Abstract
We examined the genetics of hybrid incompatibility between two closely related diploid hermaphroditic plant species. Using a set of near-isogenic lines (NILs) representing 85% of the genome of the wild species Lycopersicon hirsutum (Solanum habrochaites) in the genetic background of the cultivated tomato L. esculentum (S. lycopersicum), we found that hybrid pollen and seed infertility are each based on 5–11 QTL that individually reduce hybrid fitness by 36–90%. Seed infertility QTL act additively or recessively, consistent with findings in other systems where incompatibility loci have largely been recessive. Genetic lengths of introgressed chromosomal segments explain little of the variation for hybrid incompatibility among NILs, arguing against an infinitesimal model of hybrid incompatibility and reinforcing our inference of a limited number of discrete incompatibility factors between these species. In addition, male (pollen) and other (seed) incompatibility factors are roughly comparable in number. The latter two findings contrast strongly with data from Drosophila where hybrid incompatibility can be highly polygenic and complex, and male sterility evolves substantially faster than female sterility or hybrid inviability. The observed differences between Lycopersicon and Drosophila might be due to differences in sex determination system, reproductive and mating biology, and/or the prevalence of sexual interactions such as sexual selection.
UNDERSTANDING the genetic basis of interspecific barriers is central to elucidating mechanisms of biological speciation. This is because the tempo and mode of speciation can be strongly influenced by the number and individual effects of changes causing reproductive isolation (Barton and Charlesworth 1984; Gottlieb 1984; Coyne 1992). Classical (Stebbins 1950; Dobzhansky 1951) and more recent (e.g., Coyne and Orr 1989a; Tilley et al. 1990; Sasa et al. 1998; Price and Bouvier 2002; Mendelson 2003; Moyle et al. 2004) analyses indicate that reproductive isolation between taxa generally increases with time since divergence. Nonetheless, relatively few studies have directly examined the genetic basis of traits responsible for reproductive isolation, especially hybrid inviability and sterility occurring in the absence of ploidy differences or apparent ecological factors. Two alternative genetic mechanisms could explain the basis of these “intrinsic” incompatibility factors: chromosomal rearrangements or interlocus epistasis (commonly known as Dobzhansky-Muller interactions, after Dobzhansky 1936 and Muller 1939). Chromosomal rearrangements are thought to be particularly important in the evolution of hybrid incompatibility among plant species (e.g., Grant 1963; Rieseberg 2001) but have also been implicated in more general divergence processes (Rieseberg et al. 2000; Noor et al. 2001; Navarro and Barton 2003; but see Lu et al. 2003). In cases where interspecific barriers are due to genic Dobzhansky-Muller interactions, however, current interest is focused on identifying the specific nature of hybrid incompatibility factors (i.e., the number of loci involved, the size of their individual phenotypic effects, their mode of gene action, and their location in the genome) and what these might indicate about the forces primarily responsible for the formation of new species.
To date, the data brought to bear on these questions are overwhelmingly from Drosophila species crosses. Such studies include genome-wide mapping of hybrid incompatibilities (Dobzhansky 1951; Naveira and Fontdevila 1986; True et al. 1996; Coyne et al. 1998; Presgraves 2003), fine-scale mapping of hybrid male sterility (e.g., Tao et al. 2003a,b), and even identification of the molecular genetic basis of individual loci that contribute to hybrid breakdown (Ting et al. 1998; Barbash et al. 2003; Presgraves et al. 2003). In combination with theoretical work (e.g., Turelli and Orr 1995; 2000), these studies have elegantly demonstrated that at least three patterns appear to characterize the genetic basis of hybrid incompatibility in Drosophila. First, incompatibilities that specifically influence hybrid male sterility appear to evolve most readily, such that for any given species cross male sterility factors are much more prevalent than female sterility or hybrid inviability factors (Wu et al. 1996; Tao et al. 2003a,b; Tao and Hartl 2003 and references therein). Second, male sterility appears to be highly polygenic and complex, involving interactions between multiple loci and/or blocks of chromosomally clustered factors to generate sterility phenotypes (Davis and Wu 1996; Tao et al. 2003b and references therein). Third, hybrid incompatibilities appear on average to act recessively, such that their phenotypic effects are observed or maximized when in homozygous (or hemizygous) form, especially against a homozygous foreign genetic background (i.e., recessive-recessive incompatibilities; Presgraves 2003; Tao et al. 2003a,b).
Several explanations have been proposed for the first two patterns. First, sexual selection may accelerate rates of change in male traits, thereby accelerating evolution of interspecific differences in male fertility loci as a by-product (Wu and Davis 1993). This can lead to both more male sterility factors and a more complex genetic basis for male sterility; multiple bouts of sexual selection can act to fix many different (and comparatively more complex) changes between species than are observed in traits influenced by one or few bouts of natural selection (Orr and Coyne 1992). Another proposal is that gametogenesis evolves much faster in the heterogametic (i.e., “XY”) sex, rather than in males per se, because of more intense sex-ratio selection in the heterogametic sex (Tao and Hartl 2003). Alternatively, the process of spermatogenesis might be relatively more sensitive to perturbation than other hybrid fitness components, so that more genic changes can result in male sterility than other forms of hybrid incompatibility (Wu and Davis 1993). While the biochemical basis of the third pattern, recessivity of hybrid incompatibility factors, is not well understood (Turelli and Orr 2000), recessivity of gene action directly influences the expression of hybrid fitness phenotypes. For example, the common observation in hybrids between species with heteromorphic sex chromosomes that the weaker sex is the heterogametic sex (i.e., Haldane's rule; see Laurie 1997) could be due in part to recessivity of most hybrid incompatibility factors, such that they are differentially uncovered in the hemizygous sex (Turelli and Orr 2000). Recessivity has also been used to explain the “large-X” effect (Turelli and Orr 2000): the observation in Drosophila that the X chromosome disproportionately contributes to the expression of hybrid incompatibility (Coyne and Orr 1989b). (See also Naveira and Maside (1998) and Tao et al. (2003a) for an alternative meaning of the large-X effect and hypothesized causes of this effect, including faster male evolution.)
In combination, these patterns suggest the emergence of some general rules and common dynamics shaping the genetic basis of hybrid incompatibility in Drosophila. These rules depend especially on the influence of two related factors: sex chromosomes and sexual interactions. Patterns such as Haldane's rule and the large-X effect indicate that sex chromosomes play a special role in the evolution of hybrid incompatibility in Drosophila. The sex chromosomes can be subject to unique evolutionary dynamics in comparison to the autosomes, including potentially higher rates of substitution (see Tao et al. 2003a; Tao and Hartl 2003 and references therein). They may also facilitate the independent evolution of male and female traits, enabling faster sex-specific evolution and/or stronger sexual selection. Similarly, sexual interactions (including male-male competition and male-female antagonism) are frequently invoked to explain accelerated rates of male evolution and of reproductive traits in general (Wu et al. 1996; Swanson and Vacquier 2002). These interactions therefore appear to play a central role in the evolution of traits that could strongly influence the expression of interspecific reproductive isolation.
These patterns in Drosophila may provide expectations for hybrid incompatibility in other biological groups that have heteromorphic sex chromosomes and/or strong sexual interactions [for example, Anopheles (Slotman et al. 2004) or Aedes (Presgraves and Orr 1998) mosquitoes]. However, in biological groups where there are no heteromorphic sex chromosomes, where sex determination is not genetic, and/or where individuals are bisexual (hermaphrodites) rather than simply male or female, it is unclear that equivalent evolutionary dynamics will predominate and, therefore, that similar experimental observations are expected. These alternative sexual and mating systems are prevalent in many biological systems and dominate in groups as diverse as plants, fungi, marine invertebrates, mollusks, and reptiles (Bull 1983). Their biological differences may also act to reduce the strength, frequency, or efficacy of forces such as sexual selection and other sex-specific interactions. For example, self-fertilization may reduce the opportunity for selection to act on gamete discrimination traits and, therefore, on traits involved in reproductive isolation (Levin 2000). Accordingly, while our expectations of hybrid incompatibility in the absence of chromosomal sex determination and well-understood sexual interactions are comparatively ill defined, there are plausible biological reasons to expect they might differ from those in Drosophila.
Recently, analyses in non-Dipteran systems (e.g., Bradshaw et al. 1995; Fishman and Willis 2001; Hodges et al. 2002) including agricultural model species (e.g., Kim and Rieseberg 1999; Harushima et al. 2001) have begun to generate data on the number, location, and individual effects of loci contributing to reproductive isolation, including hybrid incompatibility. These studies clearly demonstrate that genomic mapping can be used to dissect hybrid incompatibility in species further afield than Drosophila. They also suggest a more variable genetic basis for hybrid incompatibility. For example, sunflower hybrid incompatibility appears to be largely due to extensive chromosomal rearrangements, which are perhaps associated with genic changes (Kim and Rieseberg 1999, 2001). In contrast, premating isolation is predominantly due to genic differences between wildflower Mimulus species (Bradshaw and Schemske 2003), with little or no known role for chromosomal inversions. Mapping of individual sterility factors in rice cultivar crosses indicates that sterility factors can be due both to local chromosomal rearrangements between species (e.g., Li et al. 1997) and to pairs of “complementary” (i.e., Dobzhansky-Muller) factors (e.g., Ikehashi and Araki 1988; Li et al. 1997) that influence hybrid fitness. Despite these promising investigations, however, there are still few direct genome-wide analyses of the genetic basis of interspecific hybrid incompatibility outside Drosophila. Accordingly, little comprehensive data on the relative number, individual effects, and mode of gene action of loci underlying such traits are available, compared to the current wealth of data available in Drosophila, and few grounds on which to evaluate the generality of patterns observed within this group.
Here we examine the genetics of interspecific barriers between two closely related species in the plant group Lycopersicon. Lycopersicon is an attractive plant model in which to analyze hybrid incompatibility because all nine members of this clade are closely related hermaphroditic diploids (2n = 2x = 24; Peralta and Spooner 2001; Nesbitt and Tanksley 2002) that share a high degree of synteny (Quiros 1991) and are to some degree intercrossable (Rick 1979). These features are particularly advantageous because, in addition to allowing formal genetic analysis, they indicate that species might largely be reproductively isolated by genic loci, rather than by changes in large-scale chromosomal organization, including chromosomal rearrangements (Quiros 1991). In addition to these biological advantages, Lycopersicon is the subject of extensive genomic research because it includes the cultivated tomato, Lycopersicon esculentum. The resulting genetic resources in this group, including a high-density linkage map (Tanksley et al. 1992; Frary and Tanksley 2001) and several sets of publicly available near-isogenic lines (NILs; Eshed and Zamir 1995; Monforte and Tanksley 2000) and backcross inbred lines (Doganlar et al. 2002), promise to greatly facilitate mapping and eventual molecular identification of interspecific traits, including hybrid incompatibility.
To map quantitative trait loci (QTL) associated with pollen (male) fertility and seed inviability/fertility, we measured reproductive fitness traits in a set of near-isogenic introgression lines between the cultivated species L. esculentum and a wild congener L. hirsutum. Our goals in this article are threefold: first, to identify the genomic regions associated with pollen and seed inviability in hybrids between L. esculentum and L. hirsutum and to quantify the number, genomic location, and individual effects of these hybrid incompatibility QTL; second, to determine the mode of gene action of loci underlying hybrid seed fertility, specifically the relative effects of introgressions in homozygous vs. heterozygous form on the success of seed set; and third, to evaluate the influence (if any) of the length of introgressed chromosomal regions on the expression of hybrid pollen and seed fertility.
MATERIALS AND METHODS
Study system:
Lycopersicon is a relatively small plant group consisting of nine closely related diploid species, including the domesticated tomato L. esculentum (Mill.), within the large and diverse Solanaceae family (Rick 1995). Recent taxonomic revision indicates that Lycopersicon is a monophyletic clade nested within the genus Solanum and renames Lycopersicon species accordingly (Peralta and Spooner 2001); to maintain continuity with the established literature, however, we retain the classical nomenclature in this article. The two parental species analyzed here differ in several biologically significant features. L. hirsutum (Solanum habrochaites) is a wild, short-lived herbaceous, perennial species that predominantly occurs from middle to high elevations in northwestern South America. Most populations of L. hirsutum are obligately outcrossing—a mating system strictly enforced by the gametophytic self-incompatibility system—and exhibit several features typical of outcrossers (Rick et al. 1979), including high nucleotide diversity (Miller and Tanksley 1990; Stephan and Langley 1998). In contrast, L. esculentum (S. lycopersicum, the cultivated tomato) is a domesticated, self-pollinated species with comparatively low genetic variation. The putative wild progenitor of L. esculentum is also predominantly selfing (Miller and Tanksley 1990; Kondo et al. 2002), and self-compatibility is thought to have preceded domestication (Rick 1995). Phylogenetic resolution and chronological dating of speciation events in Lycopersicon has proved difficult. However, recent estimates suggest the Lycopersicon group began its initial radiation approximately 7 million years ago (Nesbitt and Tanksley 2002). Nucleotide divergence between L. esculentum and L. hirsutum estimated from six independent noncoding regions averages 0.044 substitutions per base pair, indicating these species are closely related (Nesbitt and Tanksley 2002).
Lycopersicon is the subject of extensive genomic research due to its use as a model organism for developmental and reproductive biology and its agronomic importance and close relationship with Solanum species including potato, pepper, and eggplant (Pillen et al. 1996; see http://www.sgn.cornell.edu/Solanaceae-project/ for recent updates). In addition to the development of near-isogenic and recombinant inbred lines, the availability of a high-density genetic linkage map in Lycopersicon (Tanksley et al. 1992; Frary and Tanksley 2001) has facilitated multiple marker-association studies involving crosses within L. esculentum and between wild and cultivated Lycopersicon. To date, analyses have focused on the identification of agriculturally valuable traits (Pillen et al. 1996; Paran and Zamir 2003) with a common goal of supplementing depauperate genetic variation within cultivars with genes from wild congeners (Zamir 2001). Understanding the genetic basis of hybrid incompatibility in this group might facilitate overcoming barriers that prevent successful interspecific hybridization.
Plant material:
Each near-isogenic introgression line analyzed here contained one (or up to three) small, individual, overlapping chromosomal segment(s) of the wild species L. hirsutum (LH) in an L. esculentum (LE) background. All lines were previously generated by Monforte and Tanksley (2000) who provide a detailed description of their construction elsewhere (see Bernacchi et al. 1998a,b; Monforte and Tanksley 2000). Briefly, all lines are the advanced generation backcross progeny of a single F1 plant produced by crossing L. hirsutum accession LA1777 as the staminate (male) parent to L. esculentum cv. E6203. The F1 individual was backcrossed to the recurrent cultivated LE parental line for two generations, and a subset of the resulting BC2 plants were subjected to marker-assisted selection to identify lines with single LH introgressions of interest and to maximize the representation of the LH genome on the LE background (Bernacchi et al. 1998a). In addition, 111 additional BC2 plants were selfed through single-seed descent for a further three generations. This population of BC2S3 lines was subjected to two rounds of marker-assisted selection to minimize the number of LH introgressed segments found within each line while maximizing genome-wide LH representation. Each resulting line carries one or more marker-characterized LH introgressions in homozygous form in an isogenic LE background. The complete set of developed NILs (99 lines) is publicly available from the Tomato Genetics Resource Center at UC Davis (http://tgrc.ucdavis.edu). In combination, they cover 85% of the wild species genome. Four genomic regions are known to account for the missing genomic coverage (Monforte and Tanksley 2000). At two of these regions, the self-incompatibility (S) locus on chromosome 1 and the self-pruning (sp) locus on chromosome 6, LE alleles were deliberately fixed during the development of the lines, to facilitate cultivation by ensuring self-fertility (i.e., selection for self-compatibility) and determinate habit (i.e., selection for determinate growth) typical of the recurrent parent (Bernacchi et al. 1998a). The other two regions, on chromosomes 2 and 8, could not be fixed in homozygous form during NIL development for unknown reasons (Monforte and Tanksley 2000). Our analysis cannot address whether incompatibility or other trait factors are or are not contained within genomic regions that are unrepresented in these lines (see discussion).
For this experiment, 71 introgression lines were chosen to maximize genomic representation of L. hirsutum introgressions (i.e., 85% of the LH genome in total) in the L. esculentum genetic background, while avoiding undue repetition of any single introgressed region and minimizing the number of lines that contained >1 individual introgression. On the basis of estimates of introgression size (Monforte and Tanksley 2000 and see below), each of these 71 NILs contained, on average, 48.5 cM of introgressed LH genome (range, 4.5–135 cM); this corresponds to an average of 3.86% LH genome per NIL (range, 0.35–10.7%), assuming a genome size of ∼1260 cM (Tanksley et al. 1992). (NILs with >100 cM of introgressed LH genome contain two or three introgressed regions on different chromosomes; see below.)
Line cultivation and handling:
Each line and both parental accessions were evaluated in a replicated (3 plants per NIL, 10 plants per parental accession), randomized common garden experiment and assayed for male and female fertility and for a set of seven floral, inflorescence, and fruit morphology traits. Results for the morphological traits will be treated elsewhere (L. Moyle and E. Graham, unpublished observations). All plants were propagated under the same conditions, following standard greenhouse cultivation protocols. Seeds were treated in 0.125% sodium hypochlorite for 30 min, rinsed, placed on blotting paper, and incubated (12-hr day length, 24°) to stimulate germination. After 10 days, germinated seedlings were transplanted to soil-filled flats and hand-watered daily. Three weeks after transplant, seedlings were transferred to individual 1-gallon pots containing greenhouse potting mix and placed in a climate controlled greenhouse at the UC Davis Division of Biological Sciences greenhouse facility. Plants were watered daily via drip irrigation, fertilized twice weekly, and pruned and staked prior to flowering.
Fertility evaluation of parental and introgression lines:
Pollen and seed fertility was measured on all introgression lines and on the LE parental plants. Only pollen fertility was measured on LH parental plants, due to the extended period required for flowering and fruit development in this species and the inability to obtain selfed seed (due to gametophytic self-incompatibility in this accession). Nonetheless, extensive propagation of L. hirsutum indicates that intra-accession crosses routinely yield 40–50 seeds per fruit (R. Chetelat, personal communication), providing a baseline for comparing seed yield with the LE parental species and the introgression lines in this study.
Pollen fertility was estimated on three unopened flowers on each plant by dissecting the entire anther cone from each flower and collecting it into 400 μl of lactophenol-aniline blue histochemical stain (Kearns and Inouye 1993). Each anther cone was gently agitated to release the pollen sacs into the dye solution. Samples were vortexed before a 7-μl subsample was pipetted onto a hemacytometer. Pollen inviability was indicated by the absence of a stained cytoplasm; this is a conservative measure of pollen infertility as some grains that stain for cytoplasm may nonetheless be functionally inviable for other reasons (Kearns and Inouye 1993). Pollen fertility was quantified in two ways: total number of pollen grains (PN) and proportion of fertile pollen (PF). Because the pollen counts in each subsample are proportional to the average pollen number per flower, we refer to them here as the number of pollen grains per flower.
Seed fertility was determined indirectly by measuring seed production (total seed count) resulting from two different pollination treatments: self-pollination (“self”) and cross-pollination with LE pollen (backcross or “LE-cross”). Because all NILs used in the study contain an LH introgression in homozygous form, selfing generates seed that is homozygous for the LH introgression, whereas pollination with L. esculentum pollen produces seed that is heterozygous for the LH introgression. For self seed, at least three flowers per plant were allowed to set seed via selfing; where fruits were not set automatically, flowers were hand-selfed to ensure that morphological differences were not responsible for lack of seed set. For LE-cross seed, at least three flowers per plant were emasculated preanthesis (in the bud stage) and subsequently hand-pollinated with pollen collected and bulked from at least five plants of the LE parental accession. Pollinated flowers were tagged so that fruit from each cross type could be distinguished during harvest. Upon maturation, fruit was individually harvested from all plants over a 2-week period. Following standard seed extraction techniques in tomato (Silva et al. 1982), seeds were cut from each fruit by hand, soaked in 10% trisodium phosphate solution for 30 min to remove gel, rinsed, and air dried on paper for 24 hr. Seed fertility was determined for each cross by counting the number of visible seeds from each fruit, to give measures for self-seed set (SSS) and LE-cross seed set (SSC) for each NIL and the LE control parent.
It is important to note that seed set and pollen fertility are not fully analogous measures of hybrid incompatibility. In particular, pollen sterility estimates inviable male gamete production whereas seed set represents the composite effects of male and female fertility, in addition to early zygotic dysfunction. As such, interpretation of self-seed set requires that individual male fertility within each NIL be taken into account. Several strategies for accounting for the influence of male fertility on self-seed set are discussed under results.
QTL analysis:
The degree of association between each trait of interest and specific introgression lines was evaluated using Dunnett's mean contrast, which evaluates the mean phenotype of each NIL against the control LE accession with an experiment-wise α-level of 0.05, i.e., corrected for multiple comparisons (Dunnett 1955; Zar 1984). For lines that were significantly different from the control, results are presented as percentage difference (Δ%) from the isogenic LE control; this difference is defined as the phenotypic effect of the QTL hypothesized to reside within the introgressed segment, as in previous studies (e.g., Eshed and Zamir 1995, 1996).
The minimum number and genomic location of QTL underlying differences in each trait were inferred by comparing the positions of introgressed segments having different trait values from the control parent, using three assumptions (following Eshed and Zamir 1995; Tao et al. 2003b): (1) a QTL is counted only if the relevant NIL is significantly different from the corresponding control, (2) each NIL affecting the trait carries only a single QTL, and (3) two overlapping introgressions with a significant effect on the trait, in the same direction relative to the control, carry the same QTL. In addition, for adjacent introgressions that contain overlapping regions but whose mean trait values differed significantly, the nonoverlapping portion of the introgression was declared the probable location of the QTL of interest. A trait shift between adjacent introgressions was evidenced by a significant difference in trait values between plants carrying each introgression, such that one line was significantly phenotypically different from LE whereas the other(s) was not. In these cases, we also directly confirmed phenotypic differences between the adjacent introgression lines using standard pairwise t-tests. In at least one case, conflicting results from NILs with whole or partial overlapping regions made definitive assignment of a QTL to a specific genomic location difficult. In this case, it is likely that assumption 2 was violated; i.e., the NIL with the significant trait difference apparently carries two or more QTL (see results).
For seed set, we also estimated the dominance deviation, i.e., the difference between the LH/LE heterozygote (i.e., seeds resulting from NIL × LE crosses) and the midvalue of the homozygote LH (selfed NIL) and LE (selfed) lines; significance of this deviation was calculated by contrasting the heterozygous seed set (× +1) with the seed set from control plants (× −0.5) and the appropriate homozygous NIL (× −0.5; Eshed and Zamir 1995).
Effects of introgression size on hybrid fitness:
In addition to testing for individual introgressions of major effect (i.e., QTL), we also evaluated the strength of association between the amount of introgressed LH genome and the expression of hybrid incompatibility. If hybrid incompatibility is due to many factors of relatively small effect, an infinitesimal model of hybrid incompatibility factors, then reduced hybrid fitness is expected to be strongly positively associated with the amount of the L. hirsutum genome represented in the L. esculentum genetic background. To evaluate this expectation, we tested the strength of individual regressions of both the line means of pollen and the line means of seed sterility against the estimated genetic length (in centimorgans) of the introgressed region in each NIL.
There is presently no complete physical map of the tomato genome. Therefore the size of individual introgressions within each NIL was estimated in map units (centimorgans) using distances between markers on the published high-density linkage map between L. esculentum and L. pennellii (Frary and Tanksley 2001); this map was used because published intermarker distances in the L. esculentum × L. hirsutum cross were not available for all markers used in the NILs. As such, our size estimates may be inaccurate if relative recombination patterns vary substantially between the two different species crosses. However, given the highly conserved colinearity between Lycopersicon genomes (Quiros 1991) and the relatively small differences in mean map distances and conserved gene order observed in the L. esculentum × L. pennellii vs. L. esculentum × L. hirsutum cross (Bernacchi and Tanksley 1997), L. esculentum/L. pennellii map distances are likely to be reasonable estimates of the map units in our species cross. Distances were obtained in January 2004 by searching for the relevant genetic markers on the 2002 L. esculentum/L. pennellii map using the Solanaceae Genome Network website (http://www.sgn.cornell.edu/). Where data were available (for a subset of 15 of 71 estimated introgression sizes) we compared published intermarker distances from the L. esculentum × L. hirsutum cross with distances generated from the L. esculentum × L. pennellii map; the correlation was both strong and significant (Pearson's r = 0.9255, P < 0.0001), indicating that the proxy estimates we use from the high-density map are likely a very good match for relative map distances in the LH/LE cross and that therefore our regression analyses likely use reasonable centimorgan length estimates for introgressed regions. In this study, most (47 of 71) of the NILs contain a single introgressed region only; however, 12 contain two known introgressed regions on two different chromosomes, and another 12 contain known introgressed regions on three different chromosomes. For each of these NILs, estimated lengths of the individual regions were simply summed to give an estimate of the total length of the introgressed LH genome.
Note that the relationship between physical and genetic distance can vary 10-fold across the Lycopersicon genome, ranging from known extremes of ∼5 million base pairs per centimorgan in centromeric regions to 43 kb/cM in euchromatic regions distant from the centromere, with an estimated average of ∼750 kb/cM (Pillen et al. 1996). If individual introgressions contain a biased or nonrandom sample of gene-rich and gene-poor regions, then the relationship between introgression size and sterility predicted under an infinitesimal model may be weaker. However, although the relative gene density in each introgressed region is unknown, the set of lines used here represents most of the genome and ∼80% (57/71) of the NILs contain both gene-rich (euchromatic) and gene-poor (centromeric and/or telomeric) chromosomal regions, providing little suggestion that individual introgressions nonrandomly sample overall gene density in the Lycopersicon genome.
RESULTS
Pollen fertility:
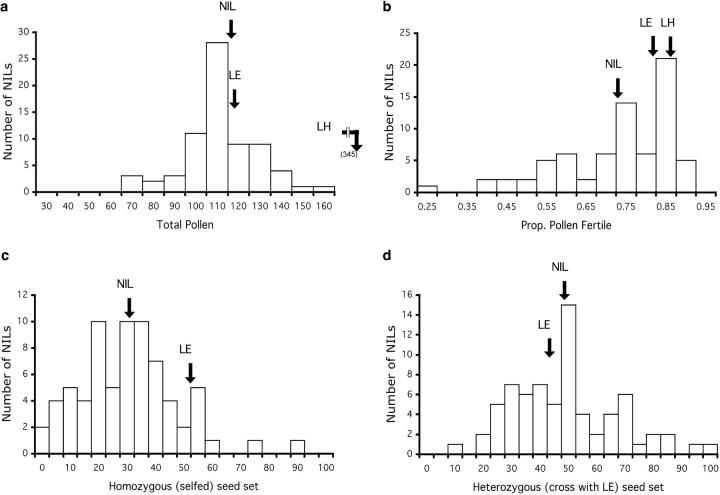
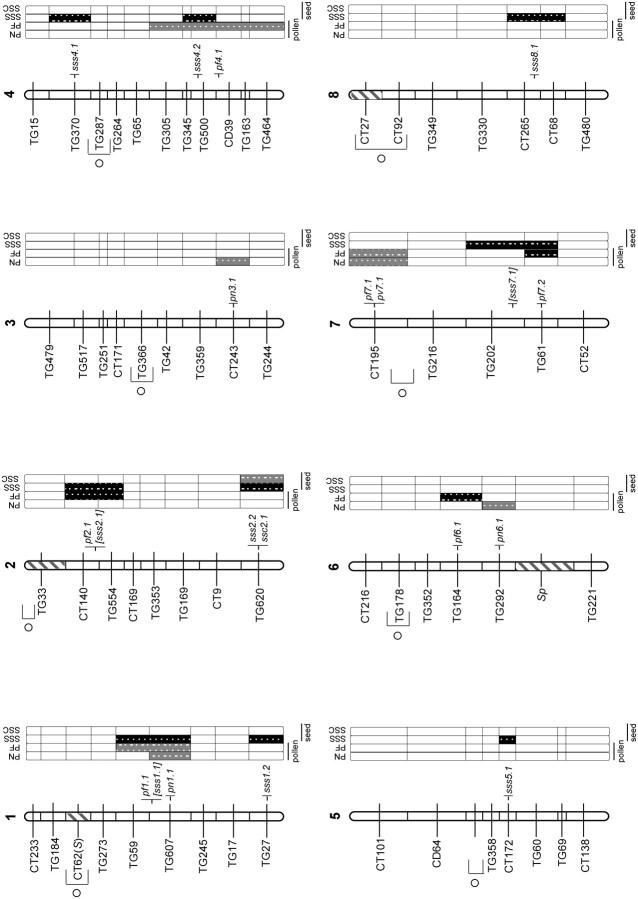
Multiple introgression lines showed significant reductions in male (pollen) fertility in comparison to both L. esculentum and L. hirsutum parental lines (Figure 1, a and b). Nonetheless, distinct components of male fertility were affected differently, with the most substantial effects shown as reduced proportion of fertile pollen, rather than as reduced total number of pollen grains, per flower (Table 1). Only one NIL showed significantly increased male fertility, in the form of increased total number of pollen grains (Table 1). QTL locations inferred from introgression line phenotypes are shown in Figure 2. In total, we detected a minimum of 12 QTL for male fertility: 8 QTL for proportion of fertile pollen (1 of which is likely due to at least two separate genetic factors; see below) and 4 QTL for total pollen number (Table 1). At all QTL for proportion of fertile pollen, the hirsutum alleles were associated with reduced pollen fertility in the esculentum background; for 3 of the 4 pollen number QTL, the hirsutum alleles reduced total pollen number. Of the 12 pollen QTL detected, 4 appeared to be colocated for the two different measures of male fertility: regions on chromosomes 1 and 7 were associated with both measures of pollen fertility (Figure 2). This modest association does not suggest substantial chromosomal clustering of traits affecting male function and is reflected in the small and nonsignificant correlation between these pollen traits (Table 2; see also below).
Figure 1.—
Distributions of NIL mean phenotypes for pollen and seed traits for 71 NILs. For each trait, the mean phenotypic value of each parental accession (LE; LH, if applicable) and the grand mean phenotypic value of all 71 NILs are indicated with arrows. (a) Total pollen grains per flower (PN); note that LH (an outcrosser) produces substantially more pollen per flower than LE (a selfer). (b) Proportion of fertile pollen grains per flower (PF). (c) Selfed seed set per fruit (SSS). (d) LE-cross seed set per fruit (SSC).
TABLE 1.
Putative QTL associated with hybrid incompatibility traits in NILs betweenL. esculentum andL. hirsutum
| Trait | QTL | Direction of effect |
Mean phenotype |
Additive effect |
Δ% | No. of NILs observed |
|---|---|---|---|---|---|---|
| Pollen no. (PN) | pn1.1 | − | 71.6 | −25.6 | −41.8 | 1 |
| pn3.1 | + | 168.2 | 22.7 | 37.0 | 1 | |
| pn6.1 | − | 78.4 | −22.2 | −36.1 | 1 | |
| pn7.1 | − | 78.4 | −24.3 | −39.6 | 1 | |
| Proportion of fertile pollen (PF) |
pf1.1 | − | 0.56 | −0.14 | −33.3 | 1 |
| pf2.1 | − | 0.49 | −0.18 | −41.7 | 1 | |
| pf4.1 | − | 0.58 | −0.13 | −31.0 | 1 | |
| pf6.1 | − | 0.45 | −0.20 | −46.4 | 1 | |
| pf7.1 | − | 0.59 | −0.13 | −29.8 | 2 | |
| pf7.2 | − | 0.51 | −0.17 | −39.3 | 3 | |
| pf9.1 | − | 0.58 | −0.13 | −31.0 | 1 | |
| pf10.1 | − | 0.49 | −0.18 | −41.7 | 4 | |
| Self-seed set (SSS) | sss1.1 | − | 9.19 | −23.2 | −83.4 | 2 |
| sss1.2 | − | 6.07 | −24.7 | −89.1 | 3 | |
| sss2.1 | − | 6.42 | −20.6 | −74.2 | 1 | |
| sss2.2 | + | 96.60 | 18.0 | 65.0 | 1 | |
| sss4.1 | − | 20.10 | −17.7 | −63.8 | 1 | |
| sss4.2 | − | 11.40 | −22.1 | −79.5 | 4 | |
| sss5.1 | − | 9.00 | −23.3 | −83.8 | 1 | |
| sss7.1 | − | 14.00 | −20.8 | −74.8 | 2 | |
| sss8.1 | − | 10.40 | −22.6 | −81.3 | 1 | |
| LE-cross seed set (SSC) | ssc2.1 | + | 103.00 | 56.8 | 121.86 | 1 |
Direction of effect describes an increase (+) or decrease (−) in the mean phenotype at each locus, compared to the LE parent. Mean phenotype is calculated from all NILs (i.e., number of NILs observed) showing the QTL phenotype at each genomic location. Additive effect is calculated as (LH/LH − LE/LE)/2. Δ% describes the percentage phenotypic change from the LE parent.
Figure 2.—
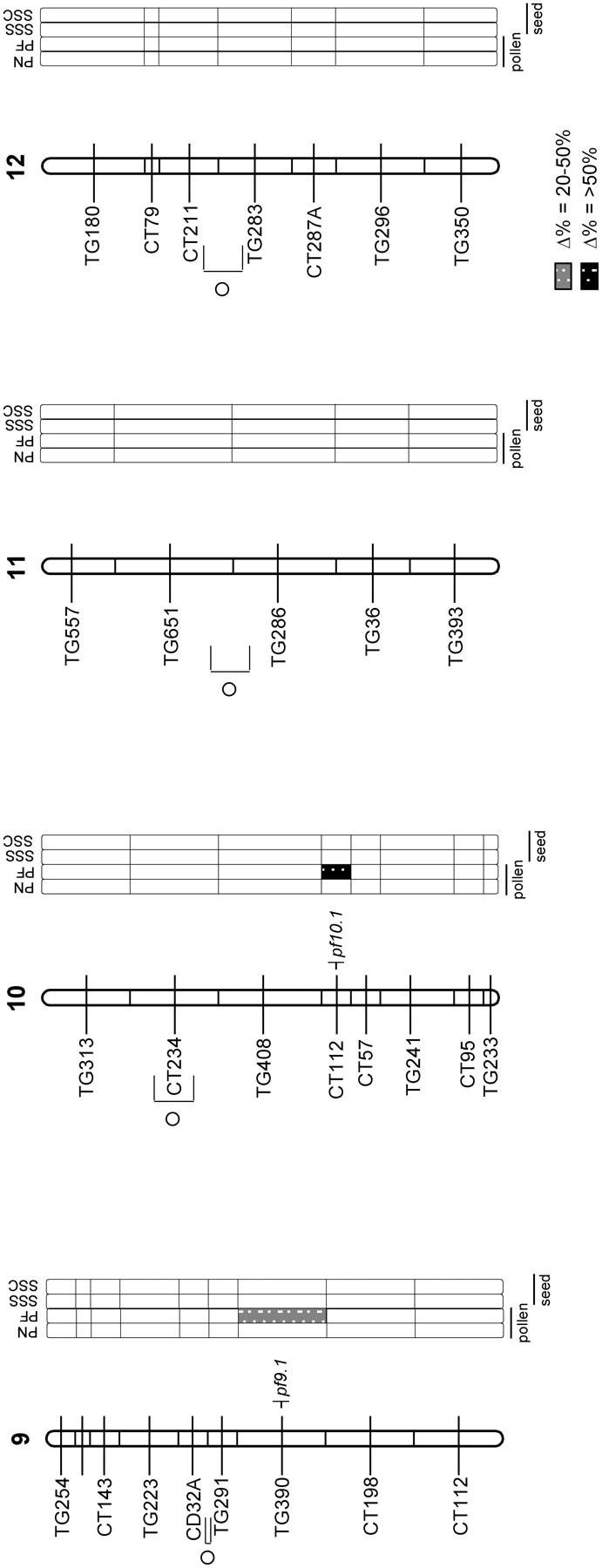
QTL for pollen and seed fertility traits, with their location on the L. esculentum × L. hirsutum linkage map. Hatched chromosomal regions on the linkage map indicate areas of the genome fixed for LE alleles (i.e., genomic regions not represented in the NIL population). The self-incompatibility (S) locus and self-pruning (sp) locus are indicated to the left of the chromosomes. The shaded bars to the right of the chromosomes show the individual analyses for the following traits: PN, total pollen count per flower; PF, proportion of fertile pollen per flower; SSS, self (homozygous) seed set; and SSC, LE-cross (heterozygous) seed set. Levels of percentage difference (Δ%) from the LE control parent for 20 > Δ < 50 and Δ > 50 are indicated by the intensity of shading (see key on figure). Putative QTL are indicated by symbols to the right of each chromosome, marked at the center of the chromosomal region showing a significant effect for the trait. Self-seed set traits in brackets are those that are nonsignificant once the effect of pollen fertility is removed statistically (see text).
TABLE 2.
Correlations between trait values from 71 NILs betweenL. esculentum andL. hirsutum
| PN | PF | SSS | |
|---|---|---|---|
| PF | 0.008 | ||
| SSS | −0.137 | 0.359** | |
| SSC | −0.087 | 0.113 | 0.537**** |
PN, total pollen count per flower; PF, proportion of fertile pollen per flower (arcsine square-root transformed); SSS, self (homozygous) seed set; SSC, LE-cross (heterozygous) seed set. *P < 0.05; **P < 0.01; ****P < 0.0001.
For one QTL, pf4.1, patterns of male sterility detected in the NILs were consistent with combined effects of two or more factors contained within a single large introgressed region (Figure 2). The NIL (LA3935) containing this single, large introgression on chromosome 4 showed a greater than threefold decrease in proportion of fertile pollen, in comparison to the LE control parent (mean PF, 0.576 vs. 0.842, respectively). However, four additional NILs containing overlapping genomic regions showed no significant decrease in pollen fertility (mean PF range, 0.852–0.774). In particular, two NILs (LA3936 and LA3937) that contained chromosomal regions representing two halves of the larger introgression individually showed mean 0.814 and 0.825 proportion of fertile pollen, giving an additive expected pollen fertility phenotype of 0.639 or multiplicative expectation of 0.672. Whether the observed pollen fertility (0.576) is substantially smaller than that expected under additive or multiplicative models—indicating synergistic negative interactions among loci contained within the larger introgression—is difficult to evaluate in this single instance. Regardless, the pattern shown at this chromosomal region does suggest that at least two factors within the large introgression contribute to the expression of male sterility.
Seed fertility/viability:
Seed fertility in introgression lines showed dramatically different results depending upon whether the resulting seed was heterozygous or homozygous for an LH introgression. For LE-cross seed (resulting from pollinations with LE pollen), which were heterozygous for LH introgressions, no lines showed a significant reduction in seed fertility in comparison to the pure LE parental line (Table 1). Indeed, the only significant line effect observed for heterozygous seed set was a twofold increase in seed production above the pure parental LE line mean (LA3923/LE heterozygote mean was 103.44 seeds per fruit vs. LE homozygote parental mean of 46.63 seeds per fruit). This resulted in a minimum of one QTL for increased heterozygous seed set (Table 1; Figure 2). In contrast, seed set per fruit resulting from self-pollination and therefore homozygous for an LH introgression was substantially reduced in multiple introgression lines in comparison to the parental LE plants (Table 1; Figure 1, c and d). In total, we detected a minimum of nine QTL for homozygous seed set (Table 1; Figure 2); the only QTL of this group that increased seed set was colocated with the QTL found to increase heterozygous seed set (Figure 2).
The absence of significant seed infertility in crosses between NILs and the LE parent is evidence that observed seed infertility is unlikely to be due to ovule (i.e., strictly female) sterility in the NIL parent (otherwise reduced seed set per fruit would be observed in infertile lines regardless of the genetic source of the pollen). Therefore, it is probable that the seed infertility QTL identified here are due to zygotic incompatibility, expressed as early hybrid seed abortion or failure and observed as reduced numbers of seeds per fruit, and that these factors are at least partially recessive (see below).
Four of the eight QTL for reduced homozygous seed set are colocated with QTL associated with reduced male fertility (Figure 2). Because homozygous seed set is the product of self-fertilization, QTL for reduced self-seed set may not always be independent of loci for male infertility, as lines with low pollen fertility may be relatively ineffective at setting selfed seed. Nonetheless, three lines of evidence support independence between pollen fertility and seed fertility for multiple QTL. First, six QTL associated with reduced pollen fertility do not show associated reductions in self-seed set (i.e., are not colocated with seed set QTL; Figure 2), indicating that reduced male fertility (measured as number and stainability of pollen) per se does not necessitate low self-seed set, under these experimental conditions. The mean pollen fertility of these QTL is 0.528 (which is comparable to the 0.535 average pollen fertility found in pollen QTL colocated with seed set QTL) yet the mean seed set associated with these regions is 36.1 seeds per fruit, which is not substantially lower than the overall NIL mean or the control LE seed set. (In comparison, the average seed set for SSS QTL colocated with PF QTL is 12.5 seeds per fruit.) Second, four QTL detected for reduced self (homozygous) set seed (sss1.2, sss4.1, sss5.1, and sss8.1) are not associated with reduced male fertility (Table 3; Figure 2) and are therefore apparently independent of male function. Finally, an ANOVA indicates that pollen fertility has a significant but very modest effect on observed variation among NILs in self-seed set (no effect was detected for LE-cross seed set); proportion of fertile pollen explains ∼5% of self-seed set variation [i.e., R2 = 0.05, P < 0.0001; ANOVA with PF (arcsine transformed) as main effect]. Total pollen number had no significant effect on self-seed set (data not shown).
TABLE 3.
Pollen fertility at genomic regions containing seed set QTL
| Seed QTL | Chromosome | Seed Δ% | PN QTL | PN Δ% | PF QTL | PF Δ% |
|---|---|---|---|---|---|---|
| Associated with pollen QTL | ||||||
| sss1.1 | 1 | −83.43 | pn1.1 | −25.83 | pf1.1 | −19.40 |
| sss2.1 | 2 | −74.17 | — | −8.82 | pf2.1 | −41.68 |
| sss4.2a | 4 | −79.50 | — | 1.84 | pf4.1 | −10.49 |
| sss7.1 | 7 | −74.77 | — | 13.70 | pf7.2 | −44.97 |
| Not associated with pollen QTL | ||||||
| sss1.2 | 1 | −89.06 | — | −14.01 | — | −28.97 |
| sss4.1 | 4 | −63.76 | — | −7.46 | — | −9.80 |
| sss5.1 | 5 | −83.78 | — | −9.00 | — | −7.96 |
| sss8.1 | 8 | −81.28 | — | 8.91 | — | 2.84 |
| sss2.2 | 2 | 64.96 | — | −14.16 | — | −5.91 |
| ssc2.1 | 2 | 121.86 | — | −14.16 | — | −5.91 |
Seed Δ% describes the percentage phenotypic difference in seeds per fruit from the LE parent. Where applicable, the QTL for total pollen count per flower (PN) or proportion of fertile pollen (PF) that is colocated with each seed QTL is identified. Pollen Δ% describes the percentage phenotypic change from the LE parent for the corresponding pollen trait at the same chromosomal region.
QTL that remain significant after the effects of proportion of fertile pollen are removed prior to analysis (see text).
To more directly address the possible influence of male fertility on the expression of self-seed set, we removed the effects of male fertility by first analyzing self-seed set with proportion of fertile pollen (arcsine square root transformed) as a main effect and then using the residual values of this analysis in a Dunnett's test of mean differences between each NIL and the control parent. Of 14 NILs originally detected as having significantly reduced self-seed set, 10 remained significant after the effects of individual male fertility were removed statistically. These correspond to five QTL: all four QTL originally identified as independent of male function and one QTL (sss4.2) that is colocated with a locus that reduces male function. The three remaining self-seed QTL (sss1.1, sss2.1, and sss7.1) are identified as dependent on male sterility in this analysis (Table 3); on the basis of this result and the other analyses above, our provisional conclusion is that these three seed infertility QTL are likely to be predominantly the product of male infertility loci located in these regions. The five remaining seed infertility QTL are regions that confer reduced seed set, partially or fully independently of any male infertility effects in the same genomic region.
In lines with a significant effect for either heterozygous or homozygous seed set, we evaluated the allelic effect of the introgression by determining the size and significance of the dominance deviation (see materials and methods). (For completeness, these were calculated for all seed set QTL whether they were associated with a pollen QTL or not.) For the one QTL that conferred increased seed set (in both homozygous and heterozygous form) the dominance deviation was significant, such that both heterozygous and homozygous seed set exceeds seed set in LE control plants (Table 4). This is consistent with the QTL contained within the corresponding genomic region acting dominantly. [Alternatively, the data may also be consistent with a QTL that directly affects female (i.e., ovule) fertility, rather than zygotic seed viability (see discussion); if this is the case, then our analysis cannot assess genic action at this QTL.] For two of the five “independent” self-seed set QTL, the dominance deviation was also significant (Table 4). These QTL for reduced hybrid seed set act recessively; that is, more heterozygous LH/LE seed was produced than expected on the basis of the midparent value of homozygous LH/LH and LE/LE seed set. The remaining three seed set QTL show patterns of gene action that do not differ from an additive model (Table 4). In addition, two of the “nonindependent” self-seed set QTL also appear to be recessive, probably due solely to differences in fertility of the pollen parent between LH/LH vs. LH/LE seeds (i.e., lowered male fertility of the LH parent; Table 4). Finally, a pattern of marginal overdominance is also shown in two SSS QTL (sss2.1 and sss5.1; Table 4). Heterozygosity could act to relieve the negative effects of slightly deleterious alleles that might have been fixed during artificial selection; accordingly, mild overdominance for fitness might be the result of inbreeding in the domesticated line.
TABLE 4.
Dominance deviations in self-seed set QTL
| Self-seed set (SSS) QTL | LH/LH phenotype (seeds) |
LH/LE phenotype (seeds) |
LH/LE midparent |
Dominance deviation (seeds) |
Gene action |
|---|---|---|---|---|---|
| Independent loci sss1.2 |
6.07 | 32.96 | 28.57 | 4.39 | (Additive) |
| sss2.2 | 91.56 | 103.44 | 71.31 | 32.14a | Dominant |
| sss4.1 | 20.11 | 30.28 | 35.59 | −5.31 | (Additive) |
| sss4.2 | 11.38 | 27.37 | 31.22 | −3.85 | (Additive) |
| sss5.1 | 9.00 | 65.50 | 30.03 | 35.47a | Recessive/overdominant |
| sss8.1 | 10.39 | 47.33 | 30.73 | 16.61a | Recessive |
| Nonindependent loci sss1.1 |
9.19 | 38.94 | 30.13 | 8.81a | Recessive |
| sss2.1 | 14.33 | 53.94 | 32.70 | 21.25a | Recessive/overdominant |
| sss7.1 | 14.00 | 35.83 | 32.53 | 3.30 | (Additive) |
Phenotypes are calculated as means of all NILs contributing to each QTL. Dominance deviation describes the difference between the observed LH/LE phenotypes (SSC) and expected phenotype based on midparent value between the observed LH/LH and LE/LE homozygous seed set.
Significant deviation from the midparent expectation; positive values indicate that LH/LE heterozygotes produce more seed than expected.
Correlations among traits:
Moderate to strong correlations were found between two pairs of traits (Table 2). First, the number of self-seed set was strongly correlated with the number of cross-seed set (Table 2), consistent with a pleiotropic mechanism underlying these physiologically and genetically related traits. Second, SSS was positively correlated with pollen fertility. While some lines with low pollen fertility could have been relatively ineffective at setting selfed seed (as outlined above), correlations between pollen and seed sterility could also be due to several other mechanisms. First, simultaneous reductions in pollen and seed fertility could be due to Dobzhansky-Muller interactions between genes, such as floral development loci, that are directly involved in the formation of pollen, ovules, and seed. Second, traits influencing fertility could be chromosomally clustered so that correlations are due to the nonrandom distribution of these traits in the genome. Third, reduced pollen and seed fertility may both indirectly result from the deleterious effect of a locus or loci that confers an overall reduction in plant physiological performance or health. Our analysis cannot differentiate direct pleiotropic effects of single Dobzhansky-Muller interactions from genomic clustering, although finer-scale mapping and eventual identification of underlying genetic factors could resolve these first two alternatives definitively. No evidence supports the third explanation, as there were no large systematic differences between plants in plant size, biomass, or general health, on the basis of greenhouse observations. Regardless of the possible mechanisms linking pollen and seed fertility, the correlation between these traits is only moderate, indicating that factors other than pollen fertility also contributed to reduced self-seed set—consistent with our detection of QTL that reduce seed set independently of reduced male function (and vice versa).
Introgression size effects on hybrid incompatibility:
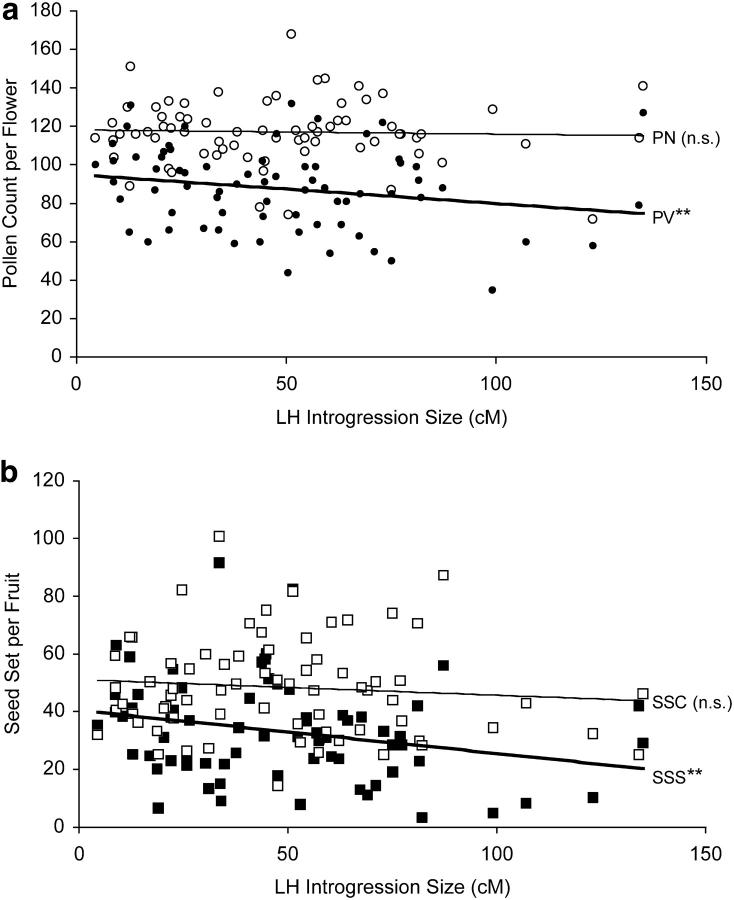
We detected statistically significant negative associations between the estimated size of the introgressed genetic region(s) in each NIL and the PF and SSS; introgression size was not associated with LE-cross seed set or total pollen count per flower (Figure 3, a and b). Nonetheless, where significant introgression “size effects” were detected, they explained a very small component of the observed variation between lines in fertility. For example, the strongest relationship observed (between introgression size and self-seed set) explained <7% of the among-line variation in this trait (Figure 3). In comparison, line effects explained 39 and 45%, respectively, of the experimental variance in PF and SSS (main effects of line in individual ANOVAs that included plant nested with line as a main effect).
Figure 3.—
Relationships between the size of LH introgression (in centimorgans) and mean NIL (a) pollen or (b) seed fertility. (a) Pollen fertility measures shown are total pollen count per flower (PN; in open circles with thin line) and (for convenience of representation) number of viable pollen per flower (PV; in solid circles with thick line). (b) Seed fertility measures are LE-cross seed set per fruit (SSC; in open squares with thin line) and self-seed set per fruit (SSS; in solid squares with thick line). Double asterisks indicate significant negative regression at P < 0.001; NS, not significant. Note that regression results for proportion of fertile pollen (PF; arcsine square-root transformed) were equivalent to PV results.
DISCUSSION
The nature of the genes that underlie reproductive incompatibility between species influences both the evolutionary dynamics of fixation of these trait changes and, more broadly, which traits are chiefly responsible for the origin of new taxonomic groups. Three substantive findings emerge from our analysis of the genetics of hybrid incompatibility in Lycopersicon. First, hybrid incompatibility (measured as pollen sterility and seed infertility) appears to be based on a relatively modest number of QTL. Second, the number of QTL influencing male (pollen) sterility and other forms of hybrid incompatibility are roughly comparable. Third, seed infertility factors appear to act recessively or at most additively in hybrid introgression lines. The first two findings differ substantially from prior analyses in Drosophila, while the third is largely in agreement with previous studies. The observed differences might be due to biological differences between Lycopersicon and Drosophila models, especially in the sex determination system, reproductive and mating biology, and the prevalence of sexual interactions such as sexual selection.
Complexity of hybrid incompatibility:
The number and distribution of phenotypic effects of incompatibility loci can suggest how many factors might be required to isolate evolving populations. Here we have shown that interspecific pollen and seed infertility could each be due to the effects of 5–11 QTL. Of the 19 QTL total that reduce hybrid pollen or seed fertility, 11 are colocated with at least 1 other incompatibility QTL, some of which could share a common physiological or genetic basis. Accounting for colocated QTL, we detect 13 individual genomic regions associated with one or more components of hybrid incompatibility. Many of these regions have relatively large individual effects on hybrid infertility, although none individually causes complete hybrid incompatibility. At least four genomic regions are associated with ∼50% hybrid male sterility. This pattern is more pronounced for seed infertility, where individual regions can reduce the number of seeds set per fruit by >80% compared to the LE parent (e.g., sss1.2, sss5.1, and sss8.1), independent of any other incompatibility factor. In general, then, we detect a relatively modest number of QTL contributing to hybrid incompatibility.
In comparison, studies in Drosophila indicate that interspecific (especially male) sterility is frequently highly polygenic and complex, being composed of many epistatically interacting factors (e.g., Wu and Davis 1993; Davis and Wu 1996). Three previous analyses (Hollocher and Wu 1996; True et al. 1996; Tao et al. 2003b) are most comparable to our study, in that they examined the effect of homozygous introgressions on a homozygous foreign genetic background and have similar genetic resolution in terms of introgression sizes. On the basis of these studies, it has been estimated that ∼60 QTL in total contribute to hybrid male sterility when D. mauritiana is introgressed into a D. simulans background (Tao et al. 2003a), a species pair separated for ∼0.3 million years. There are at least 9 loci for hybrid male sterility on ∼40% the X chromosome (reviewed in Wu and Hollocher 1998) and 19 loci on the third chromosome (Tao et al. 2003b), most with relatively modest individual effects such that at least two or three factors are simultaneously required for complete sterility.
These estimates are clearly substantially higher than those generated in our analysis, especially considering male sterility alone. Time since species divergence does not account for this difference in the number of QTL associated with hybrid incompatibility; the precise timing of the split between the ancestors of L. esculentum and L. hirsutum is unknown, but may be substantially older than 1 million years (Nesbitt and Tanksley 2002). At least some of the observed differences may be due to differences in the degree of genetic resolution between studies. Our study uses somewhat larger introgressions (mean, ∼48.5 cM; range, 4.5–135 cM) compared to the Drosophila studies [mean range from 4.7 cM (simulated estimate; True et al. 1996) to 26.8 cM (Hollocher and Wu 1996)]. However, our introgressions correspond to an average of ∼4% of the length of the hybrid genome (range 0.35–10.7%) per NIL, which is comparable to the Drosophila analyses (average of ∼0.7–7% of the length of the hybrid genome). Nonetheless, if 1 cM translates roughly to 1 Mb in tomato (Pillen et al. 1996) and if genes are ∼1 kb long on average, then even a relatively small region of 5 cM could contain hundreds of genes. As such, greater genetic resolution from smaller introgressed regions might reveal a considerably more complex genetic basis underlying each of the QTL identified here. This is borne out to some extent by our finding that pf4.1 is likely composed of at least two loci that contribute to pollen sterility. Nonetheless, three lines of evidence, discussed below, suggest that the number of loci we have detected here is a reasonable first estimate of the relative complexity of hybrid incompatibility between these Lycopersicon species.
First, the number of identified loci is roughly in line with previous analyses in plants. Estimates based on F2 segregation of male sterility (e.g., Mimulus, Fishman and Willis 2001) or patterns of marker transmission ratio distortion (rice, Harushima et al. 2001, 2002; Eucalyptus, Myburg et al. 2004) are suggestive that a modest number of Dobzhansky-Muller interactions can control hybrid incompatibility among plants. Similarly, traits such as partial embryo sac infertility (Liu et al. 2001), hybrid endosperm failure (Matsubara et al. 2003), and spikelet sterility (Li et al. 1997) in rice and hybrid pollen inviability in sunflower (Kim and Rieseberg 2001) each appear to be based on approximately three to six major QTL. Note that, unlike several of these studies (Li et al. 1997; Kim and Rieseberg 2001), the factors identified in our study are unlikely to be due to the effects of null phenotypes resulting from introgressions containing chromosomal inversions or translocations that differ between the species. Not only is Lycopersicon commonly believed to be chromosomally collinear on the basis of current cytology and marker map resolution (Stevens and Rick 1986; Quiros 1991)—a supposition that is testable with future sequencing efforts—but also we find no evidence for significant underdominance in fitness at any introgressed region. This observation is inconsistent with chromosomal rearrangements causing hybrid incompatibility (Fishman and Willis 2001); therefore chromosomal rearrangements likely play little role in reproductive isolating barriers between these two species, at least with respect to seed infertility for which we have both homozygous and heterozygous comparisons. Regardless of the precise mechanism, however, the number of factors detected in our study are in line with these limited data from other plant systems, especially as divergence times between L. esculentum and L. hirsutum are likely longer than those of most of the other crosses analyzed to date.
Second, our own analysis suggests that an infinitesimal model of hybrid incompatibility factors, where incompatibility is due to many genes of relatively small individual effect, is not consistent with detected patterns of hybrid incompatibility. Under the infinitesimal model, reduced hybrid fitness is expected to be strongly associated with the amount of one species genome represented in a foreign genetic background. This is simply because longer introgressions are likely to contain more incompatibility factors than shorter introgressions, provided each contains a comparable gene density per unit of map distance. As expected under this model, hybrid male sterility appears to increase with the size of heterozygous autosomal interspecific introgressions between Drosophila buzzatii and D. koepferae (Marin 1996; Naveira and Maside 1998) and homozygous introgressions in NILs between D. simulans and D. mauritiana (Tao et al. 2003a). (See also Naveira 1992 and Hollocher and Wu 1996 for additional evidence for introgression size effects in Drosophila.) In contrast, our analysis of pollen and seed fertility detected a very weak size effect of chromosomal introgressions for only some measures of hybrid incompatibility, which explained little of the fertility variation observed within the experiment. In comparison, genetic differences between lines explained four to five times the variation observed for these traits. There is also no evidence that hybrid incompatibility increases at some threshold of introgression size (cf. Naveira and Maside 1998). These weak (or absent) associations between introgression size and measures of hybrid incompatibility suggest that an infinitesimal model is inappropriate for our species cross and are consistent with the limited number of incompatibility QTL we detected, as well as the many genomic regions apparently unassociated with hybrid incompatibility.
Finally, single QTL have regularly been cloned to one or a few molecular genes of large effect in several plant systems, including Lycopersicon. The fine-scale genetic structure of specific QTL for hybrid incompatibility (e.g., Doi et al. 1999; Kubo et al. 2001; Sobrizal et al. 2002) and other quantitative traits (e.g., El-Assal et al. 2001; Osterberg et al. 2002; see also Remington and Purugganan 2003) has been less complex than that detected in Drosophila (e.g., Davis and Wu 1996). In Lycopersicon, large-effect QTL underlying quantitative trait expression in fruit weight and sugar content have also been fine-mapped and cloned to individual molecular loci (e.g., fw2.2, Frary et al. 2000; Brix9-2-5, Fridman et al. 2000). While we do not yet know the molecular genetic basis of the QTL identified here, if these results can be used as any indication of expectations at finer genomic scales in our species cross, then the genetic basis of each hybrid incompatibility QTL may be limited to one or a few molecular loci of moderate to large effect. Clearly, however, further genetic dissection using shorter recombinants of the current introgressions will be helpful in resolving the number of individual molecular loci contributing to each identified QTL.
In addition, there are two further caveats to interpreting the number of factors contributing to hybrid incompatibility detected here: the possible influence of either unconditionally deleterious loci or complex epistasis. The first factor could act to inflate estimates of incompatibility loci, while the second may lead us to underestimate the number of QTL involved in hybrid incompatibility. First, inbreeding depression due to unconditionally deleterious loci could account for one or more putative hybrid incompatibility QTL. This would be the case if semilethal recessive alleles, heterozygous in the normally outbreeding L. hirsutum parent, were fixed during the generation of these inbred homozygous lines; these factors would be deleterious regardless of whether the genetic background was heterospecific or homospecific. For unconditionally deleterious recessives to explain the bulk of loci identified here, the frequency of strongly deleterious recessives segregating in the L. hirsutum source population would have to be on the order of 15–19 per haploid genome. [This is because only one LH haploid genome (i.e., in the original F1 hybrid plant) contributes LH alleles to this NIL population.] L. hirsutum exhibits variable levels of inbreeding depression, depending upon the specific accession examined and its breeding system (Martin 1962); however, little is directly known about the frequency of deleterious recessives in this species. Evidence from model systems (especially Drosophila and humans), in addition to well-developed theory, indicates that the frequency of segregating deleterious recessives (lethals and steriles) in highly outbred species is on the order of 2–6 alleles per haploid genome (Crow and Kimura 1970; Hartl and Clark 1989). The potential for haploid selection against deleterious mutations makes this predicted frequency lower for genes expressed in the gametophytic stage, as appears to be the case with a large proportion of the genome in many plants (Walbot and Evans 2003), including tomato. Overall, these data suggest that the bulk of loci identified here are likely associated with true hybrid incompatibility factors rather than with strongly deleterious alleles that were sheltered in the L. hirsutum parental population. This hypothesis is testable either by the evaluation of additional introgression lines developed with an alternative L. hirsutum donor, including fertility assessment of trans-heterozygotes generated by crossing two lines with the same genomic region independently derived from different accessions (cf. Tao et al. 2003a), or by biparental inbreeding within L. hirsutum to evaluate the fitness effects of each homozygous L. hirsutum introgression region in its own homospecific genetic background.
Second, introgression lines containing single chromosomal regions are individually unable to detect complex epistasis involving multiple loci from one species in the genetic background of another. Accordingly, if expression of hybrid incompatibility is sensitive to genetic background then introgression line analysis will give an underestimate of the loci contributing to hybrid incompatibility. Numerous previous studies in Drosophila have indicated that hybrid incompatibility could be exacerbated by interactions among multiple introgressions from a single species in a foreign genetic background (Wu and Hollocher 1998; Tao et al. 2003b and references therein). Nonetheless, there is still limited and somewhat mixed (e.g., Kim and Rieseberg 2001; Slotman et al. 2004) evidence that complex epistasis is generally important in hybrid incompatibility. In this study, we identified one specific genomic region on chromosome 4 (pf4.1) in which the combined effects of two or more loci were apparently necessary for the expression of substantial pollen sterility. This single case suggests that interactions among adjacent chromosomal regions could influence the expression of hybrid sterility. Fortunately, introgression lines are powerful tools for evaluating epistasis among two or more loci, as the phenotypic effect of specific two-locus genotypes can systematically be assessed against that of single introgressions by combining NILs via crossing (Eshed and Zamir 1996). One of our forthcoming goals is to serially combine NILs containing putative hybrid incompatibility QTL, to directly assess the strength of pairwise interactions among conspecific introgressions on a foreign genetic background and to evaluate whether such interactions are typically synergistic, additive, or antagonistic in their effects on hybrid incompatibility.
Expression of male sterility vs. other hybrid incompatibilities:
The second striking result in our study is the roughly comparable numbers of male sterility QTL and QTL for other forms of hybrid incompatibility. Previous analyses in Drosophila indicate that male sterility factors are one to two orders of magnitude more abundant than loci for female sterility or hybrid inviability. For example, in a D. simulans × D. mauritiana cross, 67 of 145 introgression lines were found to be male infertile whereas no lines were female infertile or inviable (Tao et al. 2003a); comparable differences have been found in other genome-wide studies of hybrid incompatibility factors (e.g., True et al. 1996) and it is generally accepted that male sterility is the first and most rapidly evolving form of hybrid incompatibility in Drosophila (Wu and Hollocher 1998).
In our study the relative numbers of loci for male sterility and other forms of hybrid incompatibility are difficult to assess precisely. First, pollen and seed fertility are not strictly analogous measures of hybrid fertility; the former assesses inviable male gamete production whereas seed set includes the composite effects of female fertility and early zygotic dysfunction. Indeed, it is likely that seed sterility observed here is due to zygote incompatibility (lethality) causing early seed failure after fertilization, rather than ovule sterility per se. Second, some measures of hybrid incompatibility may be nonindependent, including pollen and seed sterility (as discussed above). Nonetheless, even simply assuming that there are 11 independent pollen QTL vs. 5–9 QTL for seed set, these relative counts for different components of hybrid incompatibility are a substantial departure from the orders of magnitude differences detected in previous studies. In addition, the seed infertility QTL we detected had on average much stronger negative effects on hybrid fitness than did the pollen sterility QTL (even for those putative QTL not associated with pollen traits).
These differences between our results and those typical of Drosophila may be due to biological differences between the two groups, including different modes of sex determination, different propensities for sexual interactions, different reproductive biology, or different patterns of covariation between traits underlying sex function. In Drosophila, the faster evolution of male sterility could be due to faster male evolution driven by sexual selection or sex-ratio selection or due to sex chromosome-autosome interactions that differ between hybrid males and females (Wu and Davis 1993; Wu et al. 1996; Turelli and Orr 2000; Tao and Hartl 2003). In contrast, Lycopersicon does not have heteromorphic sex chromosomes (or sex chromosomes of any kind); in addition, the relative importance of sexual interactions such as male-male competition is as yet unknown in this (and many) plant systems. Either of these biological differences could explain a slowed accumulation of male sterility factors and therefore the relative parity between pollen sterility and other forms of hybrid incompatibility in our study. Although there are little data on the relative rates of accumulation of different forms of hybrid incompatibility in other plant systems, a recent analysis of reproductive isolation among 32 species in two diverse plant genera found no evidence that hybrid pollen sterility evolved more rapidly than parental cross-compatibility (Moyle et al. 2004), reinforcing the conclusion that hybrid male sterility may not evolve especially rapidly among plant taxa. Nonetheless, whether male or female (or other) hybrid incompatibility evolves faster among plant species in general is, as yet, unknown. The evidence for faster evolution of male vs. other (especially female) hybrid incompatibility from other biological groups [e.g., butterflies and moths (Presgraves 2002), birds (Wu et al. 1996; Price and Bouvier 2002), and mosquitoes (Presgraves and Orr 1998)] is also variable, perhaps due to differences in sex determination or sexual interactions.
Alternatively, large differences in reproductive biology might plausibly explain the differences detected here. For example, differences in the sensitivity of spermatogenesis have been previously proposed to explain differences in the propensity for hybrid male sterility among animal groups (Wu et al. 1996). Lycopersicon differs considerably from Drosophila in both male and female gametogenesis and in the formation and provisioning of fertilized embryos (Walbot and Evans 2003 and references therein). Unfortunately, there are currently little data in Lycopersicon to directly assess the relative complexity of pollen vs. seed formation, the number of genes contributing directly to male and female function, and therefore their relative sensitivity to mutational change. Male sterility mutants are commonly observed in Lycopersicon (Gorman and McCormick 1997) and are approximately twice as common as female-specific sterile mutants in current Lycopersicon monogenic mutant stocks (for example, see http://zamir.sgn.cornell.edu/mutants/ and http:://tgrc.ucdavis.edu), although directly comparable estimates of the frequency of seed sterility are hard to obtain. However, our detection of multiple QTL for hybrid seed inviability is entirely consistent with previous observational data for Lycopersicon. In particular, hybrid seed failure is known to be a common early interspecific barrier among many plant species (Katsiotis et al. 1995; Lester and Kang 1998), especially within the Solanaceae (Brink and Cooper 1947; Hogenboom 1972), even in the absence of other substantial hybrid breakdown. This apparent propensity for hybrid seed incompatibility may also contribute substantially to equalizing the relative frequency of zygotic inviability and male sterility loci observed in our study. Moreover, among Solanaceous species, hybrid seed inviability has frequently been attributed to species-specific changes in gene dosage within the endosperm (the triploid tissue that provisions embryonic development within the seed; e.g., Johnston et al. 1980; Katsiotis et al. 1995), possibly leading to mismatched patterns of genomic imprinting or gene expression (Ehlenfeldt and Ortiz 1995; Masuelli 2001) and consequent seed abortion. In general, then, coordination of unique tissues such as the endosperm, with embryonic development in early zygotes, is one way in which the reproductive biology of different systems (in this case angiosperms) may facilitate very different mechanisms of early evolution of reproductive isolation.
A final alternative explanation for the rough parity between pollen and seed incompatibility QTL is that physiological and genetic covariance between male and female traits in hermaphrodites may prevent independent evolution of these classes of traits without concurrent effects on trait expression in the other sex. Drosophila mutagenesis (see Ashburner 1989) and QTL mapping (Hollocher and Wu 1996; True et al. 1996) studies show no evidence that loci influencing male and female hybrid fertility share a common genetic basis. However, in hermaphroditic flowering plants with perfect flowers (i.e., flowers with both male and female parts), male and female tissues are developmentally adjacent in both time and space, and single individuals carry complete sets of sex determination genes. Therefore one might expect greater genetic and phenotypic associations between male and female fertility factors in these species and, accordingly, stronger genetic constraints on the independent evolution of each class of traits. This expectation is supported by limited data from other studies. Among closely related Mimulus guttatus and M. nasutus, for example, significant correlations between pollen viability and seed set suggest that F2 male and female fertility partially share a common genetic basis (Fishman and Willis 2001). Data from our analysis are less clear. In several instances we have good evidence for the partial or complete independence of seed infertility from pollen sterility, most notably the observations that pollen sterility QTL are not always accompanied by seed infertility QTL and vice versa. Nonetheless, our data also show a modest correlation between pollen and seed fertility. Future fine mapping and identification of underlying genetic factors will be able to differentiate functional pleiotropy at single underlying loci from the alternative explanation that pollen and seed incompatibility QTL are nonrandomly clustered in the genome.
Finally, note that our finding of roughly comparable numbers of pollen and seed incompatibility QTL is very unlikely to be due to a bias against detecting pollen factors, because of differential selection against these during the development of the NILs. In particular, for several generations LE was used as the recurrent pollen parent during the construction of the lines (Bernacchi et al. 1998b), a breeding scheme that provides little opportunity for differential selection against male sterility factors.
The mode of gene action for hybrid seed set:
The third major finding of our study is the apparent recessivity of loci influencing hybrid seed set. To our knowledge, no previous study has directly investigated the genome-wide dominance relationships of sterility factors in plants (see Sano 1986 and Ikehashi and Araki 1988 for data from specific loci). Several studies in Drosophila have shown that hybrid male and female sterility is more severe when introgressions are homozygous rather than heterozygous (e.g., Hollocher and Wu 1996; True et al. 1996); in addition, recent analysis of hybrid inviability suggests that recessive factors are eight times more common than dominant inviability factors (Presgraves 2003). Male sterility also appears to be recessive in mosquitoes (Slotman et al. 2004). In our analysis of seed set, we routinely observed that homozygous introgressions have more than twice the effect of the corresponding heterozygous introgression on the number of seed set per fruit, indicating that seed infertility factors are often partially recessive in this Lycopersicon species cross. This is the case even for seed set QTL that are completely independent of male fertility and therefore cannot be simply due to differences between lines in male fertility. Other seed set factors showed no significant dominance deviation, consistent with additive gene action, in which the LH/LE heterozygote shows approximately half the reduced seed set seen in the LH/LH homozygote. It is interesting that only the single QTL associated with increased hybrid seed set shows gene action consistent with dominance for increased hybrid seed success. An alternative hypothesis, consistent with the observation that seed set is increased equally in self- and LE cross-pollinations involving this locus, is that this QTL increases ovule (female) fertility directly, regardless of the pollen source used to sire seeds. It is interesting that this QTL (located at the end of chromosome 2) is close to the genomic site of the molecularly cloned locus—ORFX—that controls fruit weight difference between L. esculentum and L. pennellii (Frary et al. 2000) and, perhaps, between other Lycopersicon species (Alpert et al. 1995). The ORFX locus is thought to act as a negative regulator of cell proliferation in the carpels of the developing flower, the tissues that give rise to the ovary, ovules, and fruit. Lower transcription levels correspond to more cell divisions, producing larger fruits in the domesticated tomato (Frary et al. 2000). Although, in our study, the LH introgression at this genomic region is associated with reduced fruit size that is typical of wild Lycopersicon species alleles (L. Moyle and E. Graham, unpublished observations), it is an intriguing possibility that transgressive expression of ORFX has led to a proliferation of ovules in introgression hybrids, producing consequent increases in hybrid seed number not seen in either parent species.
Finally, our conclusion that hybrid seed infertility factors are generally partially recessive or, at most, additive relies on the assumption that dominant seed incompatibility factors were not differentially lost during the development of the introgression lines we used. Very few genomic regions are missing from the analyzed NIL population (Monforte and Tanksley 2000), indicating that loss of many dominant factors is unlikely. Two of the four major missing regions, those encompassing the self-incompatibility and self-pruning loci, are unrepresented for logistical reasons (that is, they were deliberately selected during development of the NILs; see materials and methods); therefore we have no reason to expect that these regions differentially harbor dominant incompatibility factors. Of the other two missing regions, the region on chromosome 8 was introgressed in heterozygous form but could not be made homozygous during the generation of NILs (Monforte and Tanksley 2000); this strongly suggests that this region contains a locus (or loci) that acts partially or fully recessively to generate gametic or hybrid lethality. It remains possible that the second region, on chromosome 2, may harbor one or more dominant incompatibility factors.
Other influences on the expression of hybrid incompatibility:
The role of genetic self-incompatibility systems (as found in L. hirsutum and other Lycopersicon species) in the expression of interspecific crossing barriers has long been debated (de Nettancourt 2001 and references therein) with little conclusive resolution. Nonetheless, a recent study indicates that a QTL for interspecific cross-incompatibility among L. esculentum and L. hirsutum maps to the same genomic location as the self-incompatibility (S) locus (Bernacchi and Tanksley 1997). Because the S-locus was not represented in the NIL population, we were unable to assess its influence on hybrid incompatibility here. However, the data presented indicate that substantial hybrid incompatibility can be detected even in the absence of the S-locus and therefore demonstrate that factors other than this locus can strongly influence the expression of hybrid incompatibility. Finally, although one of the species used in our analysis was a cultivated species, it is unlikely that the process of domestication alone can explain our results, especially their departure from previous findings in Drosophila. As discussed, many of the general patterns detected here have counterparts in several other systems, especially in other plant crosses between wild species, a priori suggesting that domestication need not play a substantial role in either limiting the number of factors contributing to hybrid incompatibility or equalizing the relative contribution of male sterility and other hybrid incompatibilities. Nonetheless, because there are presently so few analyses of hybrid incompatibility outside Drosophila, it is difficult to independently assess the relative importance of all potentially influential factors without additional studies in more diverse biological groups, especially those with contrasting sex determination, mating, and reproductive systems.
Conclusions:
This study demonstrates the fruitfulness of using current genomic resources developed in other contexts to approach long-standing evolutionary questions. Several of our findings are intriguing in the context of current expectations of the behavior of hybrid incompatibility factors. We observed two substantive differences from patterns of hybrid incompatibility in the fruit fly, a relatively modest genetic basis and a rough parity between hybrid male sterility and other forms of hybrid incompatibility (i.e., hybrid seed inviability), that are supported by additional evidence from other diverse systems. These departures may be due to biological differences between groups, notably in sex determination, sexual interactions, and reproductive biology, but their ultimate basis remains to be determined. The relative importance of these biological differences could be readily tested with additional analyses of the genetic basis of hybrid incompatibility, using groups both with and without heteromorphic sex chromosomes, strong sexual selection, and/or separate sexes. Indirect analyses based on such contrasts (e.g., Presgraves and Orr 1998; Arnqvist et al. 2000) have provided intriguing evidence for their potential importance in shaping patterns of hybrid incompatibility. However, there are multiple promising systems both within and outside the dipterans in which it is increasingly feasible to directly examine the genetic basis of hybrid incompatibility (e.g., Fishman and Willis 2001; Slotman et al. 2004). The use of Lycopersicon and other developing model systems to understand the nature and evolution of interspecific isolating barriers promises to provide valuable insight into these unresolved questions.
Acknowledgments
We are grateful to D. Barbash, D. Bolnick, R. Chetelat, L. Fishman, M. Hahn, B. O. Jaffas, C. M. Jones, C. Langley, D. Rand, M. Turelli, and two anonymous reviewers, for comments that substantially improved earlier versions of the manuscript. R. Chetelat and the C. M. Rick Tomato Genetics Resource Center (University of California, Davis) kindly provided seed stocks of the introgression lines. L.C.M. was supported by a postdoctoral research fellowship from the Center for Population Biology, UC Davis.
References
- Alpert, K. B., S. Grandillo and S. D. Tanksley, 1995. Fw-2.2—a major QTL controlling fruit weight is common to both red-fruited and green-fruited tomato species. Theor. Appl. Genet. 91: 994–1000. [DOI] [PubMed] [Google Scholar]
- Arnqvist, G., M. Edvardsson, U. Friberg and T. Nilsson, 2000. Sexual conflict promotes speciation in insects. Proc. Natl. Acad. Sci. USA 97: 10460–10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner, M., 1989 Drosophila. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Barbash, D. A., D. F. Siino, A. M. Tarone and J. Roote, 2003. A rapidly evolving MYB-related protein causes species isolation in Drosophila. Proc. Natl. Acad. Sci. USA 100: 5302–5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton, N. H., and B. Charlesworth, 1984. Genetic revolutions, founder effects, and speciation. Annu. Rev. Ecol. Syst. 15: 133–164. [Google Scholar]
- Bernacchi, D., and S. D. Tanksley, 1997. An interspecific backcross of Lycopersicon esculentum × L. hirsutum: linkage analysis and a QTL study of sexual compatibility factors and floral traits. Genetics 147: 861–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernacchi, D., T. Beck-Bunn, D. Emmatty, Y. Eshed, S. Inai et al., 1998. a Advanced backcross QTL analysis of tomato. II. Evaluation of near-isogenic lines carrying single-donor introgressions for desirable wild QTL-alleles derived from Lycopersicon hirsutum and L. pimpinellifolium. Theor. Appl. Genet. 97: 170–180. [Google Scholar]
- Bernacchi, D., T. Beck-Bunn, Y. Eshed, J. Lopez, V. Petiard et al., 1998. b Advanced backcross QTL analysis in tomato. I. Identification of QTLs for traits of agronomic importance from Lycopersicon hirsutum. Theor. Appl. Genet. 97: 381–397. [Google Scholar]
- Bradshaw, H. D., and D. W. Schemske, 2003. Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature 426: 176–178. [DOI] [PubMed] [Google Scholar]
- Bradshaw, H. D., S. M. Wilbert, K. G. Otto and D. W. Schemske, 1995. Genetic-mapping of floral traits associated with reproductive isolation in monkeyflowers (Mimulus). Nature 376: 762–765. [Google Scholar]
- Brink, R. A., and D. C. Cooper, 1947. The endosperm in seed development. Bot. Rev. 13: 423–541. [Google Scholar]
- Bull, J. J., 1983 Evolution of Sex Determining Mechanisms. Benjamin Cummings, Reading, MA.
- Coyne, J. A., 1992. Genetics and speciation. Nature 355: 511–515. [DOI] [PubMed] [Google Scholar]
- Coyne, J. A., and H. A. Orr, 1989. a Patterns of speciation in Drosophila. Evolution 43: 362–381. [DOI] [PubMed] [Google Scholar]
- Coyne, J. A., and H. A. Orr, 1989b Two rules of speciation, pp. 180–207 in Speciation and Its Consequences, edited by D. Otte and J. A. Endler. Sinauer, Sunderland, MA.
- Coyne, J. A., S. Simeonidis and P. Rooney, 1998. Relative paucity of genes causing inviability in hybrids between Drosophila melanogaster and D. simulans. Genetics 150: 1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow, J. F., and M. Kimura, 1970 An Introduction to Population Genetics Theory. Harper & Row, New York.
- Davis, A. W., and C.-I Wu, 1996. The broom of the sorcerer's apprentice: the fine structure of a chromosomal region causing reproductive isolation between two sibling species of Drosophila. Genetics 143: 1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nettancourt, D., 2001 Incompatibility and Incongruity in Wild and Cultivated Plants. Springer, Berlin.
- Dobzhansky, T., 1936. Studies on hybrid sterility II. Localization of sterility factors in Drosophila pseudoobscura hybrids. Genetics 21: 113–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky, T., 1951 Genetics and the Origin of Species. Columbia University Press, New York.
- Doganlar, S., A. Frary, H. M. Ku and S. D. Tanksley, 2002. Mapping quantitative trait loci in inbred backcross lines of Lycopersicon pimpinellifolium (LA1589). Genome 45: 1189–1202. [DOI] [PubMed] [Google Scholar]
- Doi, K., K. Taguchi and A. Yoshimura, 1999. RFLP mapping of S20 and S21 for F1 pollen semi-sterility found in backcross progeny of Oryza sativa and O. glaberrima. Rice Genet. Newsl. 16: 65–67. [Google Scholar]
- Dunnett, C. W., 1955. A multiple comparison procedure for comparing several treatments with a control. J. Am. Stat. Assoc. 50: 1096–1121. [Google Scholar]
- Ehlenfeldt, M. K., and R. Ortiz, 1995. Evidence on the nature and origins of endosperm dosage requirements in Solanum and other angiosperm genera. Sex. Plant Reprod. 8: 189–196. [Google Scholar]
- El-Assal, S. E. D., C. Alonso-Blanco, A. J. M. Peeters, V. Raz and M. Koornneef, 2001. A QTL for flowering time in Arabidopsis reveals a novel allele of CRY2. Nat. Genet. 29: 435–440. [DOI] [PubMed] [Google Scholar]
- Eshed, Y., and D. Zamir, 1995. An introgression line population of Lycopersicon pennellii in the cultivated tomato enables the identification and fine mapping of yield-associated QTL. Genetics 141: 1147–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshed, Y., and D. Zamir, 1996. Less-than-additive epistatic interactions of quantitative trait loci in tomato. Genetics 143: 1807–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman, L., and J. H. Willis, 2001. Evidence for Dobzhansky-Muller incompatibilities contributing to the sterility of hybrids between Mimulus guttatus and M. nasutus. Evolution 55: 1932–1942. [DOI] [PubMed] [Google Scholar]
- Frary, A., and S. D. Tanksley, 2001 The molecular map of tomato, pp. 405–420 in DNA Based Markers in Plants, edited by R. L. Philips and I. K. Vasil. Kluwer Academic, Dordrecht, The Netherlands.
- Frary, A., T. C. Nesbitt, S. Grandillo, E. Van Der Knaap, B. Cong et al., 2000. fw2.2: a quantitative trait locus key to the evolution of tomato fruit size. Science 289: 85–88. [DOI] [PubMed] [Google Scholar]
- Fridman, E., T. Pleban and D. Zamir, 2000. A recombination hotspot delimits a wild-species quantitative trait locus for tomato sugar content to 484 bp within an invertase gene. Proc. Natl. Acad. Sci. USA 97: 4718–4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, V., 1963 The Origin of Adaptations. Columbia University Press, New York.
- Gorman, S. W., and S. Mccormick, 1997. Male sterility in tomato. Crit. Rev. Plant Sci. 16: 31–53. [Google Scholar]
- Gottlieb, L. D., 1984. Genetics and morphological evolution in plants. Am. Nat. 123: 681–709. [Google Scholar]
- Hartl, D. L., and A. G. Clark, 1989 Principles of Population Genetics. Sinauer Associates, Sunderland, MA.
- Harushima, Y., M. Nakagahra, M. Yano, T. Sasaki and N. Kurata, 2001. A genome-wide survey of reproductive barriers in an intraspecific hybrid. Genetics 159: 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harushima, Y., M. Nakagahra, M. Yano, T. Sasaki and N. Kurata, 2002. Diverse variation of reproductive barriers in three intraspecific rice crosses. Genetics 160: 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges, S. A., J. B. Whittall, M. Fulton and J. Y. Yang, 2002. Genetics of floral traits influencing reproductive isolation between Aquilegia formosa and Aquilegia pubescens. Am. Nat. 159: S51–S60. [DOI] [PubMed] [Google Scholar]
- Hogenboom, N. G., 1972. Breaking breeding barriers in Lycopersicon. I. The genus Lycopersicon, its breeding barriers and the importance of breaking those barriers. Euphytica 21: 221–227. [Google Scholar]
- Hollocher, H., and C.-I Wu, 1996. The genetics of reproductive isolation in the Drosophila simulans clade: X vs. autosomal effects and male vs. female effects. Genetics 143: 1243–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehashi, H., and H. Araki, 1988. Multiple alleles controlling F1 sterility in remote crosses of rice (Oryza sativa). Jpn. J. Breed. 38: 283–291. [Google Scholar]
- Johnston, S. A., T. P. N. Den Nijs, S. J. Peloquin and R. E. Hanneman, 1980. The significance of genic balance to endosperm development in interspecific crosses. Theor. Appl. Genet. 57: 5–9. [DOI] [PubMed] [Google Scholar]
- Katsiotis, A., R. E. Hanneman and R. A. Forsberg, 1995. Endosperm balance number and the polar-nuclear activation hypothesis for endosperm development in interspecific crosses of Solanaceae and Gramineae respectively. Theor. Appl. Genet. 91: 848–855. [DOI] [PubMed] [Google Scholar]
- Kearns, C. A., and D. W. Inouye, 1993 Techniques for Pollination Biologists. University of Colorado Press, Niwot, CO.
- Kim, S. C., and L. H. Rieseberg, 1999. Genetic architecture of species differences in annual sunflowers: implications for adaptive trait introgression. Genetics 153: 965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. C., and L. H. Rieseberg, 2001. The contribution of epistasis to species differences in annual sunflowers. Mol. Ecol. 10: 683–690. [DOI] [PubMed] [Google Scholar]
- Kondo, K., M. Yamamoto, R. Itahashi, T. Sato, H. Egashira et al., 2002. Insights into the evolution of self-compatibility in Lycopersicon from a study of stylar factors. Plant J. 30: 143–153. [DOI] [PubMed] [Google Scholar]
- Kubo, T., M. Eguchi and A. Yoshimura, 2001. A new gene for F1 pollen sterility located on chromosome 12 in Japonica/Indica cross of rice. Rice Genet. Newsl. 18: 54. [Google Scholar]
- Laurie, C. C., 1997. The weaker sex is heterogametic: 75 years of Haldane's rule. Genetics 147: 937–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester, R. N., and J. H. Kang, 1998. Embryo and endosperm function and failure in Solanum species and hybrids. Ann. Bot. 82: 445–453. [Google Scholar]
- Levin, D. A., 2000 The Origin, Expansion, and Demise of Plant Species. Oxford University Press, Oxford.
- Li, Z. K., S. R. M. Pinson, A. H. Paterson, W. D. Park and J. W. Stansel, 1997. Genetics of hybrid sterility and hybrid breakdown in an intersubspecific rice (Oryza sativa L.) population. Genetics 145: 1139–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. S., L. H. Zhu, J. S. Sun and Y. Chen, 2001. Mapping QTLs for defective female gametophyte development in an inter-subspecific cross in Oryza sativa L. Theor. Appl. Genet. 102: 1243–1251. [Google Scholar]
- Lu, J., W. H. Li and C.-I Wu, 2003. Comment on “Chromosomal speciation and molecular divergence—accelerated evolution in rearranged chromosomes”. Science 302: 988b. [DOI] [PubMed] [Google Scholar]
- Marin, I., 1996. Genetic architecture of autosome-mediated hybrid male sterility in Drosophila. Genetics 142: 1169–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, F. W., 1962. Distribution and interrelationships of incompatibility barriers in the Lycopersicon hirsutum Humb. and Bonpl. complex. Evolution 17: 519–528. [Google Scholar]
- Masuelli, R. W., 2001. Mitotic-cycle time and the development of embryo and endosperm in compatible and incompatible crosses in tuber-bearing Solanum species. Genome 44: 426–431. [DOI] [PubMed] [Google Scholar]
- Matsubara, K., T. Khin and Y. Sano, 2003. A gene block causing cross-incompatibility hidden in wild and cultivated rice. Genetics 165: 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson, T. C., 2003. Sexual isolation evolves faster than hybrid inviability in a diverse and sexually dimorphic genus of fish (Percidae: Etheostoma). Evolution 57: 317–327. [DOI] [PubMed] [Google Scholar]
- Miller, J. C., and S. D. Tanksley, 1990. RFLP analysis of phylogenetic relationships and genetic variation in the genus Lycopersicon. Theor. Appl. Genet. 80: 437–448. [DOI] [PubMed] [Google Scholar]
- Monforte, A. J., and S. D. Tanksley, 2000. Development of a set of near isogenic and backcross recombinant inbred lines containing most of the Lycopersicon hirsutum genome in a L. esculentum genetic background: a tool for gene mapping and gene discovery. Genome 43: 803–813. [PubMed] [Google Scholar]
- Moyle, L. C., M. S. Olson and P. Tiffin, 2004. Patterns of reproductive isolation in three angiosperm genera. Evolution 58: 1195–1208. [DOI] [PubMed] [Google Scholar]
- Muller, H. J., 1939. Reversibility in evolution considered from the standpoint of genetics. Biol. Rev. 14: 185–268. [Google Scholar]
- Myburg, A. A., C. Vogl, A. R. Griffin, R. R. Sederoff and R. W. Whetten, 2004. Genetics of postzygotic isolation in eucalyptus: whole-genome analysis of barriers to introgression in a wide interspecific cross of Eucalyptus grandis and E. globulus. Genetics 166: 1405–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro, A., and N. H. Barton, 2003. Accumulating postzygotic isolation genes in parapatry: a new twist on chromosomal speciation. Evolution 57: 447–459. [DOI] [PubMed] [Google Scholar]
- Naveira, H. F., 1992. Location of X-linked polygenic effects causing sterility in male hybrids of Drosophila simulans and D. mauritiana. Heredity 68: 211–217. [DOI] [PubMed] [Google Scholar]
- Naveira, H. F., and A. Fontdevila, 1986. The evolutionary history of Drosophila buzzatii. 12. The genetic basis of sterility in hybrids between Drosophila buzzatii and its sibling D. serido from Argentina. Genetics 114: 841–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveira, H. F., and X. R. Maside, 1998 The genetics of hybrid male sterility in Drosophila, pp. 330–338 in Endless Forms: Species and Speciation, edited by D. J. Howard and S. W. Berlocher. Oxford University Press, New York.
- Nesbitt, T. C., and S. D. Tanksley, 2002. Comparative sequencing in the genus Lycopersicon: implications for the evolution of fruit size in the domestication of cultivated tomatoes. Genetics 162: 365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor, M. A. F., K. L. Grams, L. A. Bertucci and J. Reiland, 2001. Chromosomal inversions and the reproductive isolation of species. Proc. Natl. Acad. Sci. USA 98: 12084–12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr, H. A., and J. A. Coyne, 1992. The genetics of adaptation—a reassessment. Am. Nat. 140: 725–742. [DOI] [PubMed] [Google Scholar]
- Osterberg, M. K., O. Shavorskaya, M. Lascoux and U. Lagercrantz, 2002. Naturally occurring indel variation in the Brassica nigra COL1 gene is associated with variation in flowering time. Genetics 161: 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paran, I., and D. Zamir, 2003. Quantitative traits in plants: beyond the QTL. Trends Genet. 19: 303–306. [DOI] [PubMed] [Google Scholar]
- Peralta, I. E., and D. M. Spooner, 2001. Granule-bound starch synthase (GBSSI) gene phylogeny of wild tomatoes (Solanum L. section Lycopersicon Mill. Wettst. subsection Lycopersicon). Am. J. Bot. 88: 1888–1902. [PubMed] [Google Scholar]
- Pillen, K., O. Pineda, C. B. Lewis and S. D. Tanksley, 1996 Status of genome mapping tools in the taxon Solanaceae, pp. 281–308 in Genome Mapping in Plants, edited by A. H. Paterson. R. G. Landes, Austin, TX.
- Presgraves, D. C., 2002. Patterns of postzygotic isolation in Lepidoptera. Evolution 56: 1168–1183. [DOI] [PubMed] [Google Scholar]
- Presgraves, D. C., 2003. A fine-scale genetic analysis of hybrid incompatibilities in Drosophila. Genetics 163: 955–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presgraves, D. C., and H. A. Orr, 1998. Haldane's rule in taxa lacking a hemizygous X. Science 282: 952–954. [DOI] [PubMed] [Google Scholar]
- Presgraves, D. C., L. Balagopalan, S. M. Abmayr and H. A. Orr, 2003. Adaptive evolution drives divergence of a hybrid inviability gene between two species of Drosophila. Nature 423: 715–719. [DOI] [PubMed] [Google Scholar]
- Price, T. D., and M. M. Bouvier, 2002. The evolution of F1 postzygotic incompatibilities in birds. Evolution 56: 2083–2089. [PubMed] [Google Scholar]
- Quiros, C. F., 1991 Lycopersicon cytogenetics, pp. 67–81 in Chromosome Engineering in Plants: Genetics, Breeding, Evolution, edited by P. K. Gupta and T. Tsuchiya. Elsevier, Amsterdam.
- Remington, D. L., and M. D. Purugganan, 2003. Candidate genes, quantitative trait loci, and functional trait evolution in plants. Int. J. Plant Sci. 164: S7–S20. [Google Scholar]
- Rick, C. M., 1979 Biosystematic studies in Lycopersicon and closely related species of Solanum, pp. 667–679 in The Biology and Taxonomy of the Solanaceae, edited by J. G. Hawkes, R. N. Lester and A. D. Skelding.
- Rick, C. M., 1995 Tomato, pp. 452–457 in Evolution of Crop Plants, edited by J. Smartt and N. W. Simmonds. Longman Scientific & Technical, London.
- Rick, C. M., J. F. Fobes and S. D. Tanksley, 1979. Evolution of mating systems in Lycopersicon hirsutum as deduced from genetic variation in electrophoretic and morphological characters. Plant Syst. Evol. 132: 279–298. [Google Scholar]
- Rieseberg, L. H., 2001. Chromosomal rearrangements and speciation. Trends Ecol. Evol. 16: 351–358. [DOI] [PubMed] [Google Scholar]
- Rieseberg, L. H., S. J. E. Baird and K. A. Gardner, 2000. Hybridization, introgression, and linkage evolution. Plant Mol. Biol. 42: 205–224. [PubMed] [Google Scholar]
- Sano, Y., 1986 International Rice Genetics Symposium, pp. 109–118. International Rice Research Institute, Manila, Philippines.
- Sasa, M. M., P. T. Chippindale and N. A. Johnson, 1998. Patterns of postzygotic isolation in frogs. Evolution 52: 1811–1820. [DOI] [PubMed] [Google Scholar]
- Silva, R. F., R. B. Koch and E. L. Moore, 1982. Effect of extraction procedures on tomato (Lycopersicon lycopersicum) seed germination and vigour. Seed Sci. Technol. 10: 187–191. [Google Scholar]
- Slotman, M., A. Della Torre and J. R. Powell, 2004. The genetics of inviability and male sterility in hybrids between Anopheles gambiae and Anopheles arabiensis. Genetics 167: 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrizal, Y., Y. Matsuzaki and A. Yoshimura, 2002. Mapping of pollen semi-sterility gene, S28(t), on rice chromosome 4. Rice Genet. Newsl. 19: 80–81. [Google Scholar]
- Stebbins, G. L., 1950 Variation and Evolution in Plants. Columbia University Press, New York.
- Stephan, W., and C. H. Langley, 1998. DNA polymorphism in Lycopersicon and crossing-over per physical length. Genetics 150: 1585–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, M. A., and C. M. Rick, 1986 Genetics and breeding, pp. 33–109 in The Tomato Crop: A Scientific Basis for Improvement, edited by J. G. Atherton and J. Rudich. Chapman & Hall, London.
- Swanson, W. J., and V. D. Vacquier, 2002. Reproductive protein evolution. Annu. Rev. Ecol. Syst. 33: 161–179. [Google Scholar]
- Tanksley, S. D., M. W. Ganal, J. P. Prince, M. C. Devicente, M. W. Bonierbale et al., 1992. High-density molecular linkage maps of the tomato and potato genomes. Genetics 132: 1141–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao, Y., and D. L. Hartl, 2003. Genetic dissection of hybrid incompatibilities between Drosophila simulans and D. mauritiana. III. Heterogeneous accumulation of hybrid incompatibilities, degree of dominance, and implications for Haldane's rule. Evolution 57: 2580–2598. [DOI] [PubMed] [Google Scholar]
- Tao, Y., S. N. Chen, D. L. Hartl and C. C. Laurie, 2003. a Genetic dissection of hybrid incompatibilities between Drosophila simulans and D. mauritiana. I. Differential accumulation of hybrid male sterility effects on the X and autosomes. Genetics 164: 1383–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao, Y., Z-B. Zeng, J. Li, D. L. Hartl and C. C. Laurie, 2003. b Genetic dissection of hybrid incompatibilities between Drosophila simulans and D. mauritiana. II. Mapping hybrid male sterility loci on the third chromosome. Genetics 164: 1399–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley, S. G., P. A. Verrell and S. J. Arnold, 1990. Correspondence between sexual isolation and allozyme differentiation—a test in the salamander Desmognathus ochrophaeus. Proc. Natl. Acad. Sci. USA 87: 2715–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting, C. T., S. C. Tsaur, M. L. Wu and C.-I Wu, 1998. A rapidly evolving homeobox at the site of a hybrid sterility gene. Science 282: 1501–1504. [DOI] [PubMed] [Google Scholar]
- True, J. R., B. S. Weir and C. C. Laurie, 1996. A genome-wide survey of hybrid incompatibility factors by the introgression of marked segments of Drosophila mauritiana chromosomes into Drosophila simulans. Genetics 142: 819–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli, M., and H. A. Orr, 1995. The dominance theory of Haldane's rule. Genetics 140: 389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli, M., and H. A. Orr, 2000. Dominance, epistasis and the genetics of postzygotic isolation. Genetics 154: 1663–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walbot, V., and M. M. S. Evans, 2003. Unique features of the plant life cycle and their consequences. Nat. Rev. Genet. 4: 369–379. [DOI] [PubMed] [Google Scholar]
- Wu, C.-I, and A. W. Davis, 1993. Evolution of postmating reproductive isolation—the composite nature of Haldane's rule and its genetic bases. Am. Nat. 142: 187–212. [DOI] [PubMed] [Google Scholar]
- Wu, C.-I, and H. Hollocher, 1998 Subtle is nature: the genetics of species differentiation and speciation, pp. 339–351 in Endless Forms: Species and Speciation, edited by D. J. Howard and S. W. Berlocher. Oxford University Press, New York.
- Wu, C.-I, N. A. Johnson and M. F. Palopoli, 1996. Haldane's rule and its legacy: Why are there so many sterile males? Trends Ecol. Evol. 11: 281–284. [DOI] [PubMed] [Google Scholar]
- Zamir, D., 2001. Improving plant breeding with exotic genetic libraries. Nat. Rev. Genet. 2: 983–989. [DOI] [PubMed] [Google Scholar]
- Zar, J. H., 1984 Biostatistical Analysis. Prentice Hall, Englewood Cliffs, NJ.