Abstract
Methane (CH4) contributes to the growing global background concentration of tropospheric ozone (O3), an air pollutant associated with premature mortality. Methane and ozone are also important greenhouse gases. Reducing methane emissions therefore decreases surface ozone everywhere while slowing climate warming, but although methane mitigation has been considered to address climate change, it has not for air quality. Here we show that global decreases in surface ozone concentrations, due to methane mitigation, result in substantial and widespread decreases in premature human mortality. Reducing global anthropogenic methane emissions by 20% beginning in 2010 would decrease the average daily maximum 8-h surface ozone by ≈1 part per billion by volume globally. By using epidemiologic ozone-mortality relationships, this ozone reduction is estimated to prevent ≈30,000 premature all-cause mortalities globally in 2030, and ≈370,000 between 2010 and 2030. If only cardiovascular and respiratory mortalities are considered, ≈17,000 global mortalities can be avoided in 2030. The marginal cost-effectiveness of this 20% methane reduction is estimated to be ≈$420,000 per avoided mortality. If avoided mortalities are valued at $1 million each, the benefit is ≈$240 per tonne of CH4 (≈$12 per tonne of CO2 equivalent), which exceeds the marginal cost of the methane reduction. These estimated air pollution ancillary benefits of climate-motivated methane emission reductions are comparable with those estimated previously for CO2. Methane mitigation offers a unique opportunity to improve air quality globally and can be a cost-effective component of international ozone management, bringing multiple benefits for air quality, public health, agriculture, climate, and energy.
Keywords: human health, mortality, tropospheric ozone, air quality
Tropospheric ozone (O3) is an oxidant that damages agriculture, ecosystems, and materials. Ozone also adversely affects human health and has been associated in epidemiologic studies with daily premature mortality (1–10). Surface O3 concentrations have historically increased in both polluted and remote regions and now frequently exceed regulatory standards (11–14). Global background surface O3 concentrations have roughly doubled since preindustrial times (15), primarily because of increases in anthropogenic emissions of nitrogen oxides (NOx) and methane (CH4) (16), and are projected to continue to increase (17, 18).
Tropospheric O3 is formed from photochemical reactions involving NOx and volatile organic compounds (VOCs). Although nonmethane VOCs are the dominant anthropogenic VOCs contributing to O3 formation in polluted regions, CH4 is the primary anthropogenic VOC in the global troposphere (19). Because CH4 reacts slowly (lifetime of 8–9 yr), it affects global background concentrations of O3. Because this background underlies the O3 produced on urban and regional scales, CH4 mitigation reduces O3 concentrations by roughly the same amount in polluted regions as in rural regions (19, 20).
Methane and O3 are also greenhouse gases, which rank behind only carbon dioxide (CO2) in anthropogenic radiative forcing of climate (21). Consequently, abatement of CH4 emissions both reduces surface O3 concentrations everywhere and slows greenhouse warming (19, 20). Methane abatement has been considered a low-cost means of addressing climate change (22, 23), particularly to influence the short-term rate of climate change. However, CH4 abatement has not been considered for air quality management, mainly because O3 pollution has traditionally been considered a local and regional problem, and the local benefits of local CH4 reductions are small.
Here we examine the global reduction in O3 and consequent decrease in premature human mortalities resulting from CH4 emission controls. We first estimate the global decrease in surface O3 concentration due to CH4 mitigation, using the MOZART-2 global three-dimensional tropospheric chemistry-transport model (24, 25). This spatial distribution of O3 is then overlaid on projections of population, and avoided premature mortalities are estimated by using daily O3-mortality relationships from epidemiologic studies (6–9). Results are presented as the number of avoided premature mortalities due to the CH4 reduction, the marginal cost-effectiveness per avoided mortality (using the marginal cost of CH4 mitigation), and the monetized benefit per tonne of CH4 reduced [using a value of a statistical life (VSL)].
Response of Global Surface Ozone to Methane Mitigation
Methods.
We consider a CH4 emission reduction of 65 Mt·yr−1 (1 Mt = 109 kg) (≈20% of current global anthropogenic emissions), which is assumed to be immediate in 2010 and sustained relative to the Intergovernmental Panel on Climate Change Special Report on Emissions Scenarios (SRES) A2 scenario (26) until 2030. A compilation of global CH4 abatement options in five industrial sectors (27) suggests that 65 Mt·yr−1 can be reduced by 2010 at a net cost savings, using identified abatement options.
The MOZART-2 simulations use uniform global mixing ratios of CH4, and spatially and temporally distributed emissions of other O3 precursors, as other studies have done (19, 28). We conduct four simulations with MOZART-2, as shown in Table 1. Simulations I and III use CH4 mixing ratios and emissions of other O3 precursors as specified for the Intergovernmental Panel on Climate Change AR-4 2000 and 2030 A2 atmospheric chemistry experiments (29). In the CH4 reduction cases (simulations II and IV), the decreased CH4 mixing ratios are the steady-state mixing ratios resulting from a 65 Mt·yr−1 emission reduction versus the corresponding base cases (simulations I and III), assuming a CH4 feedback factor of 1.4 (28). We do not consider any effects of changes in future climate on O3 distributions in projecting to 2030 (30, 31), nor do we consider the decrease in global mean temperature due to CH4 reductions, which could amplify the O3 decrease that we estimate. MOZART-2 has a horizontal resolution of ≈1.9° by 1.9° and 28 vertical levels. In all cases, we use meteorological fields from the National Centers for Environmental Prediction reanalysis (32), beginning in July 1998, with an 18-month initialization, before focusing on results for the meteorological year 2000.
Table 1.
Four MOZART-2 simulations conducted in this study
| Simulation | Fixed CH4 mixing ratio, ppbv | Global anthropogenic NOx emissions, Mt·yr−1 as NO2 |
|---|---|---|
| I: 2000 base case | 1,760 | 124.8 |
| II: 2000 CH4 reduction | 1,460* | 124.8 |
| III: 2030 A2 | 2,163 | 212.7 |
| IV: 2030 A2, CH4 reduction | 1,865* | 212.7 |
*Fixed global CH4 mixing ratios at steady state, corresponding to an emission reduction of 65 Mt·yr−1 of CH4.
Results.
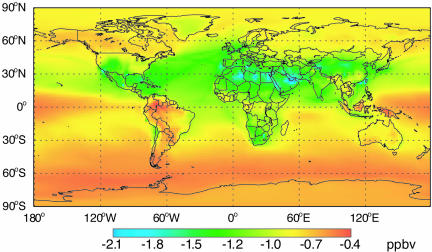
Between 2000 and 2030 (simulations I and III), we project the population-weighted global average 8-h daily maximum surface O3 mixing ratio to increase by 12.3 parts per billion by volume (ppbv) (25%) (Table 2), primarily because of projected increases in anthropogenic emissions of NOx (70%) and CH4 (48%). The 65 Mt·yr−1 CH4 emission reduction decreases the steady-state population-weighted mean 8-h O3 by 1.16 ppbv (1.9%, Table 2). This sensitivity is in agreement with other models (18, 19, 28, 33), and these results together suggest that global surface O3 responds fairly linearly to changes in CH4 (33). Decreases in O3 due to CH4 reductions are widespread globally (Fig. 1), with the largest O3 decreases occurring over the Middle East, North Africa, and Europe, because of greater down-welling from the free troposphere and greater availability of NOx. This spatial pattern is similar to previous results (19, 20), suggesting that the pattern is independent of the extent of methane abatement. Methane controls initiated in 2010 will yield ≈81% of this steady-state O3 change by 2030, assuming exponential decay with a CH4 perturbation lifetime of ≈12 yr (28).
Table 2.
Global average O3 mixing ratios (ppbv) in the 2000 and 2030 A2 base model runs (simulations I and III), and the steady-state change in O3 due to a 65 Mt·yr−1 reduction in CH4 emissions, relative to the 2030 base (simulation IV minus simulation III)
| Parameter | 2000 | 2030 A2 | ΔO3 2030 |
|---|---|---|---|
| 24-h average | 29.1 | 33.6 | −0.82 |
| 8-h daily maximum | 31.8 | 37.1 | −0.87 |
| 8-h maximum population-weighted | 49.4 | 61.7 | −1.16 |
Fig. 1.
Change in annual average daily maximum 8-h surface O3 mixing ratios, at steady state, due to a 65 Mt·yr−1 reduction in CH4 emissions relative to the 2030 A2 base case (simulation IV minus III).
The steady-state change in O3 when 65 Mt·yr−1 are reduced relative to the 2000 base case (simulation II vs. simulation I) is virtually identical to the change in Table 2 (−1.11 ppbv for population-weighted 8-h O3), indicating that the projected changes in nonmethane O3 precursors between 2000 and 2030 have little effect on the O3 sensitivity to CH4. This insensitivity presumably reflects the fact that there is little change in hydroxyl radical (OH) concentrations, because of similar emission ratios of NOx to (CO + VOCs) in 2000 and 2030 (16). Therefore, although the A2 scenario includes larger growth in emissions of O3 precursors than other SRES scenarios, and larger than the “Current Legislation” scenario of Dentener et al. (18), this high growth does not strongly affect the O3-CH4 sensitivity.
Indirect Effects of Methane Reductions on Particulate Matter (PM).
Methane reductions also indirectly affect PM concentrations through complex oxidant chemistry. MOZART-2 (25) results suggest that CH4 reductions cause a global net decrease in inorganic PM, because of decreases in hydrogen peroxide that in turn reduce sulfate production. Inorganic PM concentrations also increase at some locations, where the increased gas-phase oxidation (due to increased OH concentrations) dominates the change in sulfate production. Although the global average decrease is only ≈0.5% of the inorganic PM (sulfate, nitrate, and associated ammonium), the decrease is concentrated in populated regions. Confidence in the change in PM is lower than for O3 because of competing influences on inorganic PM, and because we have neglected changes in organic PM.
Global Mortality Benefits of Reduced Ozone
Methods.
Ozone has been associated in epidemiologic studies with adverse health effects including hospital admissions and chronic respiratory conditions, and recent research provides strong evidence for an association with daily premature mortality (1–10). We use the daily O3-mortality relationship (β) estimated by Bell et al. (6), using a distributed lag method for 95 cities in the United States, and apply this relationship globally. Because long-term effects of O3 on mortality have not been demonstrated (34), we do not consider possible chronic effects of O3 or years of life lost due to premature mortality. Bell et al. (6) directly use a large data set, and therefore their results are not subject to publication bias, which can bias meta-analyses high. The β estimated by Bell et al. (6) with a single-day lag is much smaller than the β estimated in three recent meta-analyses (7–9). However, the β of Bell et al. (6) with the distributed lag method, used in this study, is much more comparable with the meta-analyses (7–9), which are 22–36% higher. We consider the sensitivity of our results to the uncertainties reported by Bell et al. (6) and the meta-analyses (7–9). Although Bell et al. (6) focus on the United States, similar results have been reported in North America and Europe (5, 7–9). Few studies of O3 mortality have been conducted elsewhere, although some such studies suggest associations between O3 and mortality in other regions (35–37).
Although Bell et al. (6) find similar relationships between ozone and mortality over all seasons in the United States, many studies find reduced O3 impacts in winter, when O3 concentrations are often lower (5, 8, 9). However, applying seasonal differences in tropical regions is not straightforward. Available studies also show adverse effects of O3 below current standards, without identifying a clear threshold below which O3 does not affect mortality (5, 6). Rather than imposing seasonally varying relationships, we assume a low-concentration threshold of 25 ppbv, approximately the preindustrial mixing ratio (13, 15), below which we neglect any effect of O3 on mortality. We apply this threshold on each day, through all seasons, and consider the sensitivity of our results to the threshold used.
We apply β to the total nonaccident baseline mortality rates, using data for 14 world regions (38). Baseline mortality rates are applied uniformly within each region, and are assumed to be constant into the future. The spatial distribution of population is modeled consistently with the SRES A2 scenario, growing to 9.17 billion in 2030 (26).
Avoided premature mortalities are estimated daily in each model grid square, based on the maximum daily 8-h O3 mixing ratio in the A2 base and CH4 control cases. The A2 base and CH4 control cases are constructed for the period 2000–2030 by interpolating between simulations I, III, and IV. For the A2 base case, 8-h O3 mixing ratios on each day and in each grid square are interpolated between 2000 and 2030 (simulations I and III) by using a constant percent growth rate. For the CH4 control case, O3 decreases begin in 2010 and exponentially approach the steady-state change (simulation IV minus III) with the 12-yr CH4 perturbation lifetime (see the supporting information, which is published on the PNAS web site).
Results.
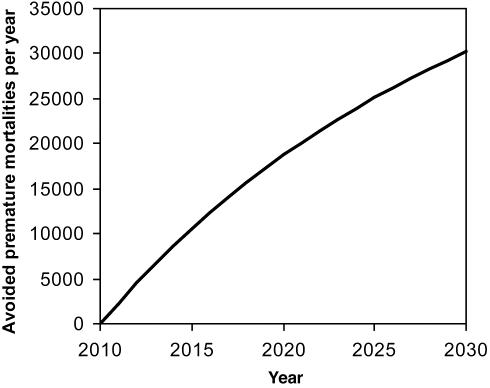
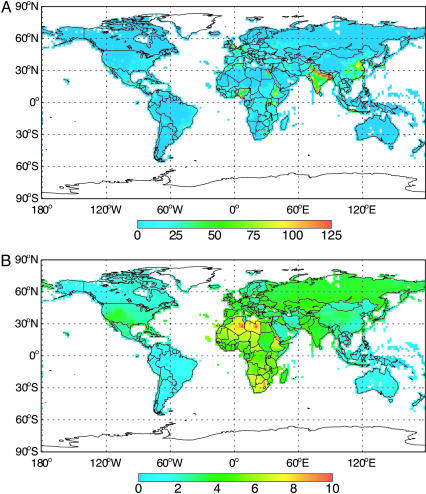
Table 3 and Fig. 2 show that reducing CH4 emissions by 65 Mt·yr−1 in 2010 would prevent ≈30,000 all-cause premature mortalities in the year 2030 (≈0.04% of the total projected mortalities), with ≈370,000 avoided premature mortalities accumulated between 2010 and 2030. These avoided mortalities are distributed globally, with the majority in highly populated regions (Table 3 and Fig. 3). Mortality benefits per million people in 2030 are highest in Africa, which has high baseline mortality rates, followed by Europe and the eastern Mediterranean.
Table 3.
Avoided premature mortalities in 2030 by world region and avoided mortalities per million people in 2030, resulting from decreases in surface O3 due to a global CH4 emission reduction of 65 Mt·yr−1
| Region | Avoided total mortalities in 2030 |
Avoided CR mortalities in 2030 |
||
|---|---|---|---|---|
| Number | Per 106 people | Number | Per 106 people | |
| Africa | 6,920 | 5.59 | 2,070 | 1.68 |
| North America | 1,110 | 2.81 | 700 | 1.77 |
| Latin America | 1,790 | 1.88 | 960 | 1.01 |
| Southeast Asia | 7,790 | 3.33 | 4,550 | 1.95 |
| Western Europe | 1,900 | 3.86 | 1,260 | 2.56 |
| Eastern Europe and former Soviet Union | 1,790 | 3.50 | 1,560 | 3.06 |
| Eastern Mediterranean | 3,150 | 3.69 | 1,660 | 1.94 |
| Western Pacific | 500 | 2.86 | 310 | 1.77 |
| East Asia | 5,250 | 2.36 | 3,610 | 1.63 |
| Global | 30,200 | 3.29 | 16,700 | 1.82 |
Fig. 2.
Avoided global premature mortalities from a 65 Mt·yr−1 CH4 emission reduction, beginning in 2010.
Fig. 3.
Estimated avoided premature mortalities in 2030. (A) Total. (B) Per million people.
Table 4 shows a large sensitivity to β over the range of uncertainties in Bell et al. (6) and three meta-analyses (7–9). The avoided mortalities also vary with the sensitivity of O3 to CH4 but are rather insensitive to the low-concentration threshold over the range considered. This insensitivity occurs because regions with low O3 typically also have low population and small changes in O3 due to CH4; O3 is below 25 ppbv on ≈12% of populated grid square-days in 2030, but the number of avoided mortalities decreases by only 2% relative to the no-threshold case.
Table 4.
Sensitivity of global 2030 avoided premature mortalities to uncertain parameters
The mortality benefits of O3 decreases are most uncertain in developing nations, where fewer epidemiologic studies exist and the general causes of death differ substantially from those in industrialized nations. As a more conservative estimate, we consider the avoided cardiovascular and respiratory (CR) mortalities, because these may be more closely linked to O3. We apply the β for CR mortalities from Bell et al. (6), which is higher than for total mortalities but not significantly different, to baseline CR mortality rates. In Table 3, ≈17,000 premature CR mortalities can be avoided globally in 2030 by the CH4 emission reduction, with the greatest per capita benefits in Europe, where relatively more people die of CR causes. Although our estimates of avoided CR mortalities may be more robust in developing nations than total mortalities, they likely miss important decreases in other causes of mortality. Henceforth, we use an uncertainty range from the estimated avoided CR mortalities (≈17,000 in 2030) to the highest number in Table 4 (≈56,000).
Effects of Changes in PM on Mortality.
By using the changes in inorganic PM in the previous section and a chronic PM-mortality relationship (34), the avoided 2030 mortalities are estimated to be less than, but comparable with, the O3 benefit (see the supporting information). The effects of CH4 reductions on both inorganic and organic PM should be further investigated, including changes in PM precursor emissions from energy sources possibly displaced because of increased CH4 availability.
Policy Analysis of Ozone Control by Means of Methane Mitigation
A compilation of global CH4 abatement measures from five industrial sectors (27) shows that ≈41 Mt·yr−1 can be reduced at a negative marginal cost (net cost-savings, through natural gas recovery), which can be justified regardless of health benefits. The 65 Mt·yr−1 reduction has a marginal cost of ≈$100 per tonne of CH4 (2000 U.S. dollars), whereas the net cost of this reduction is negative. We combine this marginal cost with the all-cause avoided premature mortalities, which we convert to a constant annualized benefit between 2010 and 2030 at a 5% yr−1 discount rate (see the supporting information), yielding $420,000 per avoided mortality ($230,000–$760,000) as the marginal cost-effectiveness of reducing 65 Mt·yr−1. The 65 Mt·yr−1 reduction would be justified, in cost-benefit terms, for any globally averaged VSL >$420,000.
If we use $1 million as a reasonable globally averaged VSL (39), the monetized benefit of reducing CH4 emissions is $240 per tonne of CH4 ($140–$450), or $12 per tonne of CO2 equivalent ($7–$22), which exceeds the marginal cost of the 65 Mt·yr−1 reduction (≈$100 per tonne of CH4). This estimate neglects increases in the VSL as incomes grow, and only considers 20 yr of benefits, whereas the O3 reductions will continue growing beyond 2030 as population also grows. The monetized benefit scales proportionally with the assumed globally averaged VSL.
Because CH4 reductions have recently traded in international markets at $10–$20 per tonne of CO2 equivalent, these results suggest that current climate-motivated CH4 emission reductions can roughly be justified by their benefits to air quality and health, irrespective of other benefits of CH4 and O3 reductions. Furthermore, although the ancillary benefits of CO2 mitigation for air quality and health have received attention (40), the ancillary benefits of CH4 mitigation have not. Our estimate for CH4 of $12 per tonne of CO2 equivalent is comparable with the range estimated previously for CO2 of $0.5–$140 per tonne of CO2 (41). Unlike the ancillary benefits of CO2 mitigation, however, the ancillary benefits of CH4 mitigation do not depend on the location or means of CH4 abatement, because the health benefits of CH4 mitigation result from reactions involving the CH4 itself, and CH4 emissions affect O3 globally regardless of emission location.
The compilation of CH4 abatement measures used in this study (27) considers five industrial sectors (coal, oil, and natural gas operations, landfills, and wastewater treatment) for which methane abatement opportunities are well understood. Because this compilation neglects abatement opportunities in the large agricultural sector, it may underestimate the availability of low-cost CH4 options, which would suggest that CH4 mitigation is more cost-effective than estimated here. On the other hand, a separate compilation by the U.S. Environmental Protection Agency (42–44) suggests that less CH4 can be reduced at low cost (see the supporting information and ref. 20).
Methane mitigation also benefits climate, because it reduces the radiative forcing of both CH4 and O3. The 65 Mt·yr−1 CH4 reduction would decrease global radiative forcing by 0.14 W·m−2, from CH4 and O3 together (at steady state). In contrast, reductions in NOx emissions decrease O3 forcing but increase CH4 forcing (45), with a net effect that could be positive or negative depending on location (46).
Methane is also an important source of global energy, and capturing half of the 65 Mt·yr−1 for energy use would provide ≈2% of current global natural gas production. The reductions in O3 concentrations would also result in benefits to human health (morbidity) and agriculture (47), which we previously estimated to be smaller than the monetized benefits of avoided mortalities estimated here (20). Methane mitigation may further benefit air quality and climate by removing other pollutants (e.g., VOCs) through the same actions that reduce CH4 emissions, and by increasing the availability of natural gas, which may reduce emissions of CO2 and air pollutants from the combustion of other fossil fuels. In addition, because the reductions in O3 are widespread globally, CH4 mitigation may increase the net primary productivity of plants, causing increased uptake of CO2 (48). Finally, methane mitigation may affect stratospheric O3, but the direction of that influence is not certain (49).
The effects of CH4 mitigation on surface O3 concentrations are widespread globally, and are delayed. These characteristics differ from other means of controlling O3, as well as most actions to manage air quality, which abate local and regional pollution over hours to weeks. Because of its global impacts, with small local benefits, CH4 mitigation for air quality purposes (as for climate) will best be implemented at national and international levels. Furthermore, the potential for reducing O3 through CH4 mitigation is limited to a few parts per billion by volume. Methane mitigation is therefore most appropriate for international and long-term (decadal) O3 management, where CH4 mitigation for background O3 is complementary to local and regional O3 management through reductions in emissions of NOx and nonmethane VOCs (20).
Important uncertainties in this study lie in the relationship between O3 and mortality, and between CH4 emissions and global surface O3 concentrations. Because CH4 affects O3 globally, this research highlights the need to improve understanding of O3 mortality in developing nations, and of the relationship between O3 and mortality at low concentration, including consideration of possible thresholds. Future research should also investigate the effects of CH4 mitigation on PM concentrations, and its implications for air quality, public health, and climate. Finally, future research should further examine opportunities to abate CH4 emissions, emphasizing the large agricultural sector.
Conclusions
As background O3 concentrations increase, meeting national O3 standards increasingly becomes an international problem (50–52). Methane mitigation reduces surface O3 everywhere, offering a unique opportunity to improve air quality globally. We estimate that reducing ≈20% of current global anthropogenic CH4 emissions, which can be achieved at a net cost-savings by using identified technologies, will reduce O3 mixing ratios globally by ≈1 ppbv and prevent ≈30,000 premature mortalities globally in 2030 and ≈370,000 mortalities between 2010 and 2030. If these mortalities are valued at $1 million each, the monetized benefit is ≈$240 per tonne of CH4, or ≈$12 per tonne of CO2 equivalent. These benefits exceed the marginal costs of the 20% anthropogenic CH4 reduction (≈$100 per tonne of CH4) and demonstrate that CH4 mitigation has ancillary benefits to air quality and human health that are comparable with those previously estimated for CO2. Methane mitigation benefits air quality, public health, agriculture, climate, and energy, and should increasingly be considered a cost-effective component of international long-term O3 management.
Supplementary Material
Acknowledgments
We thank M. Bell, J. Levy, V. Naik, and M. Oppenheimer for helpful advice and comments on earlier drafts. This work was supported by the National Oceanic and Atmospheric Administration and a National Aeronautics and Space Administration New Investigator Program grant (to D.L.M.).
Abbreviations
- CR
cardiovascular and respiratory
- PM
particulate matter
- ppbv
part(s) per billion by volume
- VOC
volatile organic compound
- VSL
value of a statistical life.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.U.S. Environmental Protection Agency . Air Quality Criteria for Ozone and Related Photochemical Oxidants. Washington, DC: Office of Research and Development, U.S. Environmental Protection Agency; 1996. EPA Publication EPA/600/P-93/004cF. [Google Scholar]
- 2.Levy J. I., Carrothers T. J., Tuomisto J. T., Hammitt J. K., Evans J. S. Environ. Health Perspect. 2001;109:1215–1226. doi: 10.1289/ehp.011091215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thurston G. D., Ito K. J. Exposure Anal. Environ. Epidemiol. 2001;11:286–294. doi: 10.1038/sj.jea.7500169. [DOI] [PubMed] [Google Scholar]
- 4.Anderson H. R., Atkinson R. W., Peacock J. L., Marston L., Konstantinou K. Meta-Analysis of Time-Series Studies and Panel Studies of Particulate Matter (PM) and Ozone (O3) Geneva: WHO; 2004. [Google Scholar]
- 5.Gryparis A., Forsberg B., Katsouyanni K., Analitis A., Touloumi G., Schwartz J., Samoli E., Medina S., Anderson H. R., Niciu E. M., et al. Am. J. Respir. Crit. Care Med. 2004;170:1080–1087. doi: 10.1164/rccm.200403-333OC. [DOI] [PubMed] [Google Scholar]
- 6.Bell M. L., McDermott A., Zeger S. L., Samet J. M., Dominici F. J. Am. Med. Assoc. 2004;292:2372–2378. doi: 10.1001/jama.292.19.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell M. L., Dominici F., Samet J. M. Epidemiology. 2005;16:436–445. doi: 10.1097/01.ede.0000165817.40152.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito K., De Leon S. F., Lippman M. Epidemiology. 2005;16:446–457. doi: 10.1097/01.ede.0000165821.90114.7f. [DOI] [PubMed] [Google Scholar]
- 9.Levy J. I., Chemerynski S. M., Sarnat J. A. Epidemiology. 2005;16:458–468. doi: 10.1097/01.ede.0000165820.08301.b3. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz J. Am. J. Respir. Crit. Care Med. 2005;171:627–631. doi: 10.1164/rccm.200407-933OC. [DOI] [PubMed] [Google Scholar]
- 11.Volz A., Kley D. Nature. 1988;332:240–242. [Google Scholar]
- 12.Marenco A., Gouget H., Nedelec P., Pages J. P., Karcher F. J. Geophys. Res. 1994;99:16617–16632. [Google Scholar]
- 13.Vingarzan R. Atmos. Environ. 2004;38:3431–3442. [Google Scholar]
- 14.Lelieveld J., van Aardenne J., Fischer H., de Reus M., Williams J., Winkler P. Science. 2004;304:1483–1487. doi: 10.1126/science.1096777. [DOI] [PubMed] [Google Scholar]
- 15.Lelieveld J., Dentener F. J. J. Geophys. Res. 2000;105:3531–3551. [Google Scholar]
- 16.Wang Y. H., Jacob D. J. J. Geophys. Res. 1998;103:31123–31135. [Google Scholar]
- 17.Prather M., Gauss M., Berntsen T., Isaksen I., Sundet J., Bey I., Brasseur G., Dentener F., Derwent R., Stevenson D., et al. Geophys. Res. Lett. 2003;30:1100. [Google Scholar]
- 18.Dentener F., Stevenson D., Cofala J., Mechler R., Amann M., Bergamaschi P., Raes F., Derwent R. Atmos. Chem. Phys. 2005;5:1731–1755. [Google Scholar]
- 19.Fiore A. M., Jacob D. J., Field B. D., Streets D. G., Fernandes S. D., Jang C. Geophys. Res. Lett. 2002;29:1919. [Google Scholar]
- 20.West J. J., Fiore A. M. Environ. Sci. Technol. 2005;39:4685–4691. doi: 10.1021/es048629f. [DOI] [PubMed] [Google Scholar]
- 21.Ramaswamy V., Boucher O., Haigh J., Hauglustaine D., Haywood J., Myhre G., Nakajima T., Shi G. Y., Solomon S. In: Climate Change 2001: The Scientific Basis. Houghton J. T., Ding Y., Griggs D. J., Noguer M., van der Linden P. J., Dai X., Maskell K., Johnson C. A., editors. Cambridge, U.K.: Cambridge Univ. Press; 2001. pp. 349–416. [Google Scholar]
- 22.Reilly J., Prinn R., Harnisch J., Fitzmaurice J., Jacoby H., Kicklighter D., Melillo J., Stone P., Sokolov A., Wang C. Nature. 1999;401:549–555. [Google Scholar]
- 23.Hayhoe K., Jain A., Pitcher H., MacCracken C., Gibbs M., Wuebbles D., Harvey R., Kruger D. Science. 1999;286:905–906. [Google Scholar]
- 24.Horowitz L. W., Walters S., Mauzerall D. L., Emmons L. K., Rasch P. J., Granier C., Tie X. X., Lamarque J. F., Schultz M. G., Tyndall G. S., et al. J. Geophys. Res. 2003;108:4784. [Google Scholar]
- 25.Tie X., Madronich S., Walters S., Edwards D. P., Ginoux P., Mahowald N., Zhang R., Luo C., Brasseur G. J. Geophys. Res. 2005;110:D03204. [Google Scholar]
- 26.Nakicenovic N., Alcamo J., Davis G., de Vries B., Fenhann J., Gaffin S., Gregory K., Grubler A., Jung T. Y., Kram T., et al. Special Report on Emissions Scenarios. Cambridge, U.K.: Cambridge Univ. Press; 2000. [Google Scholar]
- 27.International Energy Agency Greenhouse Gas R&D Programme . Building the Cost Curves for the Industrial Sources of Non-CO2 Greenhouse Gases. Cheltenham, U.K.: International Energy Agency; 2003. PH4/25. [Google Scholar]
- 28.Prather M., Ehhalt D., Dentener F., Derwent R., Dlugokencky E., Holland E., Isaksen I., Katima J., Kirchhoff V., Matson P., et al. In: Climate Change 2001: The Scientific Basis. Houghton J. T., Ding Y., Griggs D. J., Noguer M., van der Linden P. J., Dai X., Maskell K., Johnson C. A., editors. Cambridge, U.K.: Cambridge Univ. Press; 2001. pp. 239–287. [Google Scholar]
- 29.Stevenson D., Dentener F. J., Schultz M. G., Ellingsen K., van Noije T. P. C., Wild O., Zeng G., Amann M., Atherton C. S., Bell N., et al. J. Geophys. Res. in press. [Google Scholar]
- 30.Hogrefe C., Lynn B., Civerolo K., Ku J. Y., Rosenthal J., Rosenzweig C., Goldberg R., Gaffin S., Knowlton K., Kinney P. L. J. Geophys. Res. 2004;109:D22301. doi: 10.1289/ehp.7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knowlton K., Rosenthal J. E., Hogrefe C., Lynn B., Gaffin S., Goldberg R., Rosenzweig C., Civerolo K., Ku J. Y., Kinney P. L. Environ. Health Perspect. 2004;112:1557–1563. doi: 10.1289/ehp.7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalnay E., Kanamitsu M., Kistler R., Collins W., Deaven D., Gandin L., Iredell M., Saha S., White G., Woollen J., et al. Bull. Am. Meteorol. Soc. 1996;77:437–471. [Google Scholar]
- 33.Shindell D. T., Faluvegi G., Bell N., Schmidt G. A. Geophys. Res. Lett. 2005;32:L040803. [Google Scholar]
- 34.Pope C. A., Burnett R. T., Thun M. J., Calle E. E., Krewski D., Ito K., Thurston G. D. J. Am. Med. Assoc. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borja-Aburto V. H., Castillejos M., Gold D. R., Bierzwinski S., Loomis D. Environ. Health Perspect. 1998;106:849–855. doi: 10.1289/ehp.106-1533229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Neill M. S., Loomis D., Borja-Aburto V. H. Environ. Res. 2004;94:234–242. doi: 10.1016/j.envres.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Kim S. Y., Lee J. T., Hong Y. C., Ahn K. J., Kim H. Environ. Res. 2004;94:113–119. doi: 10.1016/j.envres.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization . The World Health Report 2004: Changing History. Geneva: WHO; 2004. [Google Scholar]
- 39.Markandaya A., Halsnaes K., Lanza A., Matsuoka Y., Maya S., Pan J., Shogren J., Seroa de Motta R., Zhang T. In: Climate Change 2001: Mitigation. Metz B., Davidson O., Swart R., Pan J., editors. Cambridge, U.K.: Cambridge Univ. Press; 2001. pp. 451–498. [Google Scholar]
- 40.Cifuentes L., Borja-Aburto V. H., Gouveia N., Thurston G., Davis D. L. Science. 2001;293:1257–1259. doi: 10.1126/science.1063357. [DOI] [PubMed] [Google Scholar]
- 41.Hourcade J.-C., Shukla P., Cifuentes L., Davis D., Edmonds J., Fisher B., Fortin E., Golub A., Hohmeyer O., Krupnick A., et al. In: Climate Change 2001: Mitigation. Metz B., Davidson O., Swart R., Pan J., editors. Cambridge, U.K.: Cambridge Univ. Press; 2001. pp. 499–559. [Google Scholar]
- 42.U.S. Environmental Protection Agency . U.S. Methane Emissions 1990–2010: Inventories, Projections, and Opportunities for Reductions. Washington, DC: U.S. Environmental Protection Agency; 1999. EPA Publication 430-R-99-013. [Google Scholar]
- 43.U.S. Environmental Protection Agency . International Analysis of Methane and Nitrous Oxide Abatement Opportunities: Report to the Energy Modeling Forum, Working Group 21. Washington, DC: U.S. Environmental Protection Agency; 2003. www.epa.gov/methane/pdfs/methodologych4.pdf. [Google Scholar]
- 44.Delhotal K. C., de la Chesnaye F. C., Gardiner A., Bates J., Sankovski A. Energy J. in press. [Google Scholar]
- 45.Fuglestvedt J. S., Berntsen T. K., Isaksen I. S. A., Mao H. T., Liang X. Z., Wang W. C. Atmos. Environ. 1999;33:961–977. [Google Scholar]
- 46.Naik V., Mauzerall D., Horowitz L., Schwarzkopf M. D., Ramaswamy V., Oppenheimer M. J. Geophys. Res. 2005;110:D24306. [Google Scholar]
- 47.Mauzerall D. L., Wang X. P. Annu. Rev. Energy Environ. 2001;26:237–268. [Google Scholar]
- 48.Felzer B., Kicklighter D., Melillo J., Wang C., Zhuang Q., Prinn R. Tellus. 2004;56:230–248. [Google Scholar]
- 49.World Meteorological Organization . Scientific Assessment of Ozone Depletion: 2002. Geneva: Global Ozone Research and Monitoring Project, World Meteorological Organization; 2003. Report No. 47. [Google Scholar]
- 50.Akimoto H. Science. 2003;302:1716–1719. doi: 10.1126/science.1092666. [DOI] [PubMed] [Google Scholar]
- 51.Holloway T., Fiore A., Hastings M. G. Environ. Sci. Technol. 2003;37:4535–4542. doi: 10.1021/es034031g. [DOI] [PubMed] [Google Scholar]
- 52.Keating T. J., West J. J., Farrell A. E. In: Intercontinental Transport of Air Pollution. Stohl A., editor. Berlin: Springer; 2004. pp. 295–320. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.