Abstract
SepB is an essential, conserved protein required for chromosomal DNA metabolism in Aspergillus nidulans. Homologs of SepB include yeast Ctf4p and human hAnd-1. Molecular and bioinformatic characterization of these proteins suggests that they act as molecular scaffolds. Furthermore, recent observations implicate the yeast family members in lagging-strand replication and the establishment of sister-chromatid cohesion. Here, we demonstrate that SepB functions in the A. nidulans DNA damage response. In particular, analysis of double mutants reveals that SepB is a member of the UvsCRAD51 epistasis group. In accord with this prediction, we show that UvsCRAD51 forms DNA-damage-induced nuclear foci in a manner that requires SepB function. We also provide evidence that implicates SepB in sister-chromatid cohesion, thereby suggesting that cohesion may play a role in regulating the localization and/or assembly of UvsCRAD51 complexes.
IN eukaryotic cells, the repair of damaged DNA and the maintenance of genome integrity are often dependent on homologous recombination (reviewed by Jackson 2002). The RecA homolog Rad51 performs an essential role in this process by searching for homologous sequences and catalyzing strand exchange (reviewed by Symington 2002). In vitro studies show that Rad51 forms filaments on single-strand DNA coated with replication protein A (Sung and Robberson 1995) in a process that requires Rad52 and other associated proteins (Sung 1997; Shinohara and Ogawa 1998). In Saccharomyces cerevisiae, Rad51 forms subnuclear foci in mitotic cells that have suffered DNA damage (Gasior et al. 2001; Caspari et al. 2002). These foci are thought to represent sites of ongoing recombination, and, consistent with the in vitro observations, their formation requires replication protein A and Rad52 (Miyazaki et al. 2004; Wang and Haber 2004). Additional studies show that the repair of DNA strand breaks by homologous recombination is affected by chromatin organization and the establishment of sister-chromatid cohesion (Hartsuiker et al. 2001; Sjogren and Nasmyth 2001; Jaskelioff et al. 2003). However, the role of these functions in modulating Rad51 localization in response to DNA damage has not yet been investigated.
The SepB/And-1 protein family encompasses homologs in organisms ranging from fungi to humans (Williams and McIntosh 2002). Notable features of these proteins include the presence of WD40 repeats in the amino-terminus and a central conserved region termed the SepB domain (Kohler et al. 1997; Williams and McIntosh 2002). On the basis of the known ability of WD40 repeats to adopt a β-propeller conformation (Smith et al. 1999), SepB/And-1 proteins are likely to serve as scaffolds that interact with multiple partners. In support of this notion, the yeast homologs S. cerevisiae Ctf4p and Schizosaccharomyces pombe Mcl1 interact with multiple proteins involved in lagging-strand replication, including DNA polymerase α, Rad27p, and Dna2p (Formosa and Nittis 1999; Williams and Mcintosh 2002). In addition, Ctf4p and Mcl1 have been implicated in the establishment of sister-chromatid cohesion during S phase, perhaps by facilitating polymerase switching (Hanna et al. 2001; Williams and McIntosh 2002). Although little is known about the metazoan And-1 proteins, the Xenopus homolog xAnd-1 was found to associate with interphase chromatin (Kohler et al. 1997).
We have previously reported that SepB is an essential protein required for faithful chromosome segregation in Aspergillus nidulans (Harris and Hamer 1995). In particular, the temperature sensitive (Ts) lethal sepB3 mutation causes several phenotypes suggestive of a defect in DNA metabolism, including increased mitotic recombination and chromosome nondisjunction and delayed progression through mitosis. In the process of characterizing genetic interactions between sepB3 and mutations affecting the A. nidulans DNA damage response, we noted that sepB3 also causes modest sensitivity to DNA-damaging agents. Here, we provide evidence that SepB functions in a Rad51-mediated pathway for the repair of DNA damage by homologous recombination. Notably, we report that SepB is required for the formation of DNA-damage-induced UvsCRAD51 foci. We also present results implicating SepB in sister-chromatid cohesion. Our observations suggest that cohesion may play a role in Rad51 localization.
MATERIALS AND METHODS
Strains, media, and reagents:
All strains used in this study are described in Table 1. Media used for the growth of A. nidulans include CM (1% dextrose, 0.2% peptone, 0.1% yeast extract, 0.1% casamino acids, nitrate salts, vitamins, and trace elements; pH 6.5), MAG (2% dextrose, 2% malt extract, 0.2% peptone, trace elements, and vitamins), YGV (2% dextrose, 0.5% yeast extract, and vitamins), and MNV (1% dextrose, 5% nitrate salts, trace elements, and vitamins; pH 6.5). Nitrate salts, trace elements, and vitamins were added as described in the appendix to Kafer (1977). Arginine (1 mm), uridine (5 mm), and uracil (10 mm) were added as needed. Media were solidified using 1.5% agar. When necessary, 0.01% Triton X-100 was added to restrict colony growth. Benomyl (BEN), methyl methanesulfonate (MMS; both Sigma-Aldrich Chemical, St. Louis), and phleomycin (PLM) D1 copper chelate chlorohydrate salt (CAYLA, Toulouse, France) were added to media at the appropriate concentration after autoclaving.
TABLE 1.
Strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| A28 | pabaA6 biA1 | FGSC |
| ASH15 | sepB3; argB2; wA2 | Lab collection |
| ASH49 | sepB3; pyrG89; pyroA1; wA2 | Lab collection |
| ASH60 | sepB3; pabaA6 yA2 | Lab collection |
| ASH208 | sepB3; pabaA6; wA2 | Lab collection |
| ASH201 | uvsB110; chaA1 | Lab collection |
| ASH202 | uvsB110; sepB3; chaA1 | Lab collection |
| ASH215 | uvsC114; wA2 | Lab collection |
| ASH218 | uvsC114; sepB3; pabaA1; wA2 | Lab collection |
| ASH383 | musN227; chaA1 | Lab collection |
| ASH380 | musN227; sepB3; chaA1 | Lab collection |
| ASH568 | uvsC114; pyrG89; wA1 | Lab collection |
| ASG15 | pSDW194-sepB::3xHA transformed into ASH15; integrated at argB2 |
Lab collection |
| ASG16 | pSDW194-sepB::3xHA transformed into ASH15; integrated at sepB3 |
Lab collection |
| ASG17 | pRG3-uvsC::FLAG transformed into ASH568; integrated at uvsC |
Lab collection |
| ASG19 | uvsC::FLAG; sepB3; wA1 | Lab collection |
| ASG70 | ΔsldA; wA2 | Lab collection |
| ASG72 | ΔsldA; sepB3; wA2 | Lab collection |
| ASG76 | ΔsldA; wA2 | Lab collection |
| ASG78 | ΔsldA; sepB3; wA2 | Lab collection |
| ASG80 | ΔsldB; yA2 | Lab collection |
| ASG82 | ΔsldB; sepB3; wA2 | Lab collection |
| ASG84 | ΔsldB; yA2 | Lab collection |
| ASG86 | ΔsldB; sepB3; yA2 | Lab collection |
FGSC, Fungal Genetics Stock Center, Department of Microbiology, University of Kansas Medical Center, Lawrence, Kansas.
Growth conditions and genetic manipulations were as described previously (Harris et al. 1994). The DNA sequences of oligonucleotide primers are available from the authors upon request. The corrected sequence of SepB, which includes N-terminal and C-terminal extensions of 70 and 59 amino acids, respectively, is deposited at NCBI (accession no. X86399.1).
Cloning of hAND-1 and complementation of sepB3:
RT-PCR was performed on human placental RNA (gift from Andrew Arnold, University of Connecticut Health Center) using the Ambion (Austin, TX) RETROscript kit. The 3.4-kb hAND-1 PCR product was cloned into pCR2.1-TOPO using the Invitrogen (San Diego) TOPO TA kit. The hAND-1 gene was subsequently cloned into pSDW194 (gift from Steven W. James, Gettysburg College), such that its expression is regulated by the ethanol-inducible alcA promoter. pSDW194-hAND1 was transformed into strain ASH15, and transformants in which the plasmid had integrated were identified by Southern blot analysis. Transformants were plated on MNV plates with 2% ethanol, 1% glycerol, or 1% dextrose as the inducing, nonrepressing, and repressing carbon source, respectively, or they were grown on MAG.
Cloning of the sepB3 mutant allele:
Genomic DNA was prepared from lyophilized mycelia obtained from the strains A28 and ASH60. The wild-type and sepB3 alleles were amplified by PCR and cloned using the TOPO TA kit. Three independent clones derived from each allele were pooled and sequenced.
Viability assays:
The viability of growing hyphae was measured as follows. For each strain tested, conidiospores were plated at ∼106 conidia/plate on MAG and allowed to form a uniform mycelial mat. Mycelial agar plugs were made by using the large end of a 14.6 cm × 5 mm Pasteur pipette and were subsequently placed on CM media containing appropriate concentrations of MMS or PLM. One plug was made for each strain and placed on the same plate (i.e., each plate contained wild-type, sepB3, single-mutant, and double-mutant plugs). Radial growth was measured at multiple time points up to 48 hr at 28° (permissive temperature) and 35° (semipermissive temperature). All growth was measured as percentage change in radial growth rate compared to the untreated CM control plate. Experiments were repeated three times.
The sensitivity of conidiospores to acute genotoxic stress was measured as follows. Dormant spores were harvested and diluted at ∼106 conidia/ml of water. They were subsequently inoculated with a DNA-damaging agent (0.001–0.025% MMS or 5–40 μg/ml PLM) and incubated at 37° for 45 min with constant agitation. Spores were washed with water and plated on CM + Triton X-100 (0.01%) plates at a concentration of ∼102 conidia or ∼103 conidia/plate. Viability was measured as percentage survival compared to untreated controls.
The effect of microtubule depolymerization on viability was tested as follows. Dormant spores were incubated for 6 hr at 37° in YGV (supplemented with 0.2% Tween 20 to prevent clumping) containing 10 μg/ml BEN. Spores were diluted and plated on CM + Triton X-100 plates, and viability measured as described above.
Protein extraction and Western blot assays:
Strain ASG16 was inoculated in YGV at ∼106 conidia/ml and grown for 18 hr at 28°. The cultures were treated with 50 mm hydroxyurea (HU) for 2 hr, 5 μg/ml benomyl for 2 hr, 1 μg/ml nitrosoguanidine for 10 min, 10 μg/ml PLM for 10 min, 0.05% MMS for 10 min, or left untreated. Mycelia were harvested via filtration and rinsed with stop buffer (Moreno et al. 1989), pressed dry between paper towels, frozen in liquid nitrogen, and lyophilized overnight. Lyophilized mycelia were crushed to a fine powder using a spatula. Protein extraction and immunoprecipitation was performed following the protocol provided with the Roche anti-HA affinity matrix. For Western blots, proteins were separated by SDS-PAGE and transferred to Immobilon-P PVDF transfer membrane (Millipore, Bedford, MA) by electroblotting. Membranes were probed with a 0.4 mg/ml solution of 12CA5 anti-HA antibody (Roche, Indianapolis) at a 1:400 dilution, or with a 0.45 mg/ml solution of mouse monoclonal M2 anti-FLAG (Sigma, St. Louis) at a 1:400 dilution. A 1/10,000 dilution of anti-mouse IgG A3562 (whole cell) alkaline phosphatase conjugate (Sigma ImmunoChemicals, St. Louis) was used as a secondary antibody. Western analysis was performed by enhanced chemiluminescent detection (Roche, Indianapolis).
Localization of SepB and UvsC:
A SepB-HA fusion protein was constructed by designing two complementary 117-bp oligonucleotides that contain the 3x-HA tag (YPYDVPDYAG) from pGTEPI (gift from Aaron Mitchell, Columbia University). The oligonucleotides were annealed using the protocol for adapter production from Life Technologies. The annealed product was subsequently cloned into the pCR2.1-TOPO vector. The HA tag was cloned in frame at the C terminus of sepB in pSDW194-SEPB. pSDW194-SEPB-HA was transformed into strain ASH15 to generate strains ASG15 and ASG16 (Table 1). In both cases, transformants displayed wild-type growth at 42°, demonstrating that the SepB-HA fusion protein was functional. For localization experiments, ASG16 was grown on coverslips in YGV for 12 hr.
A UvsC-FLAG fusion protein was constructed by PCR amplification using primers designed to amplify the entire uvsC gene with the FLAG epitope (DYKDDDDK; Hoffmann et al. 2001) added in frame to the C terminus. The uvsC-FLAG allele was cloned into the pRG3 (pUC19; pyr-4) vector, and the resulting pRG3-uvsC-FLAG plasmid was then transformed into strain ASH568 to generate ASG17 (Table 1). Transformants displayed wild-type resistance to ultraviolet (UV) irradiation and MMS, demonstrating that the UvsC-FLAG fusion protein was functional. ASG17 was crossed with ASH49 to generate ASG19, which introduces the sepB3 mutation into the uvsC-FLAG background (Table 1). For localization experiments, ASG17 and ASG19 were grown on coverslips in YGV for 12 hr. The coverslips were treated with 50 mm HU for 1 hr, 10 μg/ml PLM for 1 hr, 0.02% MMS for 1 hr, or left untreated.
Immunofluorescence was performed following a standard protocol (Harris et al. 1999) using the following primary antibodies: 12CA5 anti-HA mouse monoclonal (Roche) as a 0.4 mg/ml solution in 50 mm PIPES, 25 mm EGTA, 5 mm MgSo4 (PEM)-BSA or M2 anti-FLAG mouse monoclonal (Sigma) as a 0.45 mg/ml solution in PEM-BSA. A 1:200 dilution of FITC-conjugated anti-mouse IgG (Sigma) was used as the secondary antibody. Nuclei and cell wall were detected using Hoechst 33258 (Molecular Probes, Eugene, OR) and calcofluor white (Sigma), respectively, as previously described (Harris et al. 1994).
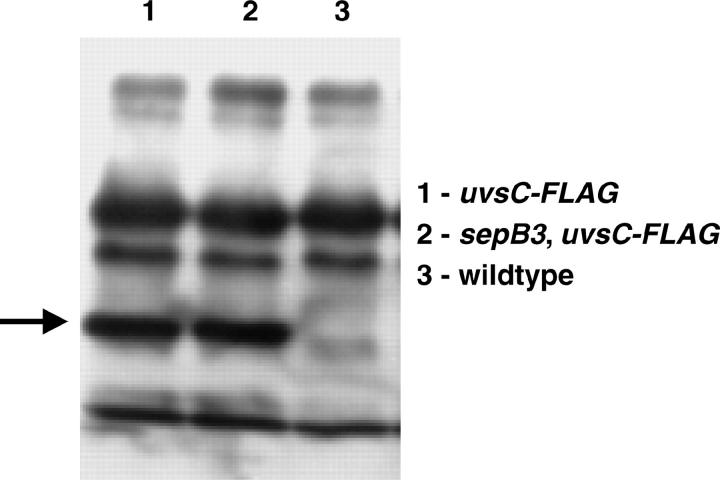
Analysis of Western blots revealed that the M2 anti-FLAG antibody detected additional bands in A. nidulans extracts (see Figure 3). Therefore, immunofluorescence experiments were also performed on a wild-type strain that does not possess the UvsC-FLAG construct (A28). These experiments show that the background cytoplasmic staining observed in uvsC-FLAG or sepB3 uvsC-FLAG hyphae is due to the additional FLAG-reactive proteins (supplemental Figure 1B at http://www.genetics.org/supplemental/). In no case was FLAG localization observed in nuclei of A28 hyphae (supplemental Figure 1B at http://www.genetics.org/supplemental/).
Figure 3.—
Expression of UvsC-FLAG in wild-type and sepB3 mutants. ASH17 (uvsC-FLAG), ASG19 (uvsC-FLAG; sepB3), and A28 (wild-type control) hyphae were incubated in YGV for 18 hr at 28°. Whole-cell extracts were precipitated with anti-FLAG agarose beads and detected by Western blot with anti-FLAG antibodies. Arrows depicts UvsC-FLAG (39 kD).
Slides were viewed using an Olympus BX51 fluorescent microscope. Images were captured with a Photometrics CoolSnap HQ CCD camera (Roper Scientific) and processed using IPLab software (Scanalytics) and Adobe PhotoShop 6.0. Confocal images were obtained with an Olympus FW500/BX61 confocal laser scanning microscope using the following laserlines: 405 nm for Hoechst 33258 and 563 nm for Cy3. Images were captured by direct acquisition with a Z step of 1–2 μm and were subsequently processed using ImageJ and Adobe PhotoShop 6.0.
RESULTS
Predicted organization of SepB/And-1 proteins:
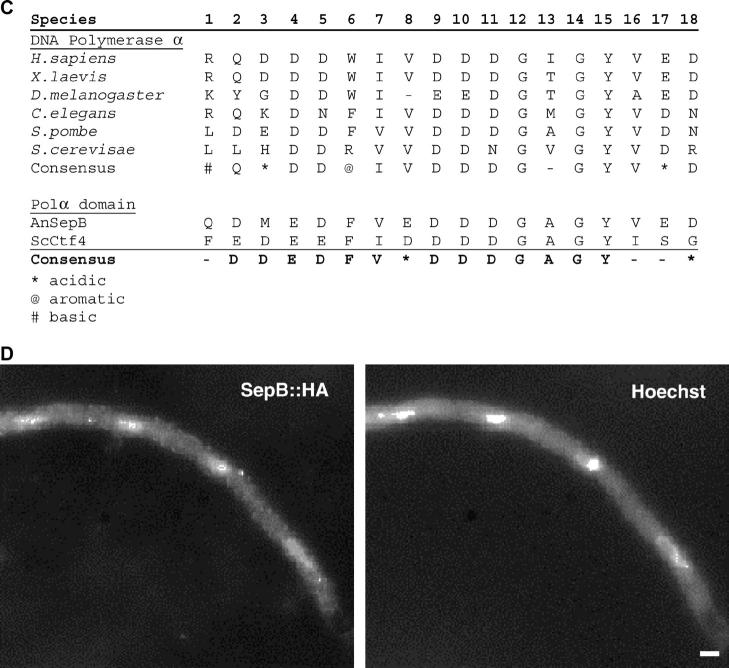
A schematic of the fungal and metazoan SepB/And-1 proteins is shown in Figure 1A. All homologs possess conserved WD40 and SepB domains. The Ts lethal sepB3 mutation (P618S) affects a conserved residue within the SepB domain (Figure 1B), emphasizing the potential functional importance of this region. Differences between the fungal and metazoan homologs include the presence of different carboxy-terminal motifs, as well as a fungal-specific region similar to the amino-terminus of the catalytic subunit of DNA polymerase α (Figure 1, A and C). To determine the extent of functional conservation between family members, we expressed hAND-1 in a sepB3 mutant. However, instead of complementation, we found that expression of hAND-1, but not sepB, exacerbated the growth defects caused by the sepB3 mutation (supplemental Table 1 at http://www.genetics.org/supplemental/). This may reflect the ability of hAnd-1 to interfere with the formation of stable SepB complexes or to cause the formation of nonfunctional complexes. This observation suggests that hAnd-1 and SepB may be able to form a heteropolymer, thereby implying that the two proteins might be functional homologs.
Figure 1.—
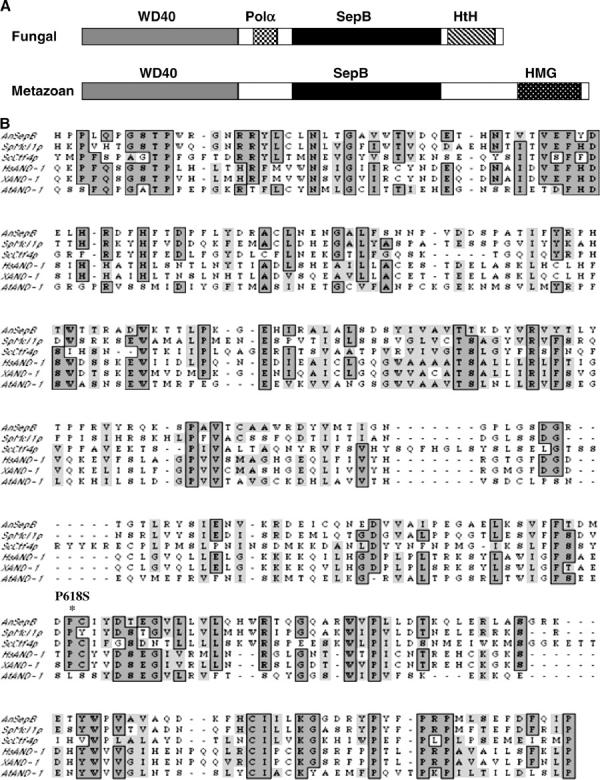
Predicted organization of the SepB/And-1 protein family. (A) A schematic of the fungal and metazoan members of the SepB/AND-1 protein family. The WD40 (shaded) and the SepB (solid) domains are conserved in all members. In contrast, the Pol α checkerboard) and the HtH (diagonal lines) domains are specific to the fungal homologs, whereas the HMG domain (stippled) is found only in the metazoan homologs. (B) The SepB Domain. The predicted protein sequence of the SepB domain from the SepB/And-1 family members AnSepB (A. nidulans; X86399.1), SpMcl1p (S. pombe; AL590605.1), ScCtf4p (S. cerevisiae; M94769.1), HsAND-1 (Homo sapiens; AJ006266.1), XAND-1 (Xenopus laevis; X98884.1), and AtAND-1 (Arabidopsis thaliana; AL138640.1) were aligned using the MacVector 7.0 software. Note that the site of the sepB3 mutation (P618S; indicated by an asterisk) is highly conserved. (C) The Pol α domain. An alignment of the 18-amino-acid Pol α domain from the catalytic subunit of DNA polymerase α and the fungal SepB homologs. A consensus sequence for the domain is shown in boldface type below the line. (D) SepB is a nuclear protein. ASG16 hyphae were grown on coverslips in YGV for 12 hr. SepB::3xHA was detected by indirect immunofluorescence using the anti-HA monoclonal antibody 12CA5. Nuclei were visualized using Hoechst 33258. Bar, 4 μm.
To examine SepB localization, a SepB-HA fusion protein was constructed and shown to complement the Ts growth defects caused by the sepB3 mutation. Like the other homologs (Kohler et al. 1997; Hanna et al. 2001; Williams and McIntosh 2002), we found that SepB localizes to the nucleus and appears to be uniformly distributed throughout the nuclear compartment (Figure 1D). The localization pattern did not change when hyphae were exposed to DNA-damaging agents (PLM, MMS) or arrested in specific cell cycle phases using either HU (S-phase arrest) or Ben (mitotic arrest).
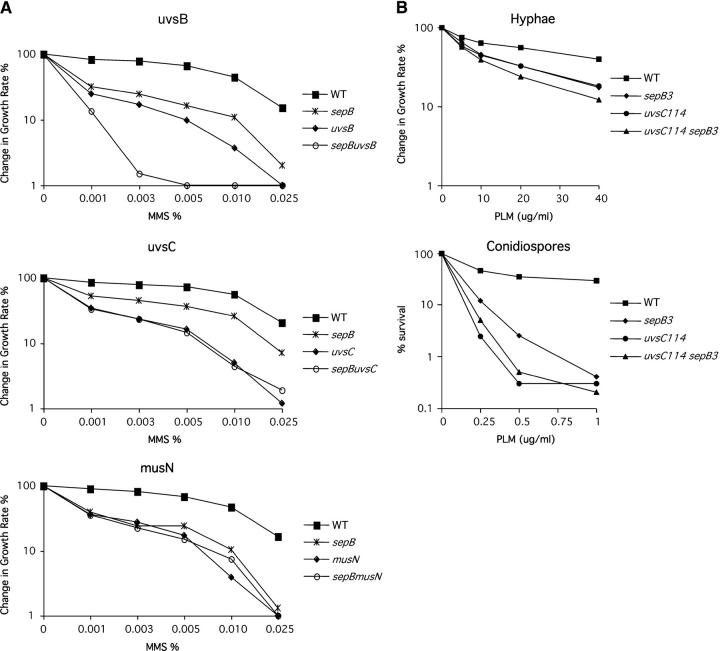
sepB3 hyphae and conidiospores display sensitivity to agents that cause DSBs:
Ctf4p and Mcl1 are each required for an uncharacterized aspect of the DNA damage response (Kouprina et al. 1992; Williams and McIntosh 2002). To determine if SepB functions in the A. nidulans DNA damage response, we used a radial colony growth assay to measure the ability of sepB3 hyphae to grow on media containing DNA-damaging agents at semipermissive temperature. These experiments revealed that growing sepB3 hyphae are approximately twofold more sensitive to PLM (20–40 μg/ml) and to MMS (0.01–0.025%) than are wild type (Figure 2). Since hyphae are composed of a relatively asynchronous population of cells, we also tested sepB3 sensitivity to DNA-damaging agents using dormant conidiospores, which are naturally synchronized in the G1 phase of the cell cycle (Bergen and Morris 1983). Spores were allowed to break dormancy at semipermissive temperature (37°) for 45 min in the presence or absence of DNA-damaging agents and were then plated at permissive temperature. Under these conditions, sepB3 mutants were extremely sensitive to PLM (Figure 2), but not to MMS (data not shown). In addition, both the mycelial and dormant spore assays demonstrated that sepB3 mutants display normal responses to UV irradiation and HU. Collectively, these observations suggest that SepB function is particularly important for the repair of PLM-induced double-strand breaks (DSBs) as cells progress through S phase. Analysis of a functional SepB-HA fusion protein expressed under control of its endogenous promoter indicated that neither its expression nor its apparent mobility is affected by exposure to PLM or other DNA-damaging agents (data not shown).
Figure 2.—
SepB is involved in the DNA damage response. (A) Viability curves for wild-type, sepB3, uvsB110, sepB3 uvsB110, musN227, sepB3 musN227, uvsC114, and sepB3 uvsC114 hyphae grown on media containing MMS at 35°. Growth was measured as percentage change in radial growth rate compared to an untreated CM control plate. (B) Viability curves for wild-type, sepB3, uvsC114, and sepB3 uvsC114 hyphae (top) or conidiospores (bottom) exposed to PLM. Hyphae were grown on media containing PLM at 35° and growth was measured as percentage change in radial growth rate compared to an untreated CM control plate. Dormant conidiospores spores were suspended in sterile water and treated with the indicated doses of PLM for 45 min at 37° with constant agitation. Conidia were plated on CM + Triton X-100 plates and viability was measured as percentage survival compared to untreated controls.
SepB is a member of the UvsC epistasis group:
In A. nidulans, genetic analysis has revealed the existence of at least four distinct DNA repair pathways (Goldman et al. 2002). To obtain additional insight into the potential role of SepB in DSB repair, we used the radial colony growth assay to test for epistatic interactions between sepB3 and mutations affecting other repair pathways. These experiments showed that sepB3 uvsC114 and sepB3 musN227 double mutants are no more sensitive to MMS than either single mutant (Figure 2). In contrast, sepB3 displayed additive interactions on MMS with other repair mutations such as uvsB110 (Figure 2). Similarly, sepB3 uvsC114 double mutants appear to display an epistatic interaction in response to PLM exposure (Figure 2). UvsCRAD51 is the A. nidulans ortholog of Rad51 and is required for the repair of DNA strand breaks (van Heemst et al. 1997). In addition, on the basis of a plasmid integration assay, uvsC mutants display severe defects in homologous recombination (Ichioka et al. 2001). MusNRECQ is the A. nidulans homolog of RecQ/Sgs1, and is also a member of the UvsC epistasis group (Kafer and Chae 1994; Hofmann and Harris 2001). By contrast, UvsBATR, which is the A. nidulans ATR/Mec1 homolog (Hofmann and Harris 2000), does not function within this pathway (Kafer and Mayor 1986). Accordingly, the epistatic interaction between the sepB3, musN227, and uvsC114 mutations suggests that SepB participates in a UvsCRAD51-mediated homologous recombination pathway required for the repair of DSBs.
SepB is required for the formation of DNA-damage-induced UvsC nuclear foci:
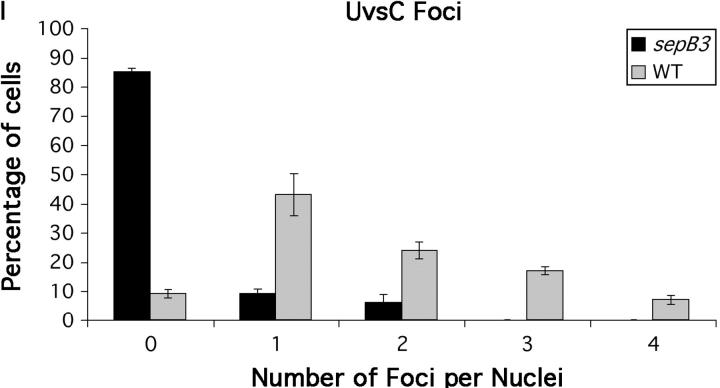
In S. cerevisiae, lagging-strand replication is required after strand invasion for the repair of a DSB by homologous recombination (Holmes and Haber 1999). Since Ctf4p, the yeast homolog of SepB, has been implicated in lagging-strand replication (Formosa and Nittis 1999), SepB may be involved in an analogous process downstream of UvsC. Alternatively, since the yeast homologs also function in sister-chromatid cohesion (Hanna et al. 2001; Williams and McIntosh 2002), which is presumably a prerequisite for strand exchange (Sjogren and Nasmyth 2001), SepB may function upstream of UvsC. We surmised that it might be possible to characterize the function of SepB in recombination-mediated repair by assessing its effect on UvsCRAD51 localization. In yeast cells, Rad51 homologs form irradiation-induced subnuclear foci (Gasior et al. 2001; Caspari et al. 2002). To determine if UvsCRAD51 localized to similar structures, we constructed a functional UvsC-FLAG fusion protein expressed under the control of uvsC promoter sequences. This protein fully complemented the UV and MMS sensitivity caused by the uvsC114 mutation (data not shown). In untreated hyphae, UvsC-FLAG was expressed (Figure 3), but failed to localize to nuclei (Figure 4, A–D). By contrast, when hyphae were exposed to PLM (10 μg/ml for 1 hr), UvsC-FLAG localized to discrete subnuclear foci (average 1.8/nucleus; Figure 4, E, F, and I; supplemental Figure 1A at http://www.genetics.org/supplemental/) that may correspond to sites of recombination-mediated repair. When examined in a sepB3 background, PLM-induced nuclear localization of UvsC-FLAG was severely reduced (average 0.3/nucleus, Figure 4, G–I; supplemental Figure 1A at http://www.genetics.org/supplemental/). Note that the background staining observed in the latter set of images appears to be due to interactions between the anti-FLAG antibody and an additional epitope in A. nidulans (supplemental Figure 1B at http://www.genetics.org/supplemental/; also see materials and methods) and likely does not reflect cytoplasmic localization of UvsCRAD51. We also observed SepB-dependent formation of UvsCRAD51 subnuclear foci in hyphae exposed to MMS or HU (C. Semighini, S. Gygax and S. Harris, unpublished results). Since UvsC-FLAG expression was not affected by the sepB3 mutation (Figure 3), these observations suggest that SepB is required for the localization of UvsCRAD51 to sites of homologous recombination.
Figure 4.—
UvsC localizes to DNA-damage-induced subnuclear foci. (A, B, E, and F) Wild-type (ASG17) hyphae. (C, D, G, and H) sepB3 (ASG19) hyphae. Strains were grown in YGV for 14 hr at 28° and then left untreated (A–D) or exposed to 10 μg/ml PLM (E–H) and examined by immunofluorescence microscopy. UvsC-FLAG was detected using anti-FLAG antibodies (A, C, E, and G), and nuclei were stained using Hoechst 33258 (B, D, F, and H). (I) The number of UvsC-FLAG foci per nucleus was determined for wild-type (ASG17) and sepB3 (ASG19) hyphae treated with 10 μg/ml PLM. UvsC-FLAG was detected as described above, and hyphae were examined by immunofluorescence microscopy. For each sample, 100 nuclei were examined. Bars, 4 μm.
sepB3 mutants display phenotypes consistent with defects in sister-chromatid cohesion:
In S. cerevisiae, mutations that affect sister-chromatid cohesion cause a characteristic set of phenotypes (i.e., see Hanna et al. 2001). These include sensitivity to spindle-deploymerizing agents such as benomyl, which triggers prolonged metaphase arrest. In wild-type cells, sister chromatids remain aligned during the arrest and segregate properly upon recovery. By contrast, sister chromatids separate precociously in cohesion mutants, which leads to random segregation and loss of viability during recovery. In addition, cohesion mutants are typically sensitive to loss of the spindle assembly checkpoint. This presumably reflects the role of cohesins in generating the tension required to establish stable bipolar attachment of sister chromatids to microtubules (Tanaka et al. 2000). In the absence of this function, the spindle assembly checkpoint maintains viability by preventing premature segregation (reviewed in Lew and Burke 2003). Because sister-chromatid cohesion defects could account for the failure to properly localize UvsCRAD51, we screened for analogous phenotypes in sepB3 mutants.
When incubated at semipermissive temperature (35°) on media containing BEN, sepB3 mutants display a striking growth defect (Figure 5A). Although BEN triggers a normal metaphase arrest in sepB3 mutants, which is reflected by the increased chromosome mitotic index (i.e., the fraction of nuclei with condensed chromatin as determined by staining with Hoechst), the first mitosis during recovery appears slower relative to untreated controls (Table 2). Moreover, when sepB3 conidiospores are treated with BEN for 6 hr and then plated at permissive temperature (28°), the fraction of viable colonies that display abnormal morphology is threefold higher compared to untreated controls (12.2% vs. 4.1%, Figure 5B; note that for wild-type conidiospores, the fraction is 0.2% for both control and treated samples). Because abnormal colony morphology is indicative of aneuploidy in A. nidulans (Kafer and Upshall 1973; Harris and Hamer 1995), this observation suggests that the sepB3 mutation causes increased chromosome loss during recovery from spindle depolymerization.
Figure 5.—
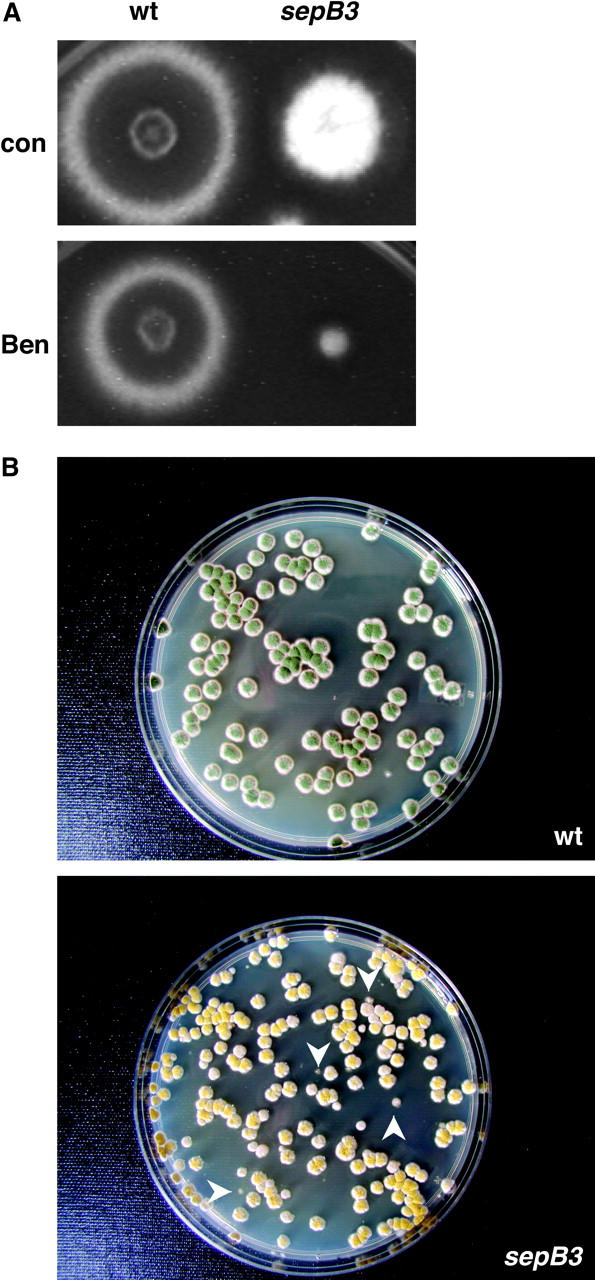
sepB3 mutants are sensitive to BEN. (A) Wild-type (A28) and sepB3 (ASH60) conidiospores were patched onto MAG plates containing either 0.4 μg/ml BEN or no drug. Plates were incubated for 60 hr at 35°. (B) Increased appearance of abnormal colonies following exposure of sepB3 mutants to Ben. Wild-type (A28) and sepB3 (ASH60) conidiospores were germinated for 6 hr at 42° in YGV or YGV + 10 μg/ml BEN. Germlings were diluted and plated on CM + Triton X-100 and incubated at 28° for 3 days. Only plates containing BEN-treated conidiospores are shown. White arrowheads indicate colonies that display abnormal morphology.
TABLE 2.
Effects of benomyl on nuclear division insepB3 mutants
|
T = 6 hr (+BEN)
|
T = 10 hr (BEN rel): |
|||
|---|---|---|---|---|
| Strain | Treatment | Chromosome mitotic index |
% ≤2 nuclei | % ≤2 nuclei |
| Wild type | Con | 7 | 62 | 0 |
| Ben | 51 | 100 | 0 | |
| sepB3 | Con | 11 | 84 | 25 |
| Ben | 36 | 100 | 49 | |
Wild-type (A28) and sepB3 (ASH60) conidiospores were germinated on coverslips at 37° in YGV or YGV + 10 μg/ml BEN. After 6 hr, one coverslip was removed (+BEN), and the remainder were washed and shifted to prewarmed YGV at 37° for an additional 4 hr (BEN release). Samples were fixed and stained with Hoechst 33258. At the 6-hr time point, the chromosome mitotic index was determined to verify that BEN triggered mitotic arrest. The experiment was performed twice, and representative results from one experiment are shown. Con, untreated control conidiospores, Ben, benomyl-treated conidiospores.
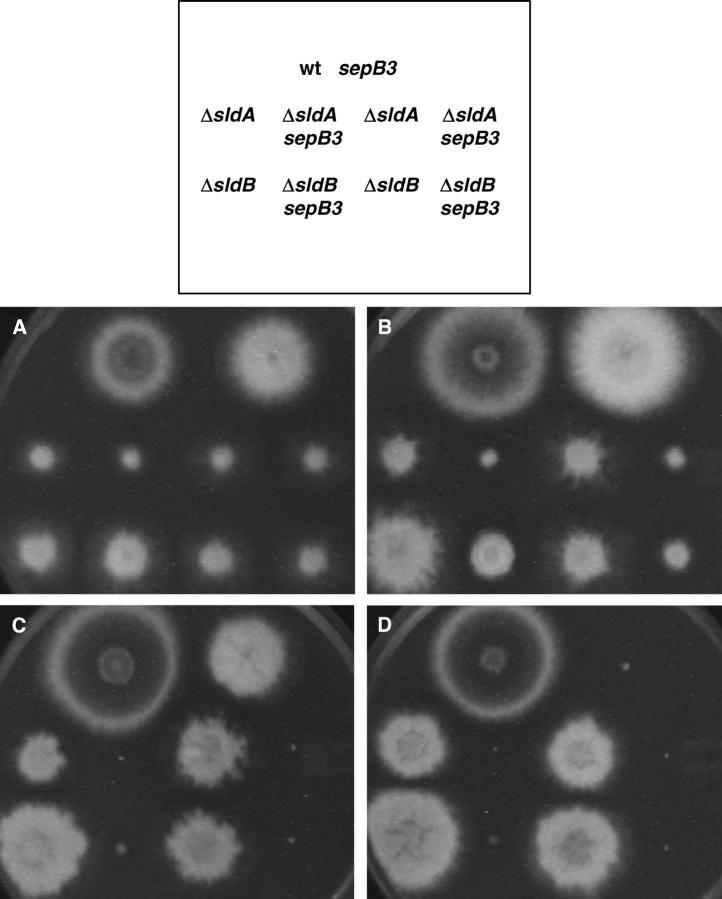
The sldA and sldB genes encode the A. nidulans homologs of the spindle assembly checkpoint proteins Bub1p and Bub3p, respectively, and are required for metaphase arrest in response to spindle assembly defects (Efimov and Morris 1998). As shown in Figure 6, sepB3 mutants exhibit a synthetic lethal interaction with deletion mutations in sldA and sldB. The double mutants fail to grow at 35° and display reduced colony formation at 32°. Notably, these interactions are more severe than those previously observed between sepB3 and mutations that compromise the DNA damage checkpoint (Harris and Kraus 1998). However, unlike the DNA damage checkpoint mutations, neither ΔsldA nor ΔsldB permits sepB3 mutants to form septa at restrictive temperature (data not shown). Because sepB3 mutants are sensitive to spindle-depolymerizing agents and cannot tolerate abrogation of the spindle assembly checkpoint, we propose that, like the yeast homologs Ctf4p and mcl1 (Hanna et al. 2001; Williams and McIntosh 2002), SepB may be required for sister-chromatid cohesion (see discussion for further comment).
Figure 6.—
sepB3 displays synthetic lethal interactions with spindle assembly checkpoint mutations. Conidia were patched onto MAG plates in the order shown. The following strains were used: top row (left to right), A28 and ASH60; middle row (left to right), ASG70, ASG72, ASG76, and ASG78; bottom row (left to right), ASG80, ASG82, ASG84, and ASG86. Plates were incubated for 60 hr at 28° (A), 32° (B), 35° (C), or 42° (D).
DISCUSSION
Molecular characterization of SepB suggests that it is a conserved protein scaffold involved in multiple aspects of chromosomal DNA metabolism. We show here that SepB functions in the A. nidulans DNA damage response. In particular, SepB is a member of the UvsCRAD51 epistasis group and is required for the formation of DNA-damage-induced UvsCRAD51 foci. Because sepB mutants display phenotypes consistent with a defect in sister-chromatid cohesion, our results raise the interesting possibility that cohesion may have a role in Rad51 localization.
SepB family members possess multiple interaction domains:
A characteristic feature of SepB/And-1 family members is the presence of two distinctive β-propeller domains. SepB, Mcl1, and And-1, but not Ctf4p, possess five to seven degenerate WD40 repeats in their respective N termini (Kohler et al. 1997; Williams and McIntosh 2002). In addition, all family members harbor a conserved ∼300-amino-acid central region termed the SepB domain (Williams and McIntosh 2002). Analysis of SepB using two different algorithms that predict protein structure (available at http://bmerc-www.bu.edu/wdrepeat/ and http://www.bmm.icnet.uk/servers/3dpssm) revealed that the SepB domain is capable of forming a four-bladed β-propeller. Therefore, SepB/And-1 family members are largely composed of multiple β-propeller platforms that presumably mediate interactions with several different partner proteins. The extensive web of genetic interactions observed for both ctf4 and mcl1 mutants supports this notion (Hanna et al. 2001; Williams and McIntosh 2002; Warren et al. 2004).
Unlike And-1, the fungal SepB homologs possess an acidic region that links the WD40 and SepB β-propeller platforms. This region displays striking homology to an N-terminal motif that is conserved in the catalytic subunit of DNA polymerase α from all eukaryotes (Wang et al. 1989). Although the role of this motif in polymerase function remains unknown, our preliminary observations show that mutations altering specific acidic residues within the motif (i.e., D9 and D10 in Figure 1C) do not affect SepB function (K. Crowley, S. Gygax and S. Harris, unpublished results). Accordingly, we propose that the crucial property of this motif is its general acidic nature. One attractive possibility is that SepB and Pol α compete for binding to a partner protein via this motif.
Role of SepB in the DNA damage response:
Our observations suggest that SepB is involved in the UvsCRAD51-mediated pathway that repairs DNA damage via homologous recombination. The yeast homologs of SepB appear to be particularly important for the repair of DSBs induced during S phase (Miles and Formosa 1992; Hanna et al. 2001; Williams and McIntosh 2002). The specific function(s) of these proteins that contribute to viability remain unclear, although involvement in lagging-strand replication or sister-chromatid cohesion are attractive possibilities (Holmes and Haber 1999; Williams and McIntosh 2002). We propose that either of these functions may account for the role of SepB in homologous recombination. At this time, we have not yet determined whether, like yeast, SepB promotes repair during S phase (i.e., attempts to construct double mutants that would permit synchronization in S phase have been unsuccessful). Nonetheless, several observations provide modest support for this conclusion. First, the characterization of sepB3 mutants clearly implicates SepB in some aspect of S-phase DNA metabolism (Harris and Hamer 1995). Second, sepB3 mutants are extremely sensitive to DSBs induced upon release from G1 arrest, which, under normal conditions, are presumably repaired during the subsequent S phase. Finally, the accumulation of subnuclear UvsCRAD51 foci in wild-type hyphae arrested in S phase with HU is abolished by the sepB3 mutation (S. Gygax and S. Harris, unpublished results)
Links between S-phase functions and sister-chromatid cohesion:
It has become increasingly apparent that sister-chromatid cohesion is linked to functions associated with DNA replication (Carson and Christman 2001). For example, several proteins involved in replication or S-phase checkpoint activity promote sister-chromatid cohesion in S. cerevisiae, including Ctf4p, Mre11p, and Xrs2p (Hanna et al. 2001; Warren et al. 2004). It has been suggested that these proteins regulate the formation of a chromatin environment required for cohesion (Warren et al. 2004). Our observations provide preliminary support for the notion that SepB also mediates sister-chromatid cohesion, although definitive proof awaits the development of appropriate fluorescent in situ hybridization protocols. Nevertheless, if substantiated, this would be the first clue suggesting the existence of a previously unsuspected link between S-phase functions, sister-chromatid cohesion, and Rad51 localization. This link may be direct, whereby cohesion facilitates Rad51 localization by holding the ends of DSBs in proximity to each other (Hartsuiker et al. 2001; Sjogren and Nasmyth 2001; Kim et al. 2002). Alternatively, a common chromatin configuration generated during S phase may independently promote both cohesion and Rad51 localization (Alexiadis and Kadonaga 2002; Jaskelioff et al. 2003).
Acknowledgments
We thank Andrew Arnold (University of Connecticut Health Center), Vladimir Efimov (University of Medicine and Dentistry of New Jersey), Steve James (Gettysburg College), and Aaron Mitchell (Columbia University) for providing reagents, strains, or plasmids. We also thank Joe Zhou and Terri Fangman (University of Nebraska) for assistance with the use of the laser scanning confocal microscope. This work was supported by an award from the American Cancer Society to S.H. (RPG-99-214-01-MBC) as well as by Fundacao de Amparo a Pesquisa do Estado de Sao Paulo and Conselho Nacional de Desenvolvimento Cientifico e Tecnologico, Brazil (G.H.G.).
Sequence data from this article have been deposited with EMBL/GenBank Data Libraries under accession no. X86399.
References
- Alexiadis, V., and J. T. Kadonaga, 2002. Strand pairing by Rad54 and Rad51 is enhanced by chromatin. Genes Dev. 16: 2767–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen, L. G., and N. R. Morris, 1983. Kinetics of the nuclear division cycle of Aspergillus nidulans. J. Bacteriol. 156: 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson, D. R., and M. F. Christman, 2001. Evidence that replication fork components catalyze cohesion between sister chromatids. Proc. Natl. Acad. Sci. USA 98: 8270–8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspari, T., J. M. Murray and A. M. Carr, 2002. Cdc2-cyclin B kinase activity links Crb2 and Rqh1-topoisomerase III. Genes Dev. 16: 1195–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimov, V. P., and N. R. Morris, 1998. A screen for dynein synthetic lethals in Aspergillus nidulans identifies spindle assembly checkpoint genes and other genes involved in mitosis. Genetics 149: 101–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formosa, T., and T. Nittis, 1999. Dna2 mutants reveal interactions with Dna polymerase α and Ctf4, a Pol α accessory factor, and show that full Dna2 helicase activity is not essential for growth. Genetics 151: 1459–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasior, S. L., H. Olivares, U. Ear, D. M. Hari, R. Weichselaum et al., 2001. Assembly of RecA-like recombinases: distinct roles for mediator proteins in mitosis and meiosis. Proc. Natl. Acad. Sci. USA 98: 8411–8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman, G. H., S. L. McGuire and S. D. Harris, 2002. The DNA damage response in filamentous fungi. Fungal Genet. Biol. 35: 183–195. [DOI] [PubMed] [Google Scholar]
- Hanna, J. S., E. S. Kroll, V. Lundblad and F. A. Spencer, 2001. Saccharomyces cerevisiae CTF18 and CTF4 are required for sister chromatid cohesion. Mol. Cell. Biol. 21: 3144–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, S. D., and J. E. Hamer, 1995. sepB: an Aspergillus nidulans gene involved in chromosome segregation ant the initiation of cytokinesis. EMBO J. 14: 5244–5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, S. D., and P. R. Kraus, 1998. Regulation of septum formation in Aspergillus nidulans by a DNA damage checkpoint pathway. Genetics 148: 1055–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, S. D., J. L. Morrell and J. E. Hamer, 1994. Identification and characterization of Aspergillus nidulans mutants defective in cytokinesis. Genetics 136: 517–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, S. D., A. F. Hofmann, H. W. Tedford and M. P. Lee, 1999. Identification and characterization of genes required for hyphal morphogenesis in the filamentous fungus Aspergillus nidulans. Genetics 151: 1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartsuiker, E., E. Vaessen, A. M. Carr and J. Kohli, 2001. Fission yeast Rad50 stimulates sister chromatid recombination and links cohesion with repair. EMBO J. 20: 6660–6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, B., W. Zuo, A. Liu and N. R. Morris, 2001. The LIS1-related protein NUDF of Aspergillus nidulans and its interaction partner NUDE bind directly to specific subunits of dynein and dynactin and to α- and γ-tubulin. J. Biol. Chem. 276: 38877–38884. [DOI] [PubMed] [Google Scholar]
- Hofmann, A. F., and S. D. Harris, 2000. The Aspergillus nidulans uvsB gene encodes an ATM-related kinase required for multiple facets of the DNA damage response. Genetics 154: 1577–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann, A. F., and S. D. Harris, 2001. The Aspergillus nidulans musN gene encodes a RecQ helicase that interacts with the PI-3K-related kinase UVSB. Genetics 159: 1595–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes, A. M., and J. E. Haber, 1999. Double-strand break repair in yeast requires both leading and lagging strand DNA polymerases. Cell 96: 415–424. [DOI] [PubMed] [Google Scholar]
- Ichioka, D., T. Itoh and Y. Itoh, 2001. An Aspergillus nidulans uvsC null mutant is deficient in homologous DNA integration. Mol. Gen. Genet. 264: 709–715. [DOI] [PubMed] [Google Scholar]
- Jackson, S. P., 2002. Sensing and repairing DNA double-strand breaks. Carcinogenesis 23: 687–696. [DOI] [PubMed] [Google Scholar]
- Jaskelioff, M., S. van Komen, J. E. Krebs, P. Sung and C. L. Peterson, 2003. Rad54p is a chromatin remodeling enzyme required for heteroduplex DNA joint formation with chromatin. J. Biol. Chem. 278: 9212–9218. [DOI] [PubMed] [Google Scholar]
- Kafer, E., 1977. Meiotic and mitotic recombination in Aspergillus and its chromosomal aberrations. Adv. Genet. 19: 33–131. [DOI] [PubMed] [Google Scholar]
- Kafer, E., and S.-K. Chae, 1994. Phenotypic and epistatic grouping of hypo- and hyper-rec mus mutants in Aspergillus. Curr. Genet. 25: 223–232. [DOI] [PubMed] [Google Scholar]
- Kafer, E., and O. Mayor, 1986. Genetic analysis of DNA repair in Aspergillus: evidence for different types of MMS-sensitive hyperrec mutants. Mutat. Res. 161: 119–134. [DOI] [PubMed] [Google Scholar]
- Kafer, E., and A. Upshall, 1973. The phenotypes of the eight disomics and trisomics of Aspergillus nidulans. J. Hered. 64: 35–38. [DOI] [PubMed] [Google Scholar]
- Kim, J.-S., T. B. Krasieva, V. LaMorte, A. M. R. Taylor and K. Yokomori, 2002. Specific recruitment of human cohesin to laser-induced DNA damage. J. Biol. Chem. 277: 45149–45153. [DOI] [PubMed] [Google Scholar]
- Kohler, A., M. S. Schmidt-Zachmann and W. W. Franke, 1997. AND-1, a natural chimeric DNA-binding protein, combines an HMG-box with regulatory WD-repeats. J. Cell Sci. 110: 1051–1062. [DOI] [PubMed] [Google Scholar]
- Kouprina, N., E. Kroll, V. Bannikov, V. Bliskovsky, R. Gizatullin et al., 1992. CTF4 (CHL15) mutants exhibit defective DNA metabolism in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 12: 5736–5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew, D. J., and D. J. Burke, 2003. The spindle assembly and spindle position checkpoints. Annu. Rev. Genet. 37: 251–282. [DOI] [PubMed] [Google Scholar]
- Miles, J., and T. Formosa, 1992. Evidence that POB1, a Saccharomyces cerevisiae protein that binds to DNA polymerase α, acts in DNA metabolism in vivo. Mol. Cell. Biol. 12: 5724–5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki, T., D. A. Bressan, M. Shinohara, J. E. Haber and A. Shinohara, 2004. In vivo assembly and disassembly of Rad51 and Rad52 complexes during double-strand break repair. EMBO J. 23: 939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, S., J. Hayles and P. Nurse, 1989. Regulation of p34cdc2 protein kinase during mitosis. Cell 58: 361–372. [DOI] [PubMed] [Google Scholar]
- Shinohara, A., and T. Ogawa, 1998. Stimulation by Rad52 of yeast Rad51-mediated recombination. Nature 391: 404–407. [DOI] [PubMed] [Google Scholar]
- Sjogren, C., and K. Nasmyth, 2001. Sister chromatid cohesion is required for post-replicative double strand break repair in Saccharomyces cerevisiae. Curr. Biol. 11: 991–995. [DOI] [PubMed] [Google Scholar]
- Smith, T. F., C. Gaitatzes, K. Saxena and E. J. Neer, 1999. The WD repeat: a common architecture for diverse functions. Trends Biochem. Sci. 24: 181–185. [DOI] [PubMed] [Google Scholar]
- Sung, P., 1997. Yeast Rad55 and Rad57 proteins form a heterodimer that functions with replication protein A to promote DNA strand exchange by Rad51 recombinase. Genes Dev. 11: 1111–1121. [DOI] [PubMed] [Google Scholar]
- Sung, P., and D. L. Robberson, 1995. DNA strand exchange mediated by a RAD51-ssDNA nucleoprotein filament with polarity opposite to that of RecA. Cell 82: 453–461. [DOI] [PubMed] [Google Scholar]
- Symington, L. S., 2002. Role of RAD52 epistasis group genes in homologous recombination and double-strand break repair. Microbiol. Mol. Biol. Rev. 66: 630–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, T., J. Fuchs, J. Loidl and K. Nasmyth, 2000. Cohesion ensures bipolar attachment of microtubules to sister centromeres and resists their precocious separation. Nat. Cell Biol. 2: 492–499. [DOI] [PubMed] [Google Scholar]
- van Heemst, D., K. Swart, E. F. Holub, R. van Dijk, H. H. Offenberg et al., 1997. Cloning, sequencing, disruption, and phenotypic analysis of uvsC, an Aspergillus nidulans homologue of yeast RAD51. Mol. Gen. Genet. 254: 654–664. [DOI] [PubMed] [Google Scholar]
- Wang, T. S., S. W. Wong and D. Korn, 1989. Human DNA polymerase alpha: predicted functional domains and relationships with viral polymerases. FASEB J. 3: 14–21. [DOI] [PubMed] [Google Scholar]
- Wang, X., and J. E. Haber, 2004. Role of Saccharomyces single-stranded DNA-binding protein RPA in the strand invasion step of double-strand break repair. PloS Biol. 2: E21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren, C. D., D. M. Eckley, M. S. Lee, J. S. Hana, A. Hughes et al., 2004. S-phase checkpoint genes safeguard high-fidelity sister chromatid cohesion. Mol. Biol. Cell 15: 1724–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, D. R., and J. R. McIntosh, 2002. mcl+, the Schizosaccharomyces pombe homologue of CTF4, is important for chromosome replication, cohesion, and segregation. Eukaryot. Cell 1: 758–773. [DOI] [PMC free article] [PubMed] [Google Scholar]