L), the native promoter was replaced with the hsp-16-2 heat-shock-inducible promoter and the codon for aspartate 205 was mutated to produce a lysine. The Q205L lesion is predicted to disrupt the GTPase activity of EGL-30, producing a constitutively active protein. (B) Halothane sensitivity of animals transformed with Ex[Gq(+)] compared to N2. The rate of body bends was measured for each animal over a 2-min period. Each data point represents the mean ±SEM of at least 10 animals. EC50 of Ex[Gq(+)] was greater than that of N2, P < 0.01 by simultaneous nonlinear regression. (C) Comparison of halothane EC50's of egl-30 gain-of-function strains. A 4-hr heat-shock induction was performed on adult animals just prior to anesthetic testing. *P < 0.05 compared to N2 by simultaneous nonlinear regression (Waud 1972; DeLean et al. 1978). (D) Comparison of aldicarb sensitivity of egl-30 gain-of-function strains. Animals were incubated on plates containing 0.1 mm aldicarb for 4 hr and then the fraction of animals that moved over the next hour was scored as the movement index. Heat-shock induction was performed on adult animals just prior to anesthetic testing. *P < 0.05 compared to N2 two-tailed test; †P < 0.05 compared to non-heat-shocked control.
Pathway for EGL-30's regulation of VA sensitivity:
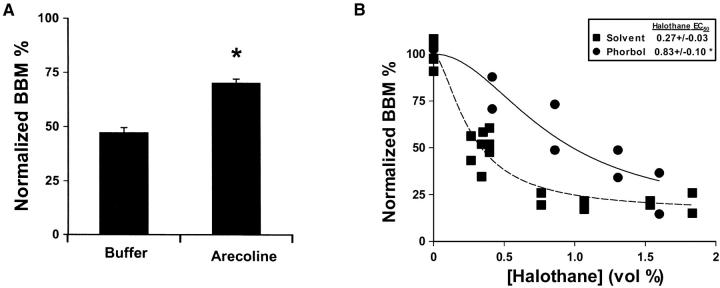
To begin to define the pathway whereby EGL-30 regulates VA sensitivity, we measured the effect of drugs previously shown to activate the EGL-30 signaling pathway. The muscarinic acetylcholine agonist, arecoline, produces hypercontraction of the C. elegans pharynx and aldicarb hypersensitivity, both effects of which are suppressed by egl-30(rf) (Brundage et al. 1996; Lackner et al. 1999). These results have led to a model where muscarinic receptors are coupled to EGL-30 and activation of muscarinic receptors stimulates EGL-30, which positively regulates acetylcholine release (Brundage et al. 1996; Lackner et al. 1999). If the muscarinic receptor is coupled to EGL-30 in the cells where VAs act, then activation of muscarinic receptors should antagonize VAs. Indeed, the muscarinic agonist arecoline produced halothane resistance (Figure 4A). Downstream of EGL-30, phorbol esters augment acetylcholine release from C. elegans motor neurons (Lackner et al. 1999; Miller et al. 1999). Both genetic and biochemical evidence implicate UNC-13 as the phorbol ester receptor acting downstream of EGL-30 to enhance acetylcholine release (Maruyama and Brenner 1991; Ahmed et al. 1992; Lackner et al. 1999). Moreover, UNC-13 has been shown to interact with the N terminus of syntaxin, making this pathway an attractive candidate for modulation of VA sensitivity by syntaxin mutants (Betz et al. 1998). Indeed, phorbol ester-treated animals were significantly resistant to halothane (Figure 4B).
Figure 4.—
Pharmacologic activation of the Gq signaling pathway confers resistance to halothane. (A) Halothane sensitivity of animals treated with the muscarinic agonist arecoline. Body bends per minute at 0 vol% and 0.3 ± vol% halothane were counted on plates containing 2.4 mg/ml arecoline. Body bends per minute (BBM) values in ∼0.3 vol% halothane were normalized to the average BBM with no added halothane. *P < 0.05, two-tailed t-test, significantly different from buffer. n = 10 animals for each condition. (B) Halothane sensitivity of phorbol ester-treated animals. After preincubation on agar plates containing phorbol ester or ethanol solvent, animals were transferred to unseeded phorbol ester or solvent plates and body bends were counted in the presence of various concentrations of halothane. BBM were normalized to the mean BBM without added halothane and then plotted against halothane concentration. *P < 0.05 by simultaneous curve fitting, EC50 significantly different from solvent. Each data point represents 1 animal.
To define genetically the pathway whereby egl-30 regulates volatile anesthetics, we tested strains carrying mutations in genes previously shown to lie downstream of egl-30 in its regulation of transmitter release. Modulation of neuromuscular transmission by egl-30 requires, at least in part, the activity of egl-8 (Lackner et al. 1999; Miller et al. 1999). egl-8 encodes a phospholipase Cβ homolog that positively regulates neuromuscular synaptic transmission (Lackner et al. 1999; Miller et al. 1999). egl-8(lf) fully suppressed the VA-resistance, aldicarb-resistance, and hyperlocomotion phenotypes of egl-30(js126) (Table 1). Thus, as for locomotion and transmitter release, we conclude that EGL-30 regulates VA sensitivity by stimulating EGL-8 PLCβ activity. However, egl-8(n488lf) itself was normally sensitive to halothane, indicating that VAs act downstream of or parallel to EGL-8.
TABLE 1.
Anesthetic and behavioral phenotypes of double mutants
| MI on aldicarbc
|
||||
|---|---|---|---|---|
| Genotype | Body bends per minutea |
Halothane EC50 (vol%)b |
0.1 mm | 0.35 mm |
| N2 (wild type) | 23.1 ± 0.87 | 0.30 ± 0.05 | 0.51 ± 0.07 | 0.07 ± 0.02 |
| egl-30(js126) | 23.4 ± 0.87 | 0.67 ± 0.05d | 0.08 ± 0.04d | 0.00 ± 0.00 |
| egl-8(n488) | 17.5 ± 1.60d | 0.34 ± 0.15 | ND | 0.32 ± 0.08d |
| egl-30(js126);egl-8(n488) | 18.4 ± 0.8e | 0.23 ± 0.02e | ND | 0.30 ± 0.07e |
| dgk-1(nu199) | 20.5 ± 1.17 | 0.53 ± 0.08d | 0.06 ± 0.04d | 0.00 ± 0.00 |
| egl-30(js126);dgk-1(nu199) | 26.8 ± 1.33f | 0.64 ± 0.10d | 0.02 ± 0.02d | 0.00 ± 0.00 |
| unc-64(md130) | 19.7 ± 1.66d | 1.27 ± 0.16d | ND | 0.95 ± 0.00d |
| egl-30(js126);unc-64(md130) | 22.4 ± 2.73 | 1.52 ± 0.23d | ND | 0.45 ± 0.05d,g |
| unc-64(js21) | 12.5 ± 0.28d | 0.30 ± 0.07 | ND | 0.58 ± 0.02d |
| egl-30(js126);unc-64(js21) | 18.0 ± 1.52d,h | 0.68 ± 0.15d,h | ND | 0.28 ± 0.02d,h |
ND, not determined.
Body bends per minute ±SEM, no anesthetic (n > 2 trials, 5–10 animals per trial).
EC50's ±SE (n > 2 trials, 10–14 animals per trial).
Movement index ±SEM on aldicarb (n > 6 trials, 90 animals per trial).
Different from N2; P < 0.05.
Different from js126; P < 0.05.
Different from nu199; P < 0.05.
Different from md130; P < 0.05.
Different from js21; P < 0.05.
Diacylglycerol (DAG) is a major product of PLCβ and positively regulates excitatory neurotransmission in vertebrates (Brose et al. 2000) and in C. elegans (Lackner et al. 1999; Miller et al. 1999). Diacylglycerol kinase inactivates DAG by converting it to phosphatidic acid. If EGL-30 regulates VA sensitivity through accumulation of DAG, loss-of-function mutations in dgk-1, which encodes a C. elegans diacylglycerol kinase that inactivates DAG (Lackner et al. 1999; Miller et al. 1999; Nurrish et al. 1999), should be VA resistant. dgk-1(nu199lf) was halothane resistant at a level similar to egl-30(js126) (Table 1). The double mutant combination of dgk-1(nu199) and egl-30(js126) was not significantly more resistant than either single mutant. This lack of additivity is consistent with egl-30 acting exclusively through DAG to regulate VA sensitivity. Notably, in a different locomotion assay (the dispersal assay) where the task requires moving a fixed distance within the assay period, dgk-1(lf) mutants are not VA resistant (van Swinderen et al. 2001). The different results in the two types of assays presumably reflects an action of anesthetics on locomotion that is measured by the dispersal but not body bends assay and that is not effectively antagonized by dgk-1(lf). Nevertheless, VA resistance in the body bends assay does demonstrate that dgk-1 regulates at least some components of VA action on the neurons controlling locomotion and is consistent with egl-30 antagonizing this VA action.
The final mediators of synaptic vesicle fusion and neurotransmitter release are thought to be the SNARE proteins, syntaxin, SNAP-25, and VAMP. To examine whether EGL-30 acts through syntaxin to regulate VA sensitivity, we tested the VA sensitivity of egl-30(js126) in the presence of two different unc-64 syntaxin mutations. unc-64(md130) has a mutation in the splice donor site of the sixth intron (Saifee et al. 1998), resulting in the synthesis of truncated syntaxin products and a reduced amount of full-length product. The reduced level of syntaxin is responsible for all of the syntaxin reduction-of-function phenotypes; the truncated products confer VA resistance. unc-64(js21) carries a missense mutation that simply reduces syntaxin function (Saifee et al. 1998) and like other mutants, with the exception of unc-64(md130), with reduced transmitter release is VA hypersensitive. The egl-30(js126);unc-64(md130) double mutant was more halothane resistant than js126 but not significantly different from md130. However, the aldicarb resistance of md130 was significantly reduced by js126, indicating that egl-30 can still positively regulate transmitter release in the presence of the md130 mutation. Given that md130 is a reduction-of-function but not null mutation in syntaxin, the ability of js126 to augment transmitter release in md130 is not surprising. The lack of additivity of the VA resistances of js126 and md130 is more informative and suggests that the effect of the md130 mutation on VA sensitivity is not modulated by the output of EGL-30 and is not dependent on the general level of cholinergic transmission. In contrast, the anesthetic phenotype of the js126;unc-64(js21) double mutant resembled the js126 single mutant and not js21; however, the aldicarb and locomotion phenotypes are intermediate between that of the single mutants. Thus, neither hyperactivity nor increased levels of synaptic acetylcholine are essential for the VA resistance of js126. The genetic interactions between unc-64 and egl-30(gf) with respect to locomotion, aldicarb, and VA sensitivities are identical to those between unc-64 and goa-1(lf), which encode a C. elegans Goα homolog (van Swinderen et al. 2001). These results are consistent with goa-1 acting antagonistically to EGL-30 to regulate cholinergic neurotransmitter release and VA sensitivity (Hajdu-Cronin et al. 1999; Lackner et al. 1999; Miller et al. 1999; Chase et al. 2001; Robatzek et al. 2001; van der Linden et al. 2001; van Swinderen et al. 2001).
DISCUSSION
Our previously reported results have shown that a truncated syntaxin produced by an intron splice donor site mutation in unc-64(md130) is capable of completely antagonizing the effects of clinical concentrations of volatile anesthetics on C. elegans locomotion (van Swinderen et al. 1999). This remarkable finding suggested two important conclusions: first, that volatile anesthetics were not the nonspecific drugs that many had proposed but in fact their action could be blocked by mutation of a single gene and, second, that at least in C. elegans the relevant anesthetic target was likely a molecule that interacted with or is regulated by the syntaxin. The experiments reported here were designed to better understand syntaxin's role in anesthetic action and potentially identify the putative anesthetic target interacting with syntaxin.
Suppressors of syntaxin reduction of function were expected to fall into one of three classes. First, informational tRNA suppressor mutations could revert the mutation in the e246 protein. However, none of the identified suppressors fall into this class. A second class would be composed of mutations in proteins that complex with syntaxin and suppress e246 by having complementary mutations in residues involved in interaction with the mutated syntaxin. The e246 mutation substitutes a valine for alanine at position 248 of syntaxin (Saifee et al. 1998). This residue has been shown by crystallography and electron spin resonance studies to lie on the hydrophobic face of the H3 helical domain of syntaxin-1A when in a complex with itself and SNAP-25 (a t-SNARE complex) or with VAMP and SNAP-25 (the final ternary SNARE complex; Poirier et al. 1998; Sutton et al. 1998; Xiao et al. 2001; Brunger and Ernst 2002). Thus, mutations in complementary residues in SNAP-25 and/or VAMP might restore function in e246 mutated syntaxin. Besides SNAP-25 and VAMP, several additional proteins have been shown to interact with syntaxin in the H3 domain (Rizo and Sudhof 2002); however, unlike the SNARE complex components, it is unclear if valine 248 interacts with or is required for the interaction with these proteins. In any case, this sort of complementing protein suppressor mutant would act allele specifically. None of the suppressor mutations act on only specific syntaxin alleles; each increases cholinergic neurotransmission of the wild-type syntaxin allele and at least one unc-64(rf) allele. In addition, none of the suppressor genes encode known syntaxin-interacting proteins. Therefore, the isolated suppressor mutants are unlikely to belong to this second class of suppressors.
The third class of suppressors could act by enhancing cholinergic neurotransmission regardless of the nature of the syntaxin allele. On the basis of the aldicarb-hypersensitive phenotype of the suppressor mutants in a wild-type syntaxin background, each suppressor appears to fall into this category. Indeed, in the case of slo-1, direct electrophysiological evidence demonstrates that slo-1(lf) enhances acetylcholine release (Wang et al. 2001), and selective expression of SLO-1 shows that it controls cholinergic neurotransmission by acting in neurons not muscle. Thus, SLO-1 acts presynaptically to inhibit transmitter release. The role of EGL-30 in transmitter release has not been confirmed by electrophysiological methods. However, genetic and pharmacological data presented here and elsewhere indicate that EGL-30 acts in motor neurons by activating phospholipase Cβ, producing DAG, which directly activates UNC-13, a syntaxin binding protein (Lackner et al. 1999; Miller et al. 1999; Brose et al. 2000).
Among the suppressor mutants, the unc-43(lf) alleles are distinct. They were sluggish, had a shrinking response to touch, and had normal sensitivity to VAs. UNC-43 CaMKII has been shown to regulate locomotion, defecation, and glutamate receptor density in the C. elegans nervous system (Reiner et al. 1999; Rongo and Kaplan 1999). The phenotypes of unc-43 gain-of-function mutations are suppressed by loss-of-function mutations in HERG-type K+ channel for defecation phenotypes and by mutations in Goα and in a cyclic-nucleotide-gated channel for locomotion and transmitter release (Reiner et al. 1999; Robatzek and Thomas 2000). The genetic interaction of goa-1 and unc-43 was explained by a model where UNC-43 acts in motor neuron terminals to activate GOA-1 and thereby inhibit excitatory neurotransmitter release (Robatzek and Thomas 2000). However, in Drosophila CaMKII has been shown to act in muscle to regulate transmitter release by inhibiting a retrograde signal (Haghighi et al. 2003). Inhibitors of Drosophila CaMKII in muscle increased transmitter release from motor neurons while constitutive activation of CaMKII reduced it. The regulation of transmitter release by CaMKII in C. elegans could also be explained by a retrograde signal coming from muscle. However, why this action would not produce VA resistance is unclear. Selective expression of UNC-43 in muscle and motor neurons coupled with electrophysiological measurements with and without VAs will be required to resolve this question.
One of the goals of characterizing the anesthetic response of our suppressor mutants was to find the putative syntaxin-interacting anesthetic target. Neither SLO-1 nor Gqα is known to interact with syntaxin. Moreover, in the case of SLO-1, if it were the sought-after target, then slo-1(null) would be expected to be at least as resistant as unc-64(md130). It is not. Given that a gain of function in egl-30 produces resistance and egl-30(null) mutations are lethal, discounting EGL-30 as a target is less definitive. However, it seems unlikely. Considering this and previous data, the most parsimonious model is that slo-1(lf) and egl-30(gf) produce VA resistance by increasing the level of excitatory neurotransmitter release, thereby indirectly antagonizing VA-induced reduction in transmitter release. The VA target(s) remains to be defined.
Acknowledgments
We thank Laura Metz for help with the anesthetic assays. We thank Kouichi Iwasaki, Lorna Brundage, and Paul Sternberg for sharing of unpublished data and reagents. Some of the strains used in this work were supplied by the Caenorhabditis Genetics Center funded by the National Institutes of Health (NIH) National Center for Research Resources. This work was supported by NIH grants to C.M.C. (National Institute of General Medical Sciences) and M.L.N. (National Institute of Neurological Disorders and Stroke) and a Public Health Service grant to O.S.
References
- Ahmed, S., I. N. Maruyama, R. Kozma, J. Lee, S. Brenner et al., 1992. The Caenorhabditis elegans unc-13 gene product is a phospholipid-dependent high-affinity phorbol ester receptor. Biochem. J. 287: 995–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery, L., 1993. The genetics of feeding in Caenorhabditis elegans. Genetics 133: 897–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, T., and S. Hekimi, 1997. The Caenorhabditis elegans avermectin resistance and anesthetic response gene unc-9 encodes a member of a protein family implicated in electrical coupling of excitable cells. J. Neurochem. 69: 2251–2260. [DOI] [PubMed] [Google Scholar]
- Betz, A., U. Ashery, M. Rickmann, I. Augustin, E. Neher et al., 1998. Munc13–1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron 21: 123–136. [DOI] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose, N., C. Rosenmund and J. Rettig, 2000. Regulation of transmitter release by Unc-13 and its homologues. Curr. Opin. Neurobiol. 10: 303–311. [DOI] [PubMed] [Google Scholar]
- Brundage, L., L. Avery, A. Katz, U. J. Kim, J. E. Mendel et al., 1996. Mutations in a C. elegans Gqalpha gene disrupt movement, egg laying, and viability. Neuron 16: 999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunger, A. T., and J. A. Ernst, 2002. High resolution structure, stability, and synaptotagmin binding of a truncated neuronal SNARE complex. J. Biol. Chem. 20: 20. [DOI] [PubMed] [Google Scholar]
- Chase, D. L., G. A. Patikoglou and M. R. Koelle, 2001. Two RGS proteins that inhibit Galpha(o) and Galpha(q) signaling in C. elegans neurons require a Gbeta(5)-like subunit for function. Curr. Biol. 11: 222–231. [DOI] [PubMed] [Google Scholar]
- Chen, Y. A., and R. H. Scheller, 2001. SNARE-mediated membrane fusion. Nat. Rev. Mol. Cell. Biol. 2: 98–106. [DOI] [PubMed] [Google Scholar]
- Crowder, C. M., L. D. Shebester and T. Schedl, 1996. Behavioral effects of volatile anesthetics in Caenorhabditis elegans. Anesthesiology 85: 901–912. [DOI] [PubMed] [Google Scholar]
- Davies, A. G., J. T. Pierce-Shimomura, H. Kim, M. K. VanHoven, T. R. Thiele et al., 2003. A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell 115: 655–666. [DOI] [PubMed] [Google Scholar]
- DeLean, A., P. J. Munson and D. Rodbard, 1978. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am. J. Physiol. 235: E97–E102. [DOI] [PubMed] [Google Scholar]
- Doi, M., and K. Iwasaki, 2002. Regulation of retrograde signaling at neuromuscular junctions by the novel C2 domain protein AEX-1. Neuron 33: 249–259. [DOI] [PubMed] [Google Scholar]
- Franks, N. P., and W. R. Lieb, 1994. Molecular and cellular mechanisms of general anaesthesia. Nature 367: 607–614. [DOI] [PubMed] [Google Scholar]
- Gamo, S., J. Tomida, K. Dodo, D. Keyakidani, H. Matakatsu et al., 2003. Calreticulin mediates anesthetic sensitivity in Drosophila melanogaster. Anesthesiology 99: 867–875. [DOI] [PubMed] [Google Scholar]
- Haghighi, A. P., B. D. McCabe, R. D. Fetter, J. E. Palmer, S. Hom et al., 2003. Retrograde control of synaptic transmission by postsynaptic CaMKII at the Drosophila neuromuscular junction. Neuron 39: 255–267. [DOI] [PubMed] [Google Scholar]
- Hajdu-Cronin, Y. M., W. J. Chen, G. Patikoglou, M. R. Koelle and P. W. Sternberg, 1999. Antagonism between G(o)alpha and G(q)alpha in Caenorhabditis elegans: the RGS protein EAT-16 is necessary for G(o)alpha signaling and regulates G(q)alpha activity. Genes Dev. 13: 1780–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, Y., E. Puil and D. A. Mathers, 1994. Effect of halothane on large-conductance calcium-dependent potassium channels in cerebrovascular smooth muscle cells of the rat. Anesthesiology 81: 649–656. [DOI] [PubMed] [Google Scholar]
- Humphrey, J. A., M. M. Sedensky and P. G. Morgan, 2002. Understanding anesthesia: making genetic sense of the absence of senses. Hum. Mol. Genet. 11: 1241–1249. [DOI] [PubMed] [Google Scholar]
- Jahn, R., and T. C. Sudhof, 1999. Membrane fusion and exocytosis. Annu. Rev. Biochem. 68: 863–911. [DOI] [PubMed] [Google Scholar]
- Jones, M. V., P. A. Brooks and N. L. Harrison, 1992. Enhancement of gamma-aminobutyric acid-activated Cl- currents in cultured rat hippocampal neurones by three volatile anaesthetics. J. Physiol. 449: 279–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurd, R., M. Arras, S. Lambert, B. Drexler, R. Siegwart et al., 2003. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB J. 17: 250–252. [DOI] [PubMed] [Google Scholar]
- Krishnan, K. S., and H. A. Nash, 1990. A genetic study of the anesthetic response: mutants of Drosophila melanogaster altered in sensitivity to halothane. Proc. Natl. Acad. Sci. USA 87: 8632–8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann, D. M., R. L. Martin and S. J. Redman, 1989. Reduction by general anaesthetics of group Ia excitatory postsynaptic potentials and currents in the cat spinal cord. J. Physiol. 412: 277–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner, M. R., S. J. Nurrish and J. M. Kaplan, 1999. Facilitation of synaptic transmission by EGL-30 Gqalpha and EGL-8 PLCbeta: DAG binding to UNC-13 is required to stimulate acetylcholine release. Neuron 24: 335–346. [DOI] [PubMed] [Google Scholar]
- Leibovitch, B. A., D. B. Campbell, K. S. Krishnan and H. A. Nash, 1995. Mutations that affect ion channels change the sensitivity of Drosophila melanogaster to volatile anesthetics. J. Neurogenet. 10: 1–13. [DOI] [PubMed] [Google Scholar]
- MacIver, M. B., A. A. Mikulec, S. M. Amagasu and F. A. Monroe, 1996. Volatile anesthetics depress glutamate transmission via presynaptic actions. Anesthesiology 85: 823–834. [DOI] [PubMed] [Google Scholar]
- Maruyama, I. N., and S. Brenner, 1991. A phorbol ester/diacylglycerol-binding protein encoded by the unc-13 gene of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 88: 5729–5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire, S. L., E. Jorgensen and H. R. Horvitz, 1993. Genes required for GABA function in Caenorhabditis elegans. Nature 364: 334–337. [DOI] [PubMed] [Google Scholar]
- Mello, C., and A. Fire, 1995 DNA transformation, pp. 451–482 in Caenorhabditis elegans: Modern Biological Analysis of an Organism, edited by H. Epstein and D. Shakes. Academic Press, San Diego.
- Miao, N., M. J. Frazer and C. Lynch, III, 1995. Volatile anesthetics depress Ca2+ transients and glutamate release in isolated cerebral synaptosomes. Anesthesiology 83: 593–603. [DOI] [PubMed] [Google Scholar]
- Mihic, S. J., Q. Ye, M. J. Wick, V. V. Koltchine, M. D. Krasowski et al., 1997. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature 389: 385–389. [DOI] [PubMed] [Google Scholar]
- Miller, K. G., M. D. Emerson and J. B. Rand, 1999. Goalpha and diacylglycerol kinase negatively regulate the Gqalpha pathway in C. elegans. Neuron 24: 323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida, S., 2000. Protein-protein interactions in neurotransmitter release. Neurosci. Res. 36: 175–182. [DOI] [PubMed] [Google Scholar]
- Morgan, P. G., and H. F. Cascorbi, 1985. Effect of anesthetics and a convulsant on normal and mutant Caenorhabditis elegans. Anesthesiology 62: 738–744. [DOI] [PubMed] [Google Scholar]
- Morgan, P. G., and M. M. Sedensky, 1994. Mutations conferring new patterns of sensitivity to volatile anesthetics in Caenorhabditis elegans. Anesthesiology 81: 888–898. [DOI] [PubMed] [Google Scholar]
- Morgan, P. G., M. M. Sedensky, P. M. Meneely and H. F. Cascorbi, 1988. The effect of two genes on anesthetic response in the nematode Caenorhabditis elegans. Anesthesiology 69: 246–251. [DOI] [PubMed] [Google Scholar]
- Morgan, P. G., M. Sedensky and P. M. Meneely, 1990. Multiple sites of action of volatile anesthetics in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 87: 2965–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa, K., and Y. Kidokoro, 1999. Halothane presynaptically depresses synaptic transmission in wild-type Drosophila larvae but not in halothane-resistant (har) mutants. Anesthesiology 90: 1691–1697. [DOI] [PubMed] [Google Scholar]
- Nishikawa, K.-I., and M. B. MacIver, 2000. Excitatory synaptic transmission mediated by NMDA receptors is more sensitive to isoflurane than are non-NMDA receptor-mediated responses. Anesthesiology 92: 228–236. [DOI] [PubMed] [Google Scholar]
- Noel, J. P., H. E. Hamm and P. B. Sigler, 1993. The 2.2 A crystal structure of transducin-alpha complexed with GTP gamma S. Nature 366: 654–663. [DOI] [PubMed] [Google Scholar]
- Nurrish, S., L. Segalat and J. M. Kaplan, 1999. Serotonin inhibition of synaptic transmission: Galpha(0) decreases the abundance of UNC-13 at release sites. Neuron 24: 231–242. [DOI] [PubMed] [Google Scholar]
- Pancrazio, J. J., W. K. Park and C. Lynch, III, 1993. Inhalational anesthetic actions on voltage-gated ion currents of bovine adrenal chromaffin cells. Mol. Pharmacol. 43: 783–794. [PubMed] [Google Scholar]
- Patel, A. J., E. Honore, F. Lesage, M. Fink, G. Romey et al., 1999. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat. Neurosci. 2: 422–426. [DOI] [PubMed] [Google Scholar]
- Perouansky, M., D. Baranov, M. Salman and Y. Yaari, 1995. Effects of halothane on glutamate receptor-mediated excitatory postsynaptic currents. Anesthesiology 83: 109–119. [DOI] [PubMed] [Google Scholar]
- Phelan, P., and T. A. Starich, 2001. Innexins get into the gap. BioEssays 23: 388–396. [DOI] [PubMed] [Google Scholar]
- Phelan, P., J. P. Bacon, J. A. Davies, L. A. Stebbings, M. G. Todman et al., 1998. Innexins: a family of invertebrate gap-junction proteins. Trends Genet. 14: 348–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock, G., and C. D. Richards, 1991. Cellular mechanisms in general anaesthesia. Br. J. Anaesth. 66: 116–128. [DOI] [PubMed] [Google Scholar]
- Poirier, M. A., W. Xiao, J. C. Macosko, C. Chan, Y. K. Shin et al., 1998. The synaptic SNARE complex is a parallel four-stranded helical bundle. Nat. Struct. Biol. 5: 765–769. [DOI] [PubMed] [Google Scholar]
- Rajaram, S., M. M. Sedensky and P. G. Morgan, 1998. Unc-1: a stomatin homologue controls sensitivity to volatile anesthetics in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 95: 8761–8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaram, S., T. L. Spangler, M. M. Sedensky and P. G. Morgan, 1999. A stomatin and a degenerin interact to control anesthetic sensitivity in Caenorhabditis elegans. Genetics 153: 1673–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand, J. B., and M. L. Nonet, 1997 Synaptic transmission, pp. 611–643 in C. elegans II, edited by D. L. Riddle, T. Blumenthal, B. J. Meyer and J. R. Priess. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed]
- Reiner, D. J., E. M. Newton, H. Tian and J. H. Thomas, 1999. Diverse behavioural defects caused by mutations in Caenorhabditis elegans unc-43 CaM kinase II. Nature 402: 199–203. [DOI] [PubMed] [Google Scholar]
- Rizo, J., and T. C. Sudhof, 2002. Snares and Munc18 in synaptic vesicle fusion. Nat. Rev. Neurosci. 3: 641–653. [DOI] [PubMed] [Google Scholar]
- Robatzek, M., and J. H. Thomas, 2000. Calcium/calmodulin-dependent protein kinase II regulates Caenorhabditis elegans locomotion in concert with a Go/Gq signaling network. Genetics 156: 1069–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robatzek, M., T. Niacaris, K. Steger, L. Avery and J. H. Thomas, 2001. eat-11 encodes GPB-2, a Gbeta(5) ortholog that interacts with G(o)alpha and G(q)alpha to regulate C. elegans behavior. Curr. Biol. 11: 288–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongo, C., and J. M. Kaplan, 1999. CaMKII regulates the density of central glutamatergic synapses in vivo. Nature 402: 195–199. [DOI] [PubMed] [Google Scholar]
- Saifee, O., L. Wei and M. L. Nonet, 1998. The Caenorhabditis elegans unc-64 locus encodes a syntaxin that interacts genetically with synaptobrevin. Mol. Biol. Cell 9: 1235–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlame, M., and H. C. Hemmings, Jr., 1995. Inhibition by volatile anesthetics of endogenous glutamate release from synaptosomes by a presynaptic mechanism. Anesthesiology 82: 1406–1416. [DOI] [PubMed] [Google Scholar]
- Sedensky, M. M., and P. M. Meneely, 1987. Genetic analysis of halothane sensitivity in Caenorhabditis elegans. Science 236: 952–954. [DOI] [PubMed] [Google Scholar]
- Starich, T. A., R. Y. Lee, C. Panzarella, L. Avery and J. E. Shaw, 1996. eat-5 and unc-7 represent a multigene family in Caenorhabditis elegans involved in cell-cell coupling. J. Cell Biol. 134: 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringham, E. G., D. K. Dixon, D. Jones and E. P. Candido, 1992. Temporal and spatial expression patterns of the small heat shock (hsp16) genes in transgenic Caenorhabditis elegans. Mol. Biol. Cell 3: 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton, R. B., D. Fasshauer, R. Jahn and A. T. Brunger, 1998. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395: 347–353. [DOI] [PubMed] [Google Scholar]
- Takenoshita, M., and T. Takahashi, 1987. Mechanisms of halothane action on synaptic transmission in motoneurons of the newborn rat spinal cord in vitro. Brain Res. 402: 303–310. [DOI] [PubMed] [Google Scholar]
- Tavernarakis, N., W. Shreffler, S. Wang and M. Driscoll, 1997. unc-8, a Deg/EnaC family member, encodes a subunit of a candidate mechanically gated channel that modulates C. elegans locomotion. Neuron 18: 107–119. [DOI] [PubMed] [Google Scholar]
- Tinklenberg, J. A., I. S. Segal, T. Z. Guo and M. Maze, 1991. Analysis of anesthetic action on the potassium channels of the Shaker mutant of Drosophila. Ann. NY Acad. Sci. 625: 532–539. [DOI] [PubMed] [Google Scholar]
- van der Linden, A. M., F. Simmer, E. Cuppen and R. H. Plasterk, 2001. The G-protein β-subunit GPB-2 in Caenorhabditis elegans regulates the Goα–Gqα signaling network through interactions with the regulator of G-protein signaling proteins EGL-10 and EAT-16. Genetics 158: 221–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Swinderen, B., O. Saifee, L. Shebester, R. Roberson, M. L. Nonet et al., 1999. A neomorphic syntaxin mutation blocks volatile-anesthetic action in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 96: 2479–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Swinderen, B., L. B. Metz, L. D. Shebester, J. E. Mendel, P. W. Sternberg et al., 2001. Goalpha regulates volatile anesthetic action in Caenorhabditis elegans. Genetics 158: 643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Swinderen, B., L. B. Metz, L. D. Shebester and C. M. Crowder, 2002. A Caenorhabditis elegans pheromone antagonizes volatile anesthetic action through a Go-coupled pathway. Genetics 161: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. W., O. Saifee, M. L. Nonet and L. Salkoff, 2001. SLO-1 potassium channels control quantal content of neurotransmitter release at the C. elegans neuromuscular junction. Neuron 32: 867–881. [DOI] [PubMed] [Google Scholar]
- Waud, D. R., 1972. On biological assays involving quantal responses. J. Pharmacol. Exp. Ther. 183: 577–607. [PubMed] [Google Scholar]
- Xiao, W., M. A. Poirier, M. K. Bennett and Y. K. Shin, 2001. The neuronal t-SNARE complex is a parallel four-helix bundle. Nat. Struct. Biol. 8: 308–311. [DOI] [PubMed] [Google Scholar]
- Yamakura, T., E. Bertaccini, J. R. Trudell and R. A. Harris, 2001. Anesthetics and ion channels: molecular models and sites of action. Annu. Rev. Pharmacol. Toxicol. 41: 23–51. [DOI] [PubMed] [Google Scholar]
- Zorychta, E., and R. Capek, 1978. Depression of spinal monosynaptic transmission by diethyl ether: quantal analysis of unitary synaptic potentials. J. Pharmacol. Exp. Ther. 207: 825–836. [PubMed] [Google Scholar]