Abstract
Recently, degradation of all existing epimers of the complexing agent iminodisuccinate (IDS) in the bacterial strain Agrobacterium tumefaciens BY6 was proven to depend on an epimerase and a C-N lyase (Cokesa et al., Appl. Environ. Microbiol. 70:3941-3947, 2004). In the bacterial strain Ralstonia sp. strain SLRS7, a corresponding C-N lyase is responsible for the initial degradation step (Cokesa et al., Biodegradation 15:229-239, 2004). The ite gene, encoding the IDS-transforming epimerase, and the genes iclB and iclS, encoding the IDS-converting BY6-lyase and SLRS7-lyase, respectively, were cloned and sequenced. The epimerase gene encodes a protein with a predicted subunit molecular mass of 47.6 kDa. The highest degree of epimerase amino acid sequence identities was found with proteins of unknown function, indicating a novel protein. For the lyases, the deduced amino acid sequences show high similarity to enzymes of the fumarase II family. A classification into a new subfamily within the enzyme family is proposed. The subunit molecular masses of the lyases were calculated to be 54.4 and 54.7 kDa, respectively. In Agrobacterium tumefaciens BY6, the ite gene was on an approximately 180-kb circular plasmid, whereas the iclB gene was chromosomal like the corresponding iclS gene in Ralstonia sp. strain SLRS7. Heterologous expression in Escherichia coli and subsequent purification revealed recombinant enzymes with in vitro activity similar to that of the corresponding enzymes from the wild-type strains.
Sodium iminodisuccinate (IDS) is a widely used synthetic, medium-strong chelator belonging to the group of aminopolycarboxylates. IDS can be used as a substitute for EDTA, which is not biodegradable according to OECD tests 301A, 301F, and 302B (19). In contrast, all three epimers of IDS (R,S-, S,S-, and R,R-IDS), are readily biodegradable (9).
The bacterial strains Ralstonia sp. strain SLRS7 and Agrobacterium tumefaciens BY6 grow on IDS as the sole source of carbon and nitrogen. Cofactor-independent C-N lyases catalyze the cleavage of IDS, generating d-aspartic acid and fumaric acid from R,S-IDS as well as l-aspartic acid and fumaric acid from S,S-IDS (9, 10). R,R-IDS metabolism is preceded by epimerization. A cofactor-independent epimerase from A. tumefaciens BY6 catalyzes the conversion of all three epimers into each other (9). For the lyases and the epimerase, the natural function is still unknown.
Here, we report sequences, cloning, recombinant expression, and purification of the IDS-converting enzymes.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
The isolation and growth conditions of A. tumefaciens BY6 and of Ralstonia sp. strain SLRS7 have been reported previously (9, 10). Escherichia coli strains were grown aerobically in Luria-Bertani (LB) or 2× tryptone-yeast extract medium (1) supplemented with final concentrations of 100 μg ml−1 ampicillin, 25 μg ml−1 chloramphenicol, and 50 μg ml−1 kanamycin according to the requirements. A list of strains and plasmids used and constructed is given in Table 1.
TABLE 1.
List of plasmids and strains
| Plasmid or strain | Relevant characteristics or genotype | Source or reference |
|---|---|---|
| Plasmids | ||
| pBluescript II SK(+) | Derived from pUC; Apr PT7 PT3 PlaclacZ′; f1(+) origin; pUC origin | Stratagene |
| pET-28a(+) | Derived from pBR322; Kmr PT7 PlaclacI; pBR origin | Novagen |
| pJoe4036 | Derived from pBTac1; Apr PT7 PrhalacZ′ rrnB ColE1 cer | J. Altenbuchner, University of Stuttgart, personal communication |
| pEBS1.0 | Apr; 1.0-kb ite PCR product in a T-tailed EcoRV site of pBluescript II SK(+) | This study |
| pEBS5.0 | Apr; 5.0-kb XhoI fragment carrying ite in pBluescript II SK(+) | This study |
| pEETN | Kmr; ite in pET-28a(+) between NdeI and HindIII, N-terminal His6 tag | This study |
| pBBS0.6 | Apr; 0.6-kb iclB PCR product in a T-tailed EcoRV site of pBluescript II SK(+) | This study |
| pBBS3.7 | Apr; 3.7-kb BamHI fragment carrying iclB in pBluescript II SK(+) | This study |
| pBC | Apr; iclB in pJoe4036 between NdeI and BamHI, C-terminal His6 tag | This study |
| pSBS0.5 | Apr; 0.5-kb iclS PCR product in a T-tailed EcoRV site of pBluescript II SK(+) | This study |
| pSBS2.6 | Apr; 2.6-kb SalI fragment carrying iclS in pBluescript II SK(+) | This study |
| pSC | Apr; iclS in pJoe4036 between NdeI and BamHI, C-terminal His6 tag | This study |
| E. coli strains | ||
| DH5α | supE44 ΔlacU169 (φ80dlacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 11 |
| JM109 | recA1 supE44 endA1 hsdR17 gyrA96 relA1 thiΔ(lac-proAB) | 32 |
| Rosetta 2(DE3)pLysS | F−ompT hsdSB(rB− mB−) gal dcm (DE3) pLysSRARE2 (Cmr) | Novagen |
Protein purification from wild-type strains.
The purification of IDS-epimerase and IDS-lyase has been described previously (9, 10). IDS-lyase from Ralstonia sp. strain SLRS7 was purified with slight modifications of the original protocol. An initial (NH4)2SO4 (40% [wt/vol]) precipitation of the lyase was applied. As an additional purification step, hydroxyapatite chromatography was performed with a Bio-Scale cHT5-1 (Bio-Rad Laboratories, Germany) column, using a linear gradient comprising 2.5 mM potassium phosphate, pH 7.5 (buffer A), and 500 mM potassium phosphate, pH 7.5 (buffer B), with +0.4% (vol/vol) buffer B per ml. Elution of IDS-lyase started with 12% (vol/vol) buffer B. A specific activity of up to 10 μmol IDS min−1 mg−1 was obtained for SLRS7-lyase.
Protein cleavage, peptide separation, and sequencing.
Trypsin digestion of 200 μg protein was based on the method of Stone et al. (24). Separation of tryptic peptides of epimerase and SLRS7-lyase by reversed-phase high-pressure liquid chromatography was performed using a 250- by 4-mm Grom-Sil ODS-5 column (where ODS is octyldecyl silane) (Grom, Germany) and an aqueous solution of 0.1% (vol/vol) trifluoroacetic acid (solvent A) and 0.085% (vol/vol) trifluoroacetic acid in 80% (vol/vol) acetonitrile (solvent B). The following gradients were applied: 0 to 7.5 ml, 7% B; 7.5 to 57.5 ml, 7 to 50% B; and 57.5 to 77.5 ml, 50 to 98% B (0.5 ml min−1; detection at 210 nm). The BY6-lyase fragments were fractionated using a 100- by 2-mm TSK-Gel Super-ODS column (Tosoh Bioscience, Germany) with the following gradients: 0 to 3 ml, 7% B; 3 to 18 ml, 7 to 50% B; and 18 to 24 ml, 50 to 98% B (0.2 ml min−1; 210 nm). Amino acid sequences were determined by automated Edman degradation by Prosequenz Bioanalytik (Germany).
Isolation of genomic and plasmid DNA.
For the preparation of genomic DNA, bacterial cells were incubated at 37°C for 2 h in Tris-EDTA buffer (10 mM Tris-HCl, 1 mM sodium EDTA, pH 8.0) with 100 μg ml−1 proteinase K (Roth, Germany) and 0.3 to 0.5% (wt/vol) sodium dodecyl sulfate (SDS). Purification of DNA was achieved by treatment with RNase A (ICN Biomedicals, Germany), phenol-chloroform extraction, and subsequent ethanol precipitation. Cells of A. tumefaciens BY6 were sometimes pretreated by incubation at 37°C for 2 h in 50 mM Tris-HCl (pH 8.0) with 1 mg lysozyme (Fluka, Switzerland) per ml. Small-scale preparations of plasmid DNA from E. coli DH5α and JM109 were performed with different commercially available preparation kits.
DNA manipulation techniques.
Restriction digestions, electrophoresis, isolations, purifications, precipitations, and ligations were performed by standard procedures (1, 13, 16, 18, 20). Transformation of E. coli was achieved based on the methods of Inoue et al. (15) and Chung et al. (8) or by electroporation with a Gene Pulser II system (Bio-Rad Laboratories, Germany).
Gene fragment amplification.
Oligonucleotides were custom synthesized according to known parts of the amino acid sequences. Fragments of ite, iclB, and iclS were amplified by PCR, cloned into T-vectors (17), and used to transform competent E. coli cells.
Southern blotting and hybridization procedures.
Digested genomic DNA was separated by agarose gel electrophoresis based on a method described by Mülhardt (18) and transferred to a Biodyne P membrane according to the method of Southern (23). The target fragments were detected by hybridization with gene-specific probes created by labeling of gene fragments with a digoxigenin DNA labeling and detection kit (Boehringer Mannheim, Germany). All labeling, hybridization, and detection procedures were performed as described in the Boehringer manual (3).
Cloning of genomic DNA fragments and identification of clones.
Genomic DNA fragments that corresponded in size to fragments identified by hybridization were excised and eluted from an agarose gel and ligated into pBluescript II SK(+). After transformation of the ligation mixture into E. coli, clones containing desired fragments were identified by colony hybridization based on a method described in the Boehringer manual (3).
DNA sequencing and sequence analyses.
The DNA sequences were determined in-house or by a commercial sequencing service. Sequence analyses were performed using Internet resources (12, 28; also http://www.ebi.ac.uk/services/) and the DNASTAR software package (DNASTAR).
Gene localization.
Separation of chromosomal DNA and megaplasmids was based on the method of Barton et al. (2). No RNase A or lysozyme was mixed into the melted agarose. Solidified gel plugs were incubated for 2.5 to 18 h at 37°C in EC buffer (2) with 20 to 40 μg ml−1 RNase A and 2 to 10 mg ml−1 lysozyme. In order to convert plasmids into linear molecules, some samples were incubated with 1 U nuclease S1 (MBI Fermentas, Germany) for 5 min at 37°C. The agarose plugs were used for contour-clamped homogeneous electric-field electrophoresis (7) in a contour-clamped homogeneous electric-field mapper system (Bio-Rad Laboratories, Germany). The agarose gels were run as described by Barton et al. (2) or with pulse times of 7 to 24 s and a linear ramp of 26 h. An alkaline method based on that of Ausubel et al. (1) was performed to blot the DNA on a Biodyne P membrane (Pall, Germany).
Cloning, heterologous expression, and protein purification.
The genes ite, iclB, and iclS were amplified by PCR using Pwo polymerase (Peqlab Biotechnologie, Germany) and primers provided with appropriate restriction sites (Table 2). Amplified DNA was cloned into expression vectors to produce pEETN, pBC, and pSC (Table 1). The sequence-verified final constructs encoded fusion proteins consisting of the full-length IDS-converting enzyme and an additional tag with the N-terminal sequence MGSSHHHHHHSSGLVPRGSH [pET-28a(+) recombinant (Table 1)] or the C-terminal sequence GSHHHHHH (pJoe4036 recombinant [Table 1]). Recombinant proteins were expressed in Rosetta 2(DE3)pLysS transformants. Cells were cultivated at 37°C to an optical density at 600 nm of 0.4 to 0.8. Growth temperature was reduced to 30°C, and expression was induced by addition of isopropyl-β-d-thiogalactopyranoside to a final concentration of 1 mM. Cells were harvested after 3 to 6 h at an optical density at 600 nm of 2 to 6.
TABLE 2.
Cloning primers for heterologous expression
| Template | Oligonucleotide sequence (5′-3′)a | Restriction site |
|---|---|---|
| ite | AAGGTTCCATATGTTCACCACAAAATT AGCCGAAAAGGTC | NdeI |
| AAGGTTCAAGCTTTTACTACGGCTGCA CCGTCTTTATACC | HindIII | |
| iclB | AAGGTTCCATATGCGTGAACGCCTTAG | NdeI |
| AAGGTTCGGATCCTGCCTCCACCGACTC | BamHI | |
| iclS | AAGGTTCCATATGGAATCGAAAGTCAG | NdeI |
| AAGGTGGATCCGGCCGCTGCCAGG | BamHI |
Restriction sites are underlined.
After resuspension in 50 mM Tris-HCl (pH 8.0)-300 mM NaCl-0 to 10 mM imidazole (lysis buffer) with DNase, cells were disrupted by three passages through a French pressure cell (Aminco; SLM Instruments) at 7 MPa. Cell extract was used for protein purification after centrifugation (30 min, 130,000 × g) and filtration through 0.22-μm filters. Filtrate was loaded onto an immobilized metal affinity chromatography gravity flow column with an appropriate volume of Ni-nitrilotriacetic acid agarose resin (QIAGEN, Germany) and washed with 3 column volumes of lysis buffer containing 25 mM imidazole and 6 volumes of lysis buffer containing 50 mM imidazole. His6-tagged protein was eluted with 150 mM imidazole. Buffer exchange to the final storage buffer was performed with pD10 columns (Amersham Biosciences, Sweden), and the proteins were concentrated in a 10-ml ultrafiltration cell with a YM10 membrane (Amicon; Millipore, Germany).
Protein quantification and activity tests.
Protein concentrations were measured by the method of Bradford (4) or by the concentration/absorption correlation factor at 280 nm calculated by PROTEAN (DNASTAR). Enzyme activity was tested at room temperature in 25 mM Tris-HCl, pH 8.5, with a starting concentration of 10 mM substrate. Syntheses of argininosuccinate were performed with 10 mM fumarate and a 25-fold excess of l-arginine. Protein concentrations were 5 to 20 μg ml−1. Possible substrates were tested with 500 μg ml−1 protein in overnight approaches using at least two different batches of each purified protein. Reaction samples were stopped by acidification.
Quantification of IDS, fumarate, and argininosuccinate.
Ion pair chromatography was performed with a Purospher RP18 endcapped, 250- by 4.0-mm, high-pressure liquid chromatography column (Merck, Germany). Buffer for isocratic elution was 12.5% (vol/vol) or 20% (vol/vol) methanol in formate buffer (15 mM sodium formate-5 mM formic acid-2 mM tetrabutylammonium hydrogen sulfate). Solutions containing IDS were diluted 10:1 with 200 mM CuSO4, pH 1.3, and others 10:1 with HCl to pH 3 or below. Substances were detected at 210, 235, or 240 nm. Synthesis or degradation of argininosuccinate was determined via fumarate. The total IDS content of a solution was measured photometrically at 705 nm after conversion to copper complexes, as described previously (10).
Nucleotide sequence accession numbers.
The open reading frames encoding the IDS-transforming epimerase (ite) and IDS-converting lyases (iclB and iclS) appear in the GenBank nucleotide sequence database under the accession numbers DQ094782, DQ104097, and DQ104098, respectively. The corresponding accession numbers of the deduced amino acid sequences are AAZ57200, AAZ80811, and AAZ80812.
RESULTS
Identification of encoding genes.
N-terminal and internal sequences from the IDS-epimerase and the BY6-lyase as well as the SLRS7-lyase were used for the design of gene-specific oligonucleotides (Table 3). The respective gene fragments from the wild-type strains were amplified with the deduced primers, with lengths of 1.0 kb (epimerase), 0.6 kb (BY6-lyase), and 0.5 kb (SLRS7-lyase), and sequence verified. Southern blot analyses of digested genomic DNA with digoxigenin-labeled probes, derived from the above-mentioned gene fragments, identified a 5.0-kb XhoI fragment (epimerase), a 3.7-kb BamHI fragment (BY6-lyase), and a 2.6-kb SalI fragment (SLRS7-lyase). E. coli clones carrying the appropriate inserts in pBluescript II SK(+) were isolated by colony hybridization. The plasmids were designated pEBS5.0, pBBS3.7, and pSBS2.6, respectively.
TABLE 3.
Sequences of N termini, tryptic peptides, and deduced primers
| Peptide | Amino acid sequence (N-C)a | Deduced oligonucleotide sequence |
|---|---|---|
| IDS-epimerase | ||
| N terminus | MFTTKLAEKVVSAWKAKISQPALKAAQDb | AARGCNGCNCARGACGG (5′-3′) |
| Peptide 1 | AAQDGVIDTVAAALGGVTEH | |
| Peptide 2 | WGVNQR | |
| Peptide 3 | F(S/X)IEANIGAAL(F/L)DGEV(S/X)LA(S/X)FEIE | |
| Peptide 4 | FDMPSETTFSGTTGY(Y/X)DIVVH | GTYTCNSWNGGCATRTCGAA (3′-5′) |
| BY6-lyase | ||
| N terminus | MRERLSASPNELIVKHLIGPRLFGNLDRDFLEM(X/S)KVNb | NGMGAYTTYYTNGAAATG (5′-3′) |
| Peptide 1 | TGELLGFDGLIENSLDAVGSR | ATNARGCCRTCRAAGCC (3′-5′) |
| Peptide 2 | NTIASVVIGNAPQLTSDE | |
| Peptide 3 | FNLEHALIEDVGAD | |
| Peptide 4 | TVMTGYTHGQPGQPI | |
| SLRS7-lyase | ||
| N terminus | MESKVS(R/X)(X/R)LNEPLAK | GTSWSSCGSCGSCTSAACGAACC (5′-3′) |
| Peptide 1 | QFAYLTEVNQAHL(L/X)MXX | |
| Peptide 2 | QVTADGLGFGSLVHN(T/X)LDA(X/V)X | |
| Peptide 3 | VAQDFYVWSTPEFGLL(X/S)F(X/P)Q(X/Q)XX | |
| Peptide 4 | (X/C)(V/L)(T/S)(L/L)(D/F)(E/F)(S/L)(V/V)(A/V)(T/G) | |
| (D/S) (V/L)(E/A) (D/P)(G/K)LVADAGLSF | ||
| Peptide 5 | ASEFADAVMPGYTHLQPAQ(P/X)ITFG | ACGGSCCGATRTGSGTRGASGTCGG (3′-5′) |
Segments used for the design of oligonucleotides for PCR are underlined.
From reference 9.
Nucleotide sequences and sequence analyses.
The inserts of pEBS5.0, pBBS3.7, and pSBS2.6 were partially sequenced. The open reading frames were identified unequivocally by the presence of fragments encoding N termini and all tryptic peptides.
The IDS-epimerase gene of A. tumefaciens BY6 was named ite. The deduced product of ite is a protein of 446 amino acids per subunit. The calculated molecular mass of 47.6 kDa corresponded with the mass determined by SDS-polyacrylamide gel electrophoresis (9). An NCBI BLAST search using the deduced amino acid sequence of the epimerase revealed the highest degree of similarity to a sequence from Paracoccus pantotrophus (NCBI accession number AAZ93603.1) and a conserved hypothetical protein from Bordetella bronchiseptica RB50 (CAE32845.1). Identities of 30.2% and 32.5%, respectively, were calculated by the EBI EMBOSS::needle pairwise sequence alignment Internet resource. An NCBI conserved domain search revealed similarity to the MmgE/PrpD family (Pfam03972). However, amino acid sequence identities in pairwise sequence alignments to MmgE (P45859) or PrpD (2-methylcitrate dehydratase; NP_414868, P74840) were less then 22%.
The gene for the BY6-lyase, designated iclB, encoded a protein consisting of 500 amino acids per subunit, which corresponds to a molecular mass of 54.4 kDa. This is in good agreement with the electrophoretically determined mass (9). A BLAST search achieved the highest degree of amino acid sequence similarity to an entry for an argininosuccinate lyase from Bacillus clausii KSM-K16 (BAD64022.1), followed by a fumarate lyase from Polaromonas sp. strain JS666 (ZP_00507505.1). An EBI::needle pairwise sequence alignment revealed identities of 36.1% and 37.8%, respectively. A conserved domain search resulted in high similarities to cd01359 (argininosuccinate lyase).
The SLRS7-lyase, encoded by the iclS gene, is composed of 499 amino acids per subunit. The calculated molecular mass of 54.7 kDa agrees sufficiently with the experimentally derived data (10). The deduced amino acid sequence is 78.6% identical to ZP_00507505.1, mentioned above, and 46.4% identical to a putative argininosuccinate lyase from Rhodobacter sphaeroides 2.4.1 (ABA81509.1). Again, similarities to cd01359 were found.
The IDS-converting lyases from A. tumefaciens BY6 and Ralstonia sp. strain SLRS7 are 37.5% identical at the protein level.
Localization of the genes.
DNA from A. tumefaciens BY6 was blotted and hybridized with the labeled 1.0-kb fragment of the epimerase gene. A hybridization signal was obtained with a 180-kb plasmid. After stripping the blot membrane and rehybridizing with the 0.6-kb probe specific for the BY6-lyase gene, a signal was received with the chromosomal DNA. The 0.5-kb SLRS7-lyase probe produced a signal with the chromosomal DNA of Ralstonia sp. strain SLRS7.
Heterologous expression and protein purification.
Tagged IDS-converting enzymes were produced in E. coli and purified. A minimum of 13 mg of purified protein was obtained per liter of culture, with an apparent molecular mass of 49 kDa per subunit for the epimerase, 55 kDa per subunit for the BY6-lyase, and 56 kDa per subunit for the SLRS7-lyase as determined by SDS-polyacrylamide gel electrophoresis. These data correspond very well with the calculated molecular masses for the His6-tagged enzymes. Activities of His6-tagged enzymes were similar to those for the enzymes purified from the wild-type strains. The maximum epimerase specific activity was 15 μmol R,S-IDS min−1 mg−1. For the recombinant lyases from A. tumefaciens BY6 and Ralstonia sp. strain SLRS7, 19 μmol IDS min−1 mg−1 and 21 μmol IDS min−1 mg−1, respectively, were obtained.
Interestingly, none of the C-terminal His6-tagged lyases catalyzed formation or cleavage of argininosuccinate. For comparison, an E. coli argininosuccinate lyase with a C-terminal His6 tag (expression and purification not described) showed activity against argininosuccinate but not against IDS.
DISCUSSION
The IDS-epimerase of Agrobacterium tumefaciens BY6 has a functional similarity to amino acid racemases. This is reasonable because IDS can be classified as a chemical derivative of aspartate. However, no sequence similarity to aspartate racemases or generally to amino acid racemases was found. The deduced amino acid sequence revealed no significant similarity to any protein of known function, thus indicating a novel enzyme. Similarity to the Pfam03972 conserved domain family, of which 2-methylcitrate dehydratase (PrpD, EC 4.2.1.79) is a member, was found. Mechanistic similarities to the epimerase seem to be limited because this dehydratase catalyzes a reaction more similar to that of class II fumarases (14) and IDS-lyases. However, the stereochemically interesting PrpD reaction (5) might give us an idea of the substrate binding of polycarboxylates. Up to now, the function of the ubiquitously found Pfam03972 conserved domains is unknown, but involvement in substrate binding, catalytic function, or formation of structural motifs is feasible. The IDS-epimerase is therefore a promising candidate for further functional studies.
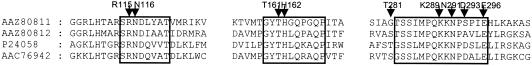
The IDS-converting lyases show sequence similarities to the fumarase II enzyme family (31). Across this family, three sequence domains show high conservation (6, 22, 26, 27). These domains are also found in the IDS-lyases (Fig. 1). Like the IDS-converting lyases (9, 10), members of the fumarase II enzyme family are homotetrameric, with a molecular mass of approximately 200 kDa (29, 31). Furthermore, there are similarities with respect to the catalyzed reactions. All reactions are reversible trans-eliminations/anti-additions at the fumaryl moiety (9, 10, 31). Within the family, the IDS-lyases are most similar to the subfamily of argininosuccinate lyases. Studies of the molecular function of argininosuccinate lyases have been performed mainly with δ-crystallin, a taxon-specific crystallin of bird and reptile eye lenses that is homologous to argininosuccinate lyase (30, 33). Crystallographic studies and mutational analyses of δ-crystallin have resulted in a model of the active site (21). The amino acid residues Asn-116, Thr-161, His-162, Thr-281, Lys-289, Asn-291, Asp-293, and Glu-296 are probably responsible for the binding of the fumaryl moiety of argininosuccinate. All of these residues, except Thr-281 and Asp-293, are conserved in the IDS-lyases (Fig. 1) and probably form the binding site for the fumaryl moiety of IDS. In contrast, conservation of those residues binding solely the amino acid moiety is 31%. Thus, the differences in conservation plus the lack of cross-reactivity of IDS-lyases and E. coli argininosuccinate lyase suggest a classification into a new subfamily within the fumarase II enzyme family.
FIG. 1.
Extracts of a CLUSTAL_X (25) alignment of duck δII-crystallin (P24058), E. coli argininosuccinate lyase (AAC76942), and the amino acid sequences AAZ80811 (BY6-lyase) and AAZ80812 (SLRS7-lyase). Three domains with high sequence similarities within the fumarase II enzyme family (6, 22, 26, 27) are boxed. Positions of δII-crystallin amino acid residues involved in binding of argininosuccinate (21) are marked with arrowheads. The figure was prepared using the program GeneDoc (http://www.psc.edu/biomed/genedoc).
The genes encoding the IDS-converting enzymes were expressed well in E. coli strains that supplied tRNAs for rare codons. This resulted in synthesis of recombinant enzymes with activity corresponding to that of the proteins of the wild-type strains. This fact unequivocally verifies the gene identifications.
The availability of two different IDS-lyases with low amino acid sequence similarity recommends these enzymes for detailed mechanistic studies. Such studies may also provide important information on the mechanistic background of the argininosuccinate lyases. For the epimerase, it is expected that further studies will provide insight into the unique epimerization reaction and the function of the ubiquitously found Pfam0397 conserved domains.
Acknowledgments
Continuous support and encouragement by H.-J. Knackmuss and G. Sprenger are gratefully acknowledged.
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 2001. Current protocols in molecular biology, vol. 1-4. John Wiley & Sons, New York, N.Y.
- 2.Barton, B. M., G. P. Hardening, and A. J. Zuccarelli. 1995. A general method for detecting and sizing large plasmids. Anal. Biochem. 226:234-240. [DOI] [PubMed] [Google Scholar]
- 3.Boehringer. 1995. DIG application manual for filter hybridization. Boehringer, Ingelheim, Germany.
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Brock, M., C. Maerker, A. Schütz, U. Völker, and W. Buckel. 2002. Oxidation of propionate to pyruvate in Escherichia coli. Involvement of methylcitrate dehydratase and aconitase. Eur. J. Biochem. 269:6184-6194. [DOI] [PubMed] [Google Scholar]
- 6.Chakraborty, A. R., A. Davidson, and P. L. Howell. 1999. Mutational analysis of amino acid residues involved in argininosuccinate lyase activity in duck δ II crystallin. Biochemistry 38:2435-2443. [DOI] [PubMed] [Google Scholar]
- 7.Chu, G., D. Vollrath, and R. W. Davis. 1986. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science 234:1582-1585. [DOI] [PubMed] [Google Scholar]
- 8.Chung, C. T., S. L. Niemela, and R. H. Miller. 1989. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc. Natl. Acad. Sci. USA 86:2172-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cokesa, Ž., H.-J. Knackmuss, and P.-G. Rieger. 2004. Biodegradation of all stereoisomers of the EDTA substitute iminodisuccinate by Agrobacterium tumefaciens BY6 requires an epimerase and a stereoselective C-N lyase. Appl. Environ. Microbiol. 70:3941-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cokesa, Ž., S. Lakner, H.-J. Knackmuss, and P.-G. Rieger. 2004. A stereoselective carbon-nitrogen lyase from Ralstonia sp. SLRS7 cleaves two of three isomers of iminodisuccinate. Biodegradation 15:229-239. [DOI] [PubMed] [Google Scholar]
- 11.Focus. 1986. New frozen competent pUC host E. coli DH5α. Focus 8.2. [Online.] http://www.lifetechnologies.com/content.cfm?pageid=63. Accessed 22 November 2005.
- 12.Gasteiger, E., C. Hoogland, A. Gattiker, S. Duvaud, M. R. Wilkins, R. D. Appel, and A. Bairoch. 2005. Protein identification and analysis tools on the ExPASy server, p. 571-607. In J. M. Walker (ed.), The proteomics protocols handbook. Humana Press, Totowa, N.J.
- 13.Heery, D. M., F. Gannon, and R. Powell. 1990. A simple method for subcloning DNA fragments from gel slices. Trends Genet. 6:173. [DOI] [PubMed] [Google Scholar]
- 14.Horswill, A. R., and J. C. Escalante-Semerena. 2001. In vitro conversion of propionate to pyruvate by Salmonella enterica enzymes: 2-methylcitrate dehydratase (PrpD) and aconitase enzymes catalyze the conversion of 2-methylcitrate to 2-methylisocitrate. Biochemistry 40:4703-4713. [DOI] [PubMed] [Google Scholar]
- 15.Inoue, H., H. Nojima, and H. Okayama. 1990. High efficiency transformation of Escherichia coli with plasmids. Gene 96:23-28. [DOI] [PubMed] [Google Scholar]
- 16.Lottspeich, F., and H. Zorbas (ed.). 1998. Bioanalytik. Spektrum, Heidelberg, Germany.
- 17.Marchuk, D., M. Drumm, A. Saulino, and F. S. Collins. 1991. Construction of T-vectors, a rapid and general system for direct cloning of unmodified PCR products. Nucleic Acids Res. 19:1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mülhardt, C. 2002. Der Experimentator: Molekularbiologie/Genomics, 3rd ed., p. 12-141. Spektrum, Heidelberg, Germany.
- 19.Rieger, P.-G., H.-M. Meier, M. Gerle, U. Vogt, T. Groth, and H.-J. Knackmuss. 2002. Xenobiotics in the environment: present and future strategies to obviate the problem of biological persistence. J. Biotechnol. 94:101-123. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 21.Sampaleanu, L. M., B. Yu, and P. L. Howell. 2002. Mutational analysis of duck δ2 crystallin and the structure of an inactive mutant with bound substrate provide insight into the enzymatic mechanism of argininosuccinate lyase. J. Biol. Chem. 277:4166-4175. [DOI] [PubMed] [Google Scholar]
- 22.Sampaleanu, L. M., P. W. Codding, Y. D. Lobsanov, M. Tsai, G. D. Smith, C. Horvatin, and P. L. Howell. 2004. Structural studies of duck δ2 crystallin mutants provide insight into the role of Thr161 and the 280s loop in catalysis. Biochem. J. 384:437-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Southern, E. M. 1975. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J. Mol. Biol. 98:503-517. [DOI] [PubMed] [Google Scholar]
- 24.Stone, K. L., M. B. LoPresti, J. M. Crawford, R. DeAngelis, and K. R. Williams. 1989. Enzymatic digestion of proteins and HPLC peptide isolation, p. 31-47. In P. T. Matsudaira (ed.), A practical guide to protein and peptide purification for microsequencing. Academic Press, San Diego, Calif.
- 25.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner, M. A., A. Simpson, R. R. McInnes, and P. L. Howell. 1997. Human argininosuccinate lyase: a structural basis for intragenic complementation. Proc. Natl. Acad. Sci. USA 94:9063-9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weaver, T. M., D. G. Levitt, M. I. Donnelly, P. P. W. Stevens, and L. J. Banaszak. 1995. The multisubunit active site of fumarase C from Escherichia coli. Nat. Struct. Biol. 2:654-662. [DOI] [PubMed] [Google Scholar]
- 28.Wheeler, D. L., D. M. Church, S. Federhen, A. E. Lash, T. L. Madden, J. U. Pontius, G. D. Schuler, L. M. Schriml, E. Sequeira, T. A. Tatusova, and L. Wagner. 2003. Database resources of the National Center for Biotechnology. Nucleic Acids Res. 31:28-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams, S. E., E. M. Woolridge, S. C. Ransom, J. A. Landro, P. C. Babbitt, and J. W. Kozarich. 1992. 3-Carboxy-cis,cis-muconate lactonizing enzyme from Pseudomonas putida is homologous to the class II fumarase family: a new reaction in the evolution of a mechanistic motif. Biochemistry 31:9768-9776. [DOI] [PubMed] [Google Scholar]
- 30.Wistow, G., and J. Piatigorsky. 1990. Gene conversion and splice-site slippage in the argininosuccinate lyases/delta-crystallins of the duck lens: members of an enzyme superfamily. Gene 96:263-270. [DOI] [PubMed] [Google Scholar]
- 31.Woods, S. A., J. S. Miles, and J. R. Guest. 1988. Sequence homologies between argininosuccinase, aspartase and fumarase: a family of structurally-related enzymes. FEMS Microbiol. Lett. 51:181-186. [Google Scholar]
- 32.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 33.Yeh, L.-S., A. Elzanowski, L. T. Hunt, and W. C. Barker. 1988. Homology of δ crystallin and argininosuccinate lyase. Comp. Biochem. Physiol. B 89:433-437. [DOI] [PubMed] [Google Scholar]