Abstract
The Drosophila selector gene cut is a hierarchal regulator of external sensory organ identity and is required to pattern the sensory and nonsensory cells of the wing margin. Cut performs the latter function, in part, by maintaining expression of the secreted morphogen encoded by wingless (wg). We find that Cut is required for wing-margin sensory organ specification in addition to and independently of Wg maintenance. In addition, we performed a genetic modifier screen to identify other genes that interact with cut in the regulation of wing-margin patterning. In total, 45 genetic loci (35 gain-of-function and 10 loss-of-function loci) were identified by virtue of their ability to suppress the wing-margin defects resulting from gypsy retrotransposon-mediated insulation of the cut wing-margin enhancer. Further genetic characterization identified several subgroups of candidate cut interacting loci. One group consists of putative regulators of gypsy insulator activity. A second group is potentially required for the regulation of Cut expression and/or activity and includes longitudinals lacking, a gene that encodes a family of BTB-domain zinc-finger transcription factors. A third group, which includes a component of the Brahma chromatin remodeling complex encoded by moira, affects the level of Cut expression in two opposing ways by suppressing the gypsy-mediated ctK phenotype and enhancing the non-gypsy ct53d phenotype. This suggests that the Brahma complex modulates both enhancer-controlled transcription and gypsy-mediated gene insulation of the cut locus.
SELECTOR genes are hierarchal regulators of developmental programs controlling tissue and cell-type diversification. The highly conserved Hox class of homeotic selector genes, which control the specification of regional identity along the anterior/posterior axis, exemplifies the ability of selector genes to instructively direct the selection between alternative developmental states. For instance, gain-of-function mutations in the Hox gene Antennapedia (Antp), resulting in the inappropriate expression of Antp protein in imaginal antennal tissue, lead to complete antenna-to-leg transformations (Schneuwly et al. 1987). It has been proposed that selector genes function by coordinating the serial activity of “realizator” genes (i.e., those genes that intimately affect basic cellular processes directing cell growth, shape, migration, proliferation, and death, among others; Garcia-Bellido 1975). Identified realizator genes include β3-tubulin (Kremser et al. 1999) and centrosomin (Heuer et al. 1995), both of which affect cyto-architectural organization, the cell adhesion molecule encoded by Connectin (Gould and White 1992), and the pro-apoptotic gene reaper (Lohmann et al. 2002). In addition to the immediate regulation of realizator gene activity, selector genes also control and integrate intermediary transcriptional and cell-signaling networks that indirectly link selector gene activity with realizator functions (reviewed in Mann and Carroll 2002; Hombria and Lovegrove 2003). Thus, to understand how selector genes define alternative developmental states, it is necessary to identify the downstream regulatory networks, as well as the realizator genes, which ultimately carry out the selected developmental program.
The Drosophila melanogaster gene cut is a neural selector gene, which establishes the developmental program directing external sensory (ES) organ identity. The peripheral nervous system is composed of diverse types of sensory organs, including ES organs (cuticular mechanosensory and chemosensory sensilla) and chordotonal organs (subcuticular proprioceptive organs). Although morphologically dissimilar, these sensory organs share molecular and developmental similarities suggestive of a common evolutionary origin (reviewed in Lai and Orgogozo 2004). During embryonic and pupal development Cut is specifically expressed in the neuroepithelial-derived sensory organ precursor cells (SOP cells) from which emerge the lineage-related cells of individual ES organs (Blochlinger et al. 1990). Loss of cut function results in the morphological and molecular transformation of ES organs into chordotonal organs (Bodmer et al. 1987; Merritt 1997). Conversely, the ubiquitous misexpression of Cut transforms chordotonal organs into ES organs (Blochlinger et al. 1991). The overexpression of Cut directs ES organ identity only in cells predetermined with proneural character (i.e., ES and chordotonal organ SOP cells) and acts in concert with factors common to these early precursor cells to direct ES organ identity. Thus cut represents a neural selector gene, the presence or absence of which is sufficient to direct alternative sensory organ fates. It is not known what downstream targets or realizator genes Cut regulates to instruct ES organ identity. The only putative Cut transcriptional target implicated in sensory organ specification is the gene bereft (bft), which is required for bristle morphogenesis (Hardiman et al. 2002).
cut is involved in the development of several embryonic and adult tissues, including the Malpighian tubules (Liu et al. 1991; Liu and Jack 1992), posterior spiracles (Hu and Castelli-Gair 1999), egg chamber (Jackson and Blochlinger 1997; Jackson and Berg 1999), flight muscles (Sudarsan et al. 2001), and wing margin (Micchelli et al. 1997). Additionally, the level of Cut expression regulates the degree of dendritic branching in a subset of multiple dendritic neurons (Grueber et al. 2003). It is not clear if cut acts as a selector gene in the development of these tissues (Liu and Jack 1992; Hu and Castelli-Gair 1999). In the developing wing, cut is required for proper patterning of the wing margin via complex interactions with multiple signaling pathways, including the Wingless (Wg) and Notch pathways (Micchelli et al. 1997). The absence of Cut activity leads to the nonautonomous degeneration of wing tissue, producing the classical “cut” wing phenotype (Jack et al. 1991). Degeneration of margin cells prefigures the development of several rows of ES organs arrayed along the anterior wing margin. It is conceivable that Cut is required early to convey a survival signal, most likely via the maintenance of Wg expression (Johnston and Sanders 2003), in addition to a later role in the specification of margin ES organ identity.
cut encodes a highly conserved homeodomain transcription factor with three novel DNA-binding domains, termed CUT repeats (Blochlinger et al. 1988; Andres et al. 1994; Moon et al. 2000). Vertebrate cut homologs, including mouse Cux1 and Cux2 (Valarche et al. 1993; Quaggin et al. 1996; Zimmer et al. 2004) and human CDP (Neufeld et al. 1992), are postulated to regulate cell growth and terminal differentiation. In diverse systems, Cut homologs functionally interact with the regulatory regions of developmentally active genes, including human histone H4 (van Wijnen et al. 1996; Gupta et al. 2003), lactoferrin (Khanna-Gupta et al. 1997, 2003), myeloid cytochrome heavy chain (gp91-phox; Skalnik et al. 1991; Lievens et al. 1995), and DNA polymerase α (Truscott et al. 2003), as well as mouse N-CAM (Valarche et al. 1993) and immunoglobulin heavy chain (IgH) (Wang et al. 1999), among others. The targeted disruption of murine Cux1 disrupts normal growth control and dermal tissue development (Ellis et al. 2001; Sinclair et al. 2001; Luong et al. 2002). Constitutive overexpression of Cux1 results in multiple organ hyperplasia, a phenotype partially attributable to the downregulation of the cyclin kinase inhibitor p27kip1 (Ledford et al. 2002). Consistent with these results, the DNA-binding ability of CDP/Cux1 is post-translationally regulated in a cell-cycle-dependent manner (Coqueret et al. 1998; Moon et al. 2001; Santaguida et al. 2001; Goulet et al. 2004), suggesting that CDP/Cux1 may act as part of a transcriptional network controlling the G1/S phase transition. Less is known about the function of murine Cux2. However, the dynamic expression pattern of Cux2 mRNA in the central nervous system, particularly in the subventricular zone and upper cortical layers of the developing telencephalon, suggests that Cux2 regulates a pool of cycling precursor cells predetermined to generate upper-layer cortical neurons (Zimmer et al. 2004). Interestingly, murine Cux1 and human CDP have been shown to functionally substitute for Drosophila Cut during embryonic development (Ludlow et al. 1996; Grueber et al. 2003), signifying a high degree of structural and functional conservation. It is not clear, however, if mammalian CDP/Cux1 genes act as selector genes in their native developmental context.
To identify genes that interact with Drosophila cut, we conducted complementary gain-of-function and loss-of-function genetic suppressor screens. For this purpose, we created >2000 new Drosophila lines, each carrying a unique insertion of the modular UAS/GAL4-based Gene Search (GS) vector (Toba et al. 1999). The GS vector contains bidirectional upstream activating sequences (UAS), which bind the transcriptional activator Gal4. Under the control of wing-margin-specific Gal4 expression, genes located near the GS vector insertion site were misexpressed and scored according to their ability to suppress the adult cut wing phenotype. Additionally, 158 deficiency chromosomes (second and third chromosomes) covering ∼50% of the genome were tested for the ability to dominantly suppress the cut allele ctK. Of the genes that were identified through these screens, the BTB-domain zinc-finger gene longitudinals lacking (lola) and several genes encoding subunits of the Brahma chromatin-remodeling complexes were investigated further with regard to their interaction with cut during wing-margin development. The genetic interactions between these genes and cut suggest that they are involved in modulating the level of Cut expression and thus act together with Cut to pattern wing-margin tissues.
MATERIALS AND METHODS
Drosophila strains and culture:
D. melanogaster stocks were reared on standard cornmeal/molasses media at room temperature or at 18°. Crosses were initiated and maintained at 25°. The cm, ct53d, and yw67c23, ct2s lines were provided by D. Dorsett. The amorphic lola5D2, lolaORE76, and lolaORE120 alleles and the decision-selective lolaORC4 and lolaORE119 alleles were provided by E. Giniger and are described elsewhere (Goeke et al. 2003). Transgenic UAS-BrmK804R, UAS-osas2, UAS-OsaRD[11c], and UAS-OsaAD[20e] lines were provided by J. Treisman and are described elsewhere (Elfring et al. 1998; Collins et al. 1999). The GS-V[1] stock was a generous gift from T. Aigaki (Toba et al. 1999). The ctK, ct6, mor1, brm2, snr1[01319], UAS-wg, UAS-p35, and all deficiency lines were obtained from the Bloomington Stock Center. The ct53d and ct2s stocks carry overlapping deletions of ∼500 bp and 1.6 kb, respectively, of the cut wing-margin enhancer, which is positioned ∼−80 kb upstream of the first exon (Liu et al. 1991; Mogila et al. 1992). The ctK and ct6 alleles result from insertions of the gypsy retrotransposon between the wing enhancer and the first exon. The ctK insertion is located −6 kb upstream of the 5′-most exon (Liu et al. 1991), whereas the ct6 insertion is located proximal to the wing enhancer (Dorsett 1993). Descriptions of the other gene mutations and deficiency chromosomes can be found on FlyBase (http://flybase.boi.indiana.edu).
The C96-Gal4 driver line carries a P{GawB} insertion at the 70D locus near the C96 gene; a gene required for viability and imaginal disc development (Gustafson and Boulianne 1996; Kim and Boulianne 1998). Flies homozygous for the P{GawB}C96 insertion are homozygous viable and wing development is normal. The P{GawB}C96 insertion directs Gal4 expression in a broad stripe straddling the dorsoventral boundary of the wing imaginal disc, which corresponds to the anlage of the adult wing margin and the cells expressing Cut. P{GawB}C96-driven Gal4 expression is unaffected by hypomorphic cut mutations.
Deficiency screen:
Prior to initiating the deficiency screen, the ctK stock was isogenized for the second and third chromosomes by first crossing females from a wild-type Oregon-R stock to males from the double balancer stock ctK/Y; Pin/CyO; D/TM6B. Individual F1 +/ctK; +/CyO; +/TM6B females were backcrossed to ctK; Pin/CyO; D/TM6B males. Stable isogenic ctK; +/+; +/+ stocks were maintained by crossing balanced F2 ctK; +/CyO; +/TM6B siblings derived from individual F1 females. The ctK stock was again isogenized after the initial screen and interacting deficiencies were rechecked. The use of two different isogenic stocks controlled for any phenotypes caused by differences in genetic background. To test for genetic interactions, female flies homozygous for ctK were crossed to male flies containing the deficiency chromosome (Df) over a marked balancer. The wings of male progeny that were ctK/Y; Df/+ were examined for a decrease in the penetrance of the ctK phenotype (see below).
Generation and screening of GS vector insertion lines:
The GS vector as described by Toba et al. (1999) contains two copies of the sequence UAS (originating form Saccharomyces cerevisiae) adjacent to a core promoter. UAS/core promoter sites are proximal to the terminal inverted repeat sequences located at either end of the P-element vector and oriented as to mediate transcription outward in both directions. Independent GS vector insertion lines were generated by mobilizing the GS vector, located on a CyO chromosome, with Δ2-3 transposase (Robertson et al. 1988). The reinsertion of the mobilized GS vector was identified via the expression of the mini-white gene. Independent reinsertion events in the second and third chromosomes were balanced with SM5-TM6, a reciprocal translocation balancer. Stable stocks were maintained over the SM5-TM6 balancer or, if possible, in a homozygous state.
To identify genetic loci that suppress the ctK wing phenotype, three male flies from each of 2066 individual GS lines, with insertions on the second or third chromosomes, were crossed to six females of the genotype w,ctK;C96-Gal4. The penetrance of the cut wing phenotype of male progeny of the genotype ctK/Y;GS*/C96-Gal4 was compared to ctK/Y;C96-Gal4/UAS-lacZ and ctK/Y;C96-Gal4/+ controls. The controls showed a completely penetrant (>99%) ctK wing phenotype, as demonstrated by numerous discontinuities (i.e., gaps) in the regular array of wing-margin sensory bristles and frequent incisions of wing tissue. The expressivity of the ctK wing phenotype was identical for both controls. In the initial testing of all 2066 GS lines and the 158 deficiencies, 20–40 males of the genotypes ctK;GS*/C96-Gal4 or ctK;Df/+ were examined. In addition, females were examined for dominant effects. Due to the prevalence of dominant effects that enhanced the ctK phenotype resulting from the overexpression of the various GS insertions, we opted not to characterize these lines further. Those lines (GS or deficiency) that were found to suppress the ctK phenotype were retested. GS lines were retested by crossing to both ctK; C96-Gal4 and ctK alone to determine if suppression resulted from overexpression or from gene disruption. In all cases, >100 ctK males were screened in the retests. Identical but independent crosses produced similar results in the majority of cases. To confirm the interaction with cut, GS lines were secondarily tested for the ability to interact with ct53d; C96-Gal4. The presence of discontinuities in the anterior wing margin of ct53d/Y;C96-Gal4/UAS-lacZ and ct53d/Y;C96-Gal4/+ controls were less penetrant (∼50–60%) than with ctK, allowing increases (enhancement) or decreases (suppression) in penetrance to be scored. To maintain consistency, all crosses were performed with cut alleles in the presence of the P{GawB}C96 insertion. The presence of P{GawB}C96 did not influence the interaction between any of the loss-of-function mutations and cut.
Individual GS insertion lines were scored according to their ability to suppress or enhance the penetrance of the cut wing phenotype. For our purposes, only a discrete region of the anterior wing margin, consisting of the region stretching from the proximal-most point of the anterior wing margin to the distally located intersection of the L1 and L2 wing veins (see Figure 1C), was scored. This region was chosen because it encompasses most of the triple row of innervated sensory bristles and can be easily examined in anesthetized intact animals with their wings tucked back in the resting position. In cases in which discontinuities were not observed within this region of individual experimental wings, the cut wing phenotype was considered suppressed. Conversely, in cases in which discontinuities were observed, regardless of the number of bristles affected, the cut wing phenotype was considered not suppressed. The scoring system is based on “all or none” suppression and therefore does not take into account individual variation in either the frequency or the severity of margin bristle loss of individual wings. The degree of penetrance correlated well with the degree of severity. In Table 2, “suppression of ctK” was calculated for each genotype by dividing the number of wings suppressed by the total number of wings scored. The ability of deficiency chromosomes to affect ctK and ct53d was scored in a similar manner. To quantify phenotypes, dissected wings of each genotype were dehydrated in absolute ethanol, mounted in Canada Balsam:methyl-salicylate (1:3), and photographed at 10× magnification using a digital camera (Canon Power Shot S45) mounted on a compound microscope (Zeiss, Axioplan). For each genotype, a representative image of a median wing phenotype was selected from a photographic series.
Figure 1.—
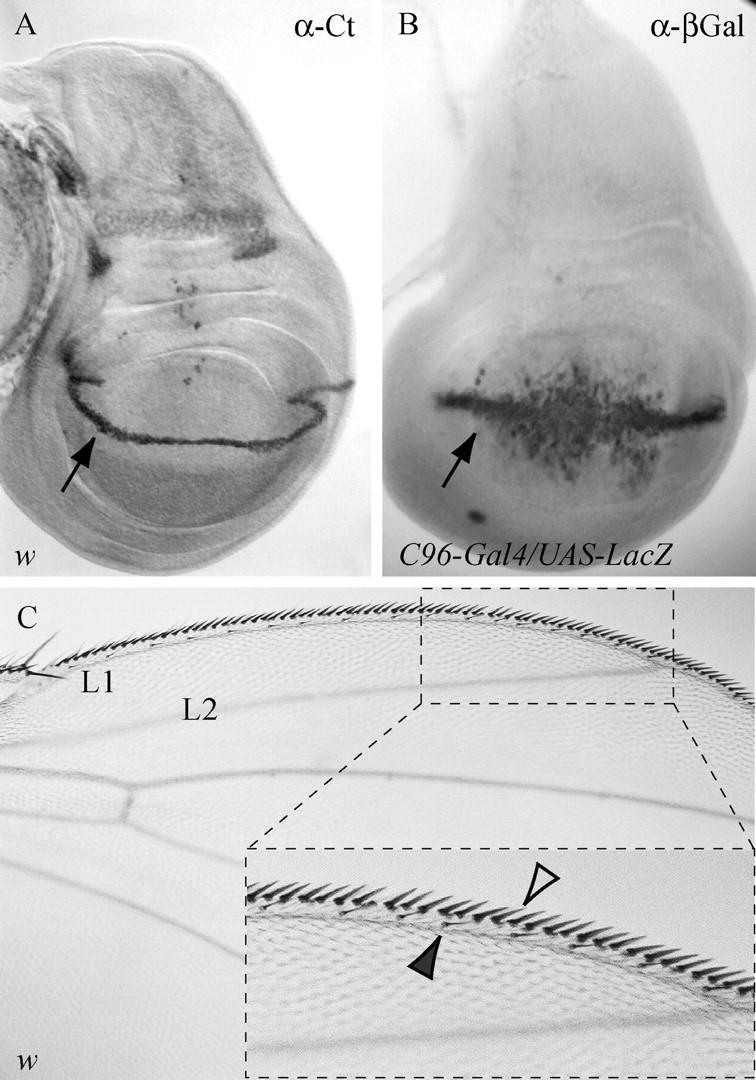
Cut is expressed in the wing margin and is required for the development of the margin sensory bristles. (A) In the third larval instar wing disc, Cut is expressed in a stripe of cells 3–4 cells wide (arrow), corresponding to the Wg organizer. These Cut-expressing cells are the precursor cells of the stout and slender margin mechanosensory bristles. (B) C96-Gal4 drives expression in a pattern overlapping the Cut-expressing cells. (C) The anterior wing margin, delimited by the L1 wing vein, is composed of a triple row of sensory bristles. Positioned on the dorsal wing surface is a single row of slender-shaft recurved chemosensory bristles (inset, solid arrowhead) adjacent to a row of stout-shaft mechanosensory bristles (inset, open arrowhead). Ventral and out of the plane of focus is the third bristle row composed of slender mechano- and chemosensory bristles.
TABLE 2.
Summary of gain- and loss-of-function GS vector insertion lines and deficiency chromosomes: molecular data and genetic interactions withcut
| Locusa | Cytology | Insertion-site flanking sequenceb | GS vector insertion |
Molecular function/domain structure |
Suppression of ctKc (%) |
Genetic interactions: cut wing allelesd |
|---|---|---|---|---|---|---|
| Gain-of-function GS vector lines | ||||||
| lolae | 47A11-13 | ACGCTTTTTTCCAACGAGAC_ | GS[A916] | Transcription factor/zinc finger/BTB domain |
94 | Group A (ct6, ct53d, ct2s sup.) |
| brat | 37C1-6 | TCCTCTCGAAAGTTCTGCGG_ | GS[A2233] | Transcription factor/B-box zinc finger |
77 | Group A (ct53d sup.) |
| CG14757 | 44B9 | AACTCGAACTCACACCAAAC_ | GS[A2066] | — | 60 | Group A (ct53d sup.) |
| CycE | 35D | AGTGAAGGAAAGAGCGGGAG_ | GS[A1869] | Cyclin-dependent protein kinase regulator |
4 | Group A (ct53d sup.) |
| CG12340d | 47C1 | GTCTTCGTCG _(TTTAAGGG)2CA | GS[A2450] | — | ∼ct rescue | Group B (ct53d enh.) |
| squeeze (sqz) | 91F8-9 | _TGTCGCCCCCAACAAAAGAG | GS[A2967] | Transcription factor/zinc finger | ∼ct rescue | Group B (ct53d enh.) |
| eukaryotic initiation factor 4E (eIF-4E)e | 67B3 | _CGCATACCACACGTTTTCAG | GS[A2783] | Translation initiation factor | 55 | Group B (ct53d enh.) |
| eukaryotic initiation factor 4a (eIF-4a) | 26B2 | _GTTCACACGCTGCGGTAAAA | GS[A2207] | Translation initiation factor/ DEAD-box/helicase |
49 | Group B (ct53d enh.) |
|
Adult enhancer factor 1 (Aef1) |
78D2 | GCCACAGATAATGCTGTGAG_ | GS[A2724] | Transcription factor/zinc finger | 45 | Group B (ct53d enh.) |
| lesswright (lwr) | 21E1 | _AGTGAGACCCTTTGTGTAGA | GS[A2612] | Ubiquitin-like conjugating enzyme |
35 | Group B (ct53d enh.) |
| fusilli (fus) | 52B3-5 | ATAAGCGGCCCACGCACACC_ | GS[A1497] | EGFR-signaling pathway/RNA binding |
22 | Group B (ct53d enh.) |
| posterior sex combs (psc) | 49E6 | _GGCCGAGCCACGACGACACG | GS[A2026] | Chromatin-remodeling/RING finger domain |
16 | Group B (ct53d enh.) |
| CG5390 | 31D1 | _GGCTGAGACTTAAGATTGAA | GS[A2688] | Serine protease | 11 | Group B (ct53d enh.) |
| Sphingomyelin synthase-related (SMSr) | 65F7-9 | _CTCTGAACGGAACAACTGAG | GS[A2330] | Sphingomyelin biosynthesis | 8 | Group B (ct53d enh.) |
| chickadee (chic) | 26A5-B2 | TCAAAATCGGTTTATGGTTC_ | GS[A2665] | Cytoskeleton constituent/actin-binding domain | 7 | Group B (ct6 sup./ct53d enh.) |
| apontic (apt)e | 59F1-4 | ATTGTCATTA_(CTTTTGGC)2CC | GS[A960] | Transcription factor/Myb domain |
∼ct rescue | Group C (ctK sup.) |
| hairy (h) | 66D1 | TATATATAGCGCAACCATCC_ | GS[A1546] | Transcription factor/HLH dimerization domain |
∼ct rescue | Group C (ctK sup.) |
| hephaestus (heph) | 100D3-E1 | _ATCCAGCGGAAAGAGAGCGG | GS[A1768] | Polypyrimidine tract binding | ∼ct rescue | Group C (ctK sup.) |
| CG7752 | 78C4-5 | TCCGTCGAGAACTGCTACAG_ | GS[A1165] | Transcription factor/zinc finger | 61 | Group C (ctK sup.) |
|
G protein α-subunit 65A (G-iα65A) |
65D5 | _ATTCCGGTATTTCCCCCCTT | GS[A2888] | Guanine-nucleotide-binding protein, α-subunit | 37 | Group C (ctK sup.) |
| CG30497 | 43E13-16 | _GCACGGAACGTAGAACGCAG | GS[A1957] | — | 25 | Group C (ctK sup.) |
| CG10373 | 37A1 | CATTGCTTGTTAGTCAGCAC_ | GS[A1703] | Amino acid transport | 25 | Group C (ctK sup.) |
| Ssl1 | 80B2 | _AGCCGGCGCATTTTATTTAG | GS[A1702] | Transcription factor/TFIIH complex |
23 | Group C (ctK sup.) |
| schnurri (shn) | 47D6-E1 | _ACTATAAGTTAGCAAACAAA | GS[A2114] | Transcription factor/zinc finger | 17 | Group C (ctK sup.) |
| CG31782 | 36A10-12 | _GTCCGAAGGCTTATACAGAA | GS[A2197] | Transcription factor | 13 | Group C (ctK sup.) |
| CG1888 | 45F1 | _AATGTCTACATACGCGTACA | GS[A2442] | — | 10 | Group C (ctK sup.) |
| CG7920 | 99D1 | _GGCGAACCAGTTGCAAATTT | GS[A828] | Acetyl-CoA hydrolase/ transferase |
7 | Group C (ctK sup.) |
| regular (rgr) | 44D4 | GTAAGTTAATCACCGCCGCC_ | GS[A1998] | Transcription factor/zincfinger | 6 | Group C (ctK sup.) |
|
Activin like protein at 23B (Alp23B) |
23B1-2 | _GTCTATAGTCATAAATCGAG | GS[A1800] | Signaling ligand/TGFβ-like | 4 | Group C (ctK sup.) |
| anterior open (aop) | 22D1 | GCTCCGCTTTACGGCTGGCA_ | GS[A1685] | Transcription factor/ Et-domain |
4 | Group C (ctK sup.) |
| Serine palmitoyltransferase subunit I (Spt-I) | 49F4 | GATATTTCACGCCTTTTGCC_ | GS[A2040] | Aminotransferase/ pyridoxal 5′-phosphate (PLP)- dependent transferase |
4 | Group C (ctK sup.) |
| trx | 88B1 | _GTTAGAATTTTCGTTTATCT | GS[A2270] | Chromatin-remodeling/PHD domain |
4 | Group C (ctK sup.) |
| 14-3-3ζ | 46E6-8 | GTTAAGTTGTAGGCGCGGAC_ | GS[A2789] | Protein kinase C inhibitor | 4 | Group C (ctK sup.) |
| CG15236 | 42D4-6 | ATGAATGCCAGACCCAGAGC_ | GS[A2203] | – | 4 | Group C (ctK sup.) |
| CG17075 | 21B6-7 | TACGAACCTATAACTGCGCC_ | GS[A1942] | – | 3 | Group C (ctK sup.) |
| Loss-of-function GS vector lines | ||||||
| mor | 89A11 | _GATTCGCCAGTGGCTGCAGA | GS[A897] | Chromatin-remodeling/Myb domain |
79 | Group B (ct53d enh.) |
| Vha68-2 | 34A3 | GAGAAAAGCAGCAATCACAC_ | GS[A1548] | Cation transport | 24 | Group B (ct53d enh.) |
| thioredoxin-2 (Trx-2) | 30C1 | _GATGTGCCAATCGGTCAATC | GS[A866] | Thiol-disulfide exchange intermediate |
40 | Group C (ctK sup.) |
| CG6907 | 25E5 | _GTGTGCCCCCATTGGCAGCC | GS[A945] | — | 28 | Group C (ctK sup.) |
| CG9270 | 38F6 | _CCTCGGGCACTCCGTAAACG | GS[A839] | ABC transporter | 16 | Group C (ctK sup.) |
| mitochondrial ribosomal protein L4 (mRpL4) | 35F11 | ACATTTTTCG_(TGTCACGG)2TG | GS[A961] | Mitochondrial large ribosomal subunit |
14 | Group C (ctK sup.) |
| stathmin (stai) | 26B9 | AAGCCCAGCTGGTGCTCACC_ | GS[A1533] | Microtubule-associated protein | 8 | Group C (ctK sup.) |
| Deficiency chromosomes | ||||||
| Df(2L)Prl | 32F-3; 33R1-2 |
100 | ND | |||
| Df(3L)Cat | 75C1-2; 75F1 |
100 | ND | |||
| Df(3R)p25, Df(3R)P2 | 85A3; 85B1, 89D9-E1; 89E2-3 |
100 | ND | |||
ND, not determined.
Gene or predicted gene located closest to the insertion site and positioned in the 5′–3′ orientation relative to the GS vector.
Genomic sequence of the coding strand (5′–3′ orientation) flanking the site of GS vector insertion (underscore).
GS insertion lines were crossed to ctK; C96-Gal4 females. Male progeny of the genotype ctK/Y; GS[*]/C96-Gal4 were scored. The percentage of suppression is equal to the number of wings displaying a complete suppression of ctK-associated gaps in the anterior margin sensory bristles divided by the total number of wings scored. Cases in which ctK suppression resembled the dominant phenotype resulting from the overexpression of Cut are represented by “∼cut rescue.” The suppression of ctK for all GS vector insertion lines is significant (P ≤ 0.01). Less than 1.0% of negative control males (ctK/Y; UAS-lacZ/C96-Gal4) were suppressed.
Genetic interactions with cut wing alleles ct6 (gypsy), ct53d (non-gypsy), and ct2s (non-gypsy) are summaried.
Multiple unique GS vector insertions were identified within locus.
Molecular analysis of GS vector insertion lines:
Genomic sequences flanking the 5′-end and/or the 3′-end of the GS vector insertions were recovered by inverse PCR. Total DNA isolated from individual GS vector insertion lines was digested with Sau3AI (with 5′ primer set) or MspI (with 3′ primer set) and ligated under dilute conditions according to the protocol available from the Berkeley Drosophila Genome Project. Genomic DNA immediately flanking the 5′- and 3′-ends of the GS vector was amplified by PCR using the following GS-vector-specific primer sets: GS[5′] (GS[5′R]—5′-CCG TAG ACG AAG CGC CTC TAT TTA TAC T-3′ and GS[5′L]—5′-CCT CTC AAC AAG CAA ACG TGC ACT GAA) and GS[3′] (GS[3′R]—5′-CGC TGT CTC ACT CAG ACT CAA TAC GAC A-3′ and GS[3′L]—5′-GCT TAG CTT TCG CTT AGC GAC GTG TTC A-3′). PCR products were sequenced using the GS[5′L] or GS[3′R], as used in the initial amplification reaction. Sequence analysis was performed using the BLASTN program administered by the National Center for Biotechnology. This allowed GS vector insertion sites to be precisely located and known or predicted genes immediately flanking the insertion site to be identified. The Apollo Genome Annotation and Curation Tool, Version 1.3.5 (Lewis et al. 2002) was used to establish the proximity of individual GS insertions to flanking genes. The GS vector insertion site was determined for 66 of 79 GS lines that suppressed the ctK phenotype. The “locus” heading in Table 2 represents the known or predicted gene closest to and downstream of the GS insertion.
In situ hybridization, immunohistochemistry, and X-Gal staining:
In situ hybridization to third instar wing discs was performed as described by Sturtevant et al. (1993) using digoxigenin (DIG)-labeled RNA probes and visualized using alkaline phosphatase conjugated α-DIG antibody (Roche; 1:200). To generate lola and pipsqueak (psq) RNA probes, coding DNA sequences from both loci were amplified by PCR using gene-specific primer sets lola[766bp] (lola[A]—5′-GTC CTC GTC ATC GCC TTG-3′ and lola[B]—5′-GAA CAG TAC GAC AAA CAT CC-3′) and psq[644bp] (psq[A]—5′-GTA GCG ATA GCG TGC CAG-3′; and psq[B]—5′-GCT GCT GAA ACA CGG ACG-3′)]. PCR products were cloned into the pGEM-T Easy Vector (Promega, Madison, WI). Immunohistochemistry on third instar wing discs was performed according to standard techniques. Dissected third instar wing discs were fixed with 4% formaldehyde/NaPO4, washed with PBS/0.5% Triton X-100 (PBST), and blocked with PBST/4% BSA. Antibodies were used at the following dilutions: α-Wg (1:50; 4D4, Developmental Studies Hybridoma Bank), α-Ct (1:20; 2B10, DSHB), α-βGal (1:40; 40-1a, DSHB), and horseradish peroxidase conjugated α-mouse (1:200; Bio-Rad, Richmond, CA). In situ detection of β-galactosidase activity in dissected third instar wing imaginal discs was carried out as described by Glaser et al. (1986).
RESULTS
Cut is required independently of Wingless maintenance to specify wing-margin sensory organs:
The presumptive wing margin of the third instar larval wing disc consists of a stripe of Cut-expressing cells located at the dorsal/ventral boundary, a region corresponding to the Wg organizer (Figure 1A). Patterning of the wing margin, which contains an organized array of chemosensory and mechanosensory bristles (Figure 1C), is regulated in part by the secreted morphogen, Wg (Phillips and Whittle 1993; Johnston and Edgar 1998; Johnston and Sanders 2003; Duman-Scheel et al. 2004). Cut activity is required to maintain Wg expression in the presumptive wing margin, which otherwise degenerates cell nonautonomously. Since degeneration of wing tissue prefigures the development of the margin sensory bristles, it has been difficult to resolve the autonomy of Cut function in margin sensory organ specification. To determine a Wg-independent requirement for Cut in wing-margin development, we prevented degeneration of margin tissue in cut mutants by (1) maintaining Wg expression ectopically and (2) preventing apoptotic cell death through the misexpression of the baculovirus caspase inhibitor p35.
The ctK, ct6, and ct2s alleles display a cut wing phenotype characterized by incised wing-blade tissue and decreased numbers of margin bristles (Figure 2, A–C). Whereas the ctK allele primarily disrupts margin bristle development, ct6 and ct2s disrupt both blade tissue and margin bristle development. The margin-specific overexpression of UAS-cut directed by the C96-Gal4 driver significantly rescues the cut wing phenotype (Figure 2, D–F). In hemizygous ctK/Y mutant males, the large discontinuities in the regular array of anterior margin bristles are rescued by Cut overexpression, but the total number of margin bristles remains less than that of wild type (Figure 2D). This may be interpreted as an incomplete rescue. However, since a similar reduction in margin bristle number is observed when Cut is overexpressed in heterozygous ctK/+ females (Figure 2J), which under normal conditions have wings of wild-type morphology, we prefer the alternative interpretation that a complete rescue is confounded by a dominant negative effect resulting from Cut overexpression (see also Ludlow et al. 1996). Cut overexpression also partially restores blade tissue and margin bristles of the ct6 and ct2s alleles (Figure 2, E and F). In particular, the number of stout mechanosensory bristles is significantly rescued (Table 1).
Figure 2.—
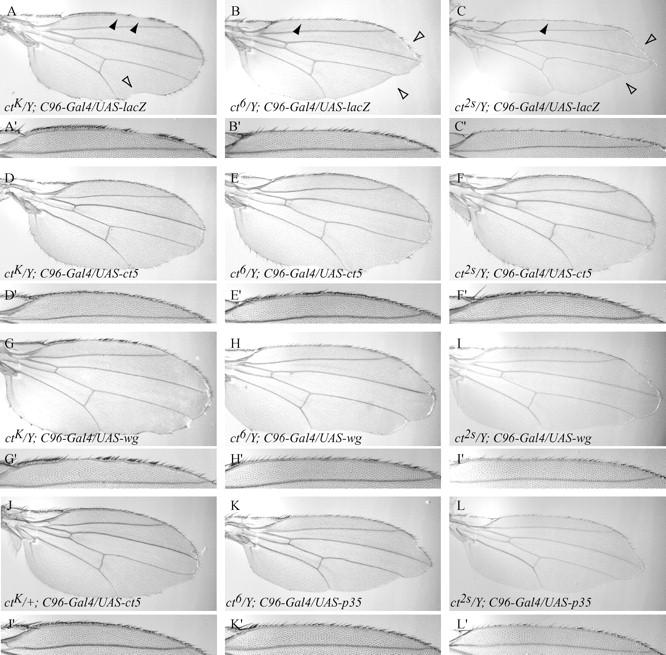
The requirement for cut in patterning the wing margin is independent of its role in maintaining Wg expression. All genotypes were reared at 25°, excepting G–I, which were raised at 18°. (A–C) The Lethal I cut allele, ctK, and the two cut alleles, ct6 and ct2s, display large discontinuities in the margin bristles (solid arrowheads), in addition to incised margin and blade tissue (open arrowheads). (D–F) C96-Gal4-directed overexpression of UAS-ct5 suppresses the wing defects in cut mutants. Although the large discontinuities in the anterior margin bristles of ctK are suppressed, the total number of sensory bristles is only partially restored, likely reflecting a dominant Cut misexpression phenotype (see J). (G–I) Overexpression of UAS-wg suppresses the degeneration of wing-blade tissue in cut mutants, but is unable to restore margin bristles. (J) The overexpression of UAS-ct5 in heterozygous ctK females disrupts anterior margin bristle development. A similar effect was observed in wild-type individuals. (K and L) Blocking apoptosis by overexpression of UAS-p35 partially suppresses the loss of blade and margin tissue, but is unable to suppress the loss of margin bristles.
TABLE 1.
Rescue of anterior wing-margin sensory bristles
| Crossed to:
|
||||
|---|---|---|---|---|
|
ct2s; C96-Gal4
|
ct6; C96-Gal4
|
|||
| Stout-shaft | Slender-shaft | Stout-staft | Slender-shaft | |
| UAS-lacZ | 7.7 ± 0.6 (n = 15) | 21.7 ± 0.6 (n = 15) | 12.2 ± 0.7 (n = 19) | 22.3 ± 0.6 (n =1 9) |
| UAS-p35 | 9.9 ± 0.5 (n = 15)** | 28.1 ± 0.7 (n = 15)*** | 16.1 ± 0.9 (n = 15)*** | 28.8 ± 0.6 (n = 15)*** |
| UAS-wg | 6.6 ± 0.6 (n = 11) | 40.1 ± 0.8 (n = 11)*** | 10.0 ± 3.0 (n = 2) | 43.5 ± 2.5 (n = 2)*** |
| UAS-ct5 | 35.4 ± 4.0 (n = 9)*** | 35.6 ± 3.1 (n = 9)*** | 40.9 ± 1.8 (n = 14)*** | 43.7 ± 1.8 (n = 14)*** |
| lolaGS[A916] | 34.0 ± 1.3 (n = 16)*** | 35.4 ± 1.5 (n = 16)*** | 35.9 ± 2.9 (n = 9)*** | 37.4 ± 1.5 (n = 9)*** |
Summation of dorsal and ventral slender-shafted mechano- and chemosensory bristles and stout-shafted mechanosensory bristles located within the region stretching from the hinge-proximal anterior wing margin to the L1/L2 wing-vein intersect. Standard error of mean is given. Wild-type wings display an average of 68.8 ± 0.6 dorsal and ventral slender bristles and 69.8 ± 0.6 stout-shafted bristles. Statistical significance was determined using Student's t-test; **, P < 0.01; ***, P < 0.001. Although there is some evidence of improvement in slender bristle number resulting from the misexpression of either UAS-p35 or UAS-wg misexpression, the number of stout bristles is only minimally affected in either instance.
Ectopically supplying Wg in the presumptive wing margin of ctK, ct6, and ct2s mutants also suppresses the loss of wing-blade tissues, including the slender recurved chemosensory bristles, but does not rescue the loss of the stout mechanosensory bristles (Figure 2, G–I, and Table 1). In several hypomorphic cut mutations, although Cut expression is disrupted in the mechanosensory and non-innervated bristles of the wing margin, expression in the precursor cells of the slender chemosensory bristles is unaffected (Jack et al. 1991). It has been proposed that loss of Cut expression in the wing margin results in the failure of the mechanosensory and non-innervated bristles to differentiate, followed by the cell nonautonomous degeneration of the margin. Therefore, it is possible that the loss of the slender chemosensory bristles is a secondary effect resulting from degeneration of the margin, rather than a cell autonomous effect resulting from the loss of Cut expression. We propose that by ectopically expressing Wg in cut mutants we suppress the degeneration of wing-margin tissue, which includes the slender chemosensory bristles, resulting from the failure of Cut-dependent maintenance of Wg expression. Only those cells that actually fail to express Cut (i.e., the precursor cells of the stout mechanosensory bristles) fail to be rescued by the misexpression of Wg. Thus, we propose that Wg is unable to promote sensory bristle development independently of Cut function and suggest that Cut is required autonomously for sensory bristle specification in a manner independent of its role in maintaining Wg expression. However, the possibility that a Wg signal, in addition to Cut activity, is required for margin bristle specification cannot be excluded.
Gain- and loss-of-function suppressor screens of the cut wing phenotype:
Having determined the dual requirement of cut to maintain margin cell survival and to specify margin bristle identity, we carried out complementary loss-of-function and gain-of-function suppression screens of the ctK phenotype to identify genes that interact with cut during wing-margin patterning. ctK is classified as a Lethal I cut allele, as defined by its failure to complement all cut mutant alleles except for the kinked femur class (Jack 1985). Placed in trans to a cut null allele, ctK is characterized by both semilethality and the transformation of embryonic ES organs into chordotonal organs. Unlike other Lethal I alleles, however, adult males that are hemizygous for the ctK allele are viable and display a completely penetrant cut wing-margin phenotype (Figure 2A). Because of these characteristics, we reasoned that ctK would provide a uniquely sensitized background in which genetic suppressor screens could be designed to identify genes involved in both wing-margin patterning and sensory bristle specification.
In an initial approach, we carried out a dominant loss-of-function suppression screen using available cytological deficiencies covering ∼50% of the genome. Male flies from each deficiency line were crossed to females homozygous for ctK, and the cut wing phenotype of the resulting male progeny—hemizygyous for ctK and heterozygous for the deficiency chromosome—was scored. The capacity of each deficiency chromosome to dominantly suppress ctK was quantitatively assessed according to their ability to reduce the overall penetrance of ctK-associated discontinuities in the anterior wing-margin sensory bristles (materials and methods). The results of this screen are summarized in Table 2. Df(2L)Prl, Df(3L)Cat, and Df(3R)p25-Df(3R)P2 completely suppressed ctK. Unlike the Df(3R)p25-Df(3R)P2 dual deficiency chromosome, neither Df(3R)p25 nor Df(3R)P2 alone was able to suppress ctK. It is possible that the two deficient genomic regions cooperate to suppress ctK, or an unrelated second-site mutation present only in the dual deficiency may be responsible for the suppression. A fourth deficiency, Df(3R)sbd105, partially suppressed ctK as assessed by a decrease in the severity of margin bristle loss, but did not reduce the overall penetrance of the phenotype. Df(3R)sbd105 covers moira (mor), a gene encoding a core subunit of the Brahma (BRM) chromatin-remodeling complex. Genetic interactions between cut and components of the BRM complex are examined below.
The ctK allele results from the insertion of a gypsy retrotransposon into the cut locus ∼6 kb upstream of the first exon, where it partially disrupts the regulation of embryonic and adult Cut expression (Jack 1985; Jack and DeLotto 1995). In wing tissue, the gypsy element functions by insulating the activity of the distal wing-margin enhancer from the proximal promoter, resulting in the loss of Cut expression specifically in the wing margin. At least two genes are known to be directly required for gypsy-mediated gene insulation: Suppressor of Hairy wing [Su(Hw)] and modifier of mdg 4 [mod(mdg4)] (Hoover et al. 1992; Gause et al. 2001; Ghosh et al. 2001). The ctK allele is unusual in that it contains a mutated gypsy insulator with a partial deletion of the Su(Hw)-binding region, which presumably makes it more sensitive to moderate decreases in the activity of Su(Hw) and mod(mdg4) (Hoover et al. 1992). As part of the deficiency screen, two deficiencies, Df(3R)red1 and Df(3R)e-N19, respectively covering Su(Hw) and mod(mdg4), were tested for an interaction with ctK. Although loss-of-function mutations in both genes have been shown to dominantly suppress ctK (Hoover et al. 1992; Gause et al. 2001), our screen failed to identify either deficiency. In the case of mod(mdg4), however, mutations that suppress ctK behave as antimorphic alleles in that they suppress the wing phenotype more strongly than null alleles do. This could account for why we did not identify Df(3R)e-N19 as a dominant suppressor of ctK. Similarly, the chromosome deficiencies Df(2R)vg-B, Df(2R)Px4, and Df(2R)nap1, covering loci previously shown to encode positive regulators of Cut expression and including the genes vestigial, Chip, and Nipped-B, respectively, were tested for an interaction with ctK. Only Df(2R)nap1 showed an interaction in the wing. Df(2R)nap1 enhanced the wing phenotype of hemizygous ctK males and produced a mild dominant cut wing phenotype in heterozygous ctK females. This is consistent with previous evidence suggesting that Nipped-B facilitates the activation of cut expression (Rollins et al. 1999, 2004). Deficiencies covering other regulators of cut expression, including scalloped and mastermind, were not tested (Morcillo et al. 1996).
We also conducted a complementary gain-of-function screen using the modular GS system of misexpression (Toba et al. 1999). The margin-specific Gal4 driver, C96-Gal4, was used to drive expression of genes located proximal to 2066 unique insertions of the GS vector (materials and methods). The ability of individual GS lines to suppress ctK was scored as described above. In total, 3.8% of the GS vector insertions (79/2066), representing at least 42 distinct loci, were found to suppress the ctK phenotype (Table 2). Insertions at 35 loci suppressed the ctK phenotype in response to Gal4-dependent misexpression. The seven remaining loci suppressed without the C96-Gal4 driver and presumably act as dominant loss-of-function suppressors of ctK. In addition, a large number of gain-of-function GS lines (319/2066) enhanced the ctK phenotype, as determined by an increase in the severity of margin tissue loss. Due to the large number of ctK-enhancing loci, we opted not to characterize them further and instead focused on the suppressing loci.
As previously stated, it is possible that genes identified by the ability to suppress ctK may result from an interaction with the cut regulatory region or, alternatively, with the gypsy insulator or a gene required for gypsy insulator activity. To distinguish between these possibilities and to further characterize the interaction with cut, we examined the ability of the candidate GS suppressor lines to modify the wing phenotype of the weak ct53d allele. In contrast to ctK, the ct53d allele results from a partial deletion (∼500 bp) of the minimal cut wing-margin enhancer (defined as a region of ∼2.7 kb) and does not contain gypsy-derived elements (Jack et al. 1991; Mogila et al. 1992). The ct53d allele disrupts Cut expression primarily in the presumptive wing tip, which corresponds to a severe loss of wing tissue in the distal-most region of the adult wing. The genetic interaction data with ct53d are summarized in Table 2.
Candidate suppressor loci were classified into three groups according to the genetic interaction with ctK and ct53d. Group A loci suppress both ctK and ct53d and are expected to represent candidate regulators or effectors of cut activity. The interaction with ct53d indicates that group A loci do not suppress the ctK wing phenotype simply by interfering with gypsy activity. In contrast, group B loci suppress ctK and enhance ct53d, suggesting a more complex interaction with the cut locus during wing-margin patterning. This may include direct interference with gypsy insulator activity, in addition to being required for cut wing enhancer activity. It should be noted that all group B loci, except a subgroup that suppressed the ctK equivalent to the UAS-Cut rescue, do not adversely affect wing development when misexpressed in heterozygous cut mutant females. Thus, enhancement of the wing phenotype in hemizygous ct53d mutant males is unlikely due to misexpression alone. Finally, group C includes candidate loci that suppress only ctK and are therefore presumed to interfere with gypsy activity. For instance, one gain-of-function suppressor contains a GS insertion near trithorax (trx), a gene previously shown to enhance gypsy insulator activity when mutated (Gerasimova and Corces 1998).
Group A consists of four candidate loci that suppress both ctK and ct53d and includes brain tumor (brat), CyclinE (CycE), and lola. brat encodes a tumor-suppressor protein (Frank et al. 2002) and CycE a cell cycle regulator controlling the G1/S phase transition (Richardson et al. 1993, 1995). Thus, both may act to suppress the wing phenotype by influencing cell growth and proliferation. The genetic interaction with lola is explored further below.
Group B consists of 12 candidate loci, including two genes encoding components of the eukaryotic translation initiation factor 4F complex (eIF-4F), eIF-4A and eIF-4E (reviewed in Gebauer and Hentze 2004). eIF-4A and eIF-4E regulate translation downstream of the insulin/target of rapamycin signaling pathway and as such act globally to regulate cell growth and proliferation (Miron et al. 2003). Overexpression of eIF-4E and eIF-4A may relieve putative cell growth or survival deficits associated with the loss of cut activity by enhancing the translation of Cut target genes. Similarly, group B candidate genes lesswright (lwr) and fussilli (fus) are also involved in regulating cell growth or proliferation of wing imaginal tissue. Some heterozygous mutants of lwr, a gene encoding a ubiquitin-like conjugating enzyme, exhibit wings severely reduced in size (Epps and Tanda 1998). Lwr has been shown to be required for the nuclear import of Bicoid during early embryogenesis (Epps and Tanda 1998). It is possible that Lwr plays a role in the nuclear import of Cut or its downstream targets. The RNA-binding protein Fus is involved in regulating cell growth in the wing disc and, similarly to eIF-4F, may affect the translation of Cut target genes (Wakabayashi-Ito et al. 2001; Raisin et al. 2003). The differential interaction of group B candidates with various cut alleles likely reflects either direct or indirect effects on both gypsy insulator activity and cut wing-enhancer-mediated transcription. It will be of interest to determine if group B loci regulate gene insulation and transcription via common mechanisms.
A subgroup of gain-of-function candidate genes, including Hephaestus (heph), suppress the ctK wing-margin patterning defects to a degree comparable to the UAS-Cut rescue; the large discontinuities in the triple-row bristles are mitigated, but the total number of sensory bristles is less than normal. Heph is expressed in the presumptive wing and encodes a polypyrimidine tract binding protein that binds to and regulates RNA stability (Dansereau et al. 2002). Heph appears to attenuate Notch signaling downstream of the binding of the Notch ligand, Delta, and heph− clones cause the nonautonomous formation of wing-margin structures (Dansereau et al. 2002). How the overexpression of Heph and presumably the attenuation of Notch signaling suppresses ctK is not clear. It is possible that Heph may affect the activity of the gypsy insulator, since overexpression of Heph did not produce an appreciable alteration of the ct53d wing phenotype.
longitudinals lacking is required for cut-dependent wing-margin patterning:
Twenty-one GS vector lines with insertions at 12 unique locations proximal to the coding region of lola were identified by their ability to suppress the ctK allele (lolaGS[A916]; Figure 3, A and B). All lolaGS insertions require the C96-Gal4 driver, indicating that suppression results from GS-vector-mediated overexpression. lolaGS[A916]-mediated suppression of the ctK phenotype is robust with 94% of ctK mutant wings displaying a normal triple row of sensory bristles (Table 2). The ct53d, ct6, and ct2s alleles are also strongly suppressed by lolaGS[A916] (Figure 3, C–H, and Table 1), demonstrating that the interaction with cut in the wing margin is not allele specific. In addition to reversing the loss of sensory bristles, lolaGS[A916] suppresses the loss of blade tissue, a phenotype thought to result from a failure of Cut to maintain Wg expression (Micchelli et al. 1997). lolaGS[A916] may interact with cut both during Wg-dependent patterning of the wing margin and during the specification of margin bristles.
Figure 3.—
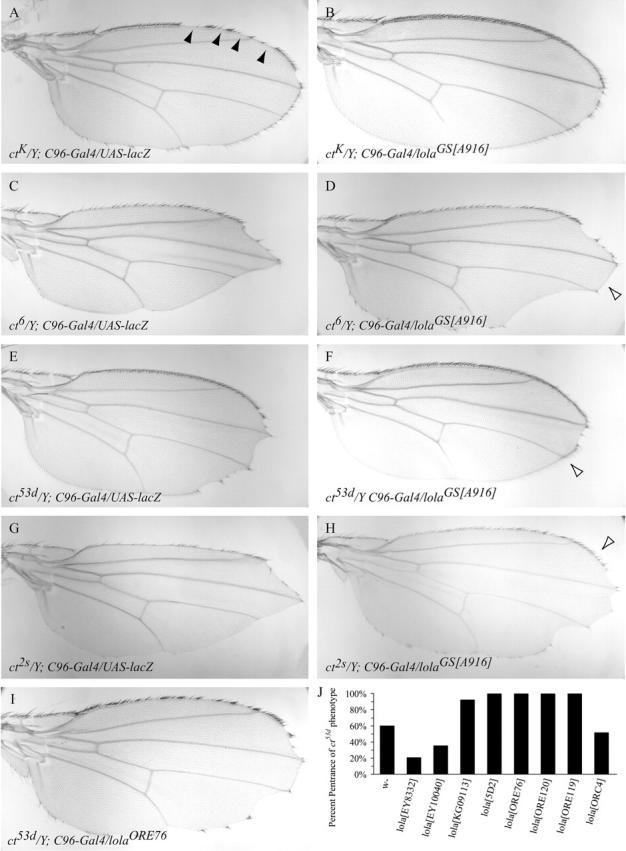
lola interacts genetically with cut during wing-margin development. (A–H) The overexpression of lolaGS[A916] can suppress the wing phenotype of ctK (B), ct6 (D), ct53d (F), and ct2s (H); compare to UAS-lacZ negative control wings (A, C, E, and G). Solid arrowheads in A represent discontinuities in the anterior wing margin; open arrowheads in D, F, and H represent rescue of margin tissues. (I and J) Loss-of-function lola alleles enhance sensory bristle loss in the anterior wing margin of ct53d. (I) A single copy of the amorphic lolaORE76 allele aggravates the ct53d wing phenotype (compare to E). (J) Penetrance of ct53d-associated gaps in the sensory bristles of the anterior margin of wings heterozygous for various lola alleles. lolaEY8332 and lolaEY10040 are gain-of-function insertions of the EPgy2 P element; lolaKG09113 is a loss-of-function insertion of the suppressor P element; lola5D2, lolaORE76, and lolaORE120 are amorphic alleles; and lolaORE119 and lolaORC4 are decision-selective alleles.
lola and its neighboring gene psq are both positioned in the proper orientation to be overexpressed by insertions of the bidirectional GS vector proximal to the 5′ region of lola. Using in situ hybridization with both lola- and psq-specific RNA probes, we found the expression of both genes to be elevated in wing imaginal discs in response to C96-Gal4-driven overexpression of the lolaGS[A916] line (Figure 4, A and B). However, semiquantitative RT-PCR revealed that only lola mRNA transcripts are consistently elevated in response to GS-vector-directed overexpression driven by heatshock-Gal4 (data not shown). Thus, suppression of the cut mutant wing phenotype is most likely due to the overexpression of Lola.
Figure 4.—
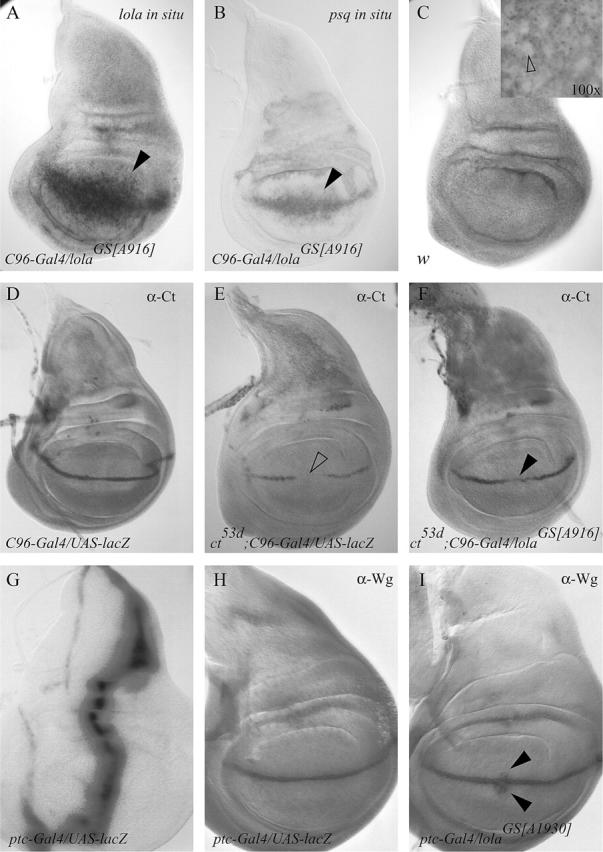
Overexpression of lola in the wing imaginal disc rescues Cut expression and induces ectopic Wg expression. (A and B) In situ hybridization demonstrates that the expression of both lola and psq mRNA are induced in response to C96-Gal4-directed misexpression of lolaGS[A916] (solid arrowheads). (C) lola mRNA is expressed ubiquitously in wild-type wing imaginal tissue. Staining is largely restricted to the cytoplasm of wing disc cells and excluded from the nuclei (inset, open arrowhead), indicting that the ubiquitous staining is not the result of nonspecific binding of the lola riboprobe. The lola-specific riboprobe used in A and C recognizes all lola mRNA isoforms. (D–F) Overexpression of lolaGS[A916] rescues Cut expression in ct53d mutant wing imaginal tissue. (D) In third instar wing imaginal disc tissue, wild-type Cut expression at the dorsoventral boundary is unaffected by a single copy of the P{GawB}C96 insertion (i.e., C96-Gal4). (E) Cut expression is reduced at the presumptive distal wing tip (open arrowhead) in ct53d mutants. (F) C96-Gal4-directed overexpression of lolaGS[A916] rescues Cut expression at the presumptive distal wing tip where Cut expression is normally lost in ct53d mutants (solid arrowhead). (G and H) Overexpression of lolaGS[A916] induces ectopic Wg expression. (G) ptc-Gal4 drives expression along the anteroposterior axis of the wing disc. (H) The Wg expression domain overlaps that of Cut at the dorsoventral boundary. (I) ptc-Gal4-directed misexpression of lolaGS[A916] results in ectopic Wg expression in cells adjacent to the dorsoventral boundary (solid arrowheads).
lola encodes a family of BTB-domain zinc-finger transcription factors previously shown to regulate multiple aspects of peripheral and central neuron axonal guidance (Giniger et al. 1994; Madden et al. 1999; Crowner et al. 2002). The lola locus is complex, encoding at least 20 different protein isoforms, each expressed in a partially distinct pattern (Goeke et al. 2003; Horiuchi et al. 2003). Seventeen of the isoforms each contain unique zinc-finger domains, indicating that each isoform may regulate a unique set of target genes. To determine if lola is involved in margin development, and if lola mutations interact with the cut locus, we examined the modifying effects of heterozygous lola loss-of-function alleles on the wing-margin phenotype of ct53d. The amorphic lola mutations lolaORE76, lolaORE120, and lola5D2 contain disruptions in the open reading frame of the N-terminal constant region present in all Lola isoforms and disrupt all known lola function (Goeke et al. 2003). The presence of one mutant copy of lolaORE76, lolaORE120, or lola5D2 results in a dramatic enhancement in the severity of the ct53d phenotype in that the anterior margin bristles show multiple discontinuities (Figure 3, I and J). Wing-blade tissue adjacent to the area of missing margin bristles is minimally affected by lola mutations, indicating that margin cells with compromised cut activity have the greatest sensitivity to disruptions in lola. Of the decision-selective alleles, lolaORE119, but not lolaORC4, enhances the ct53d phenotype (Figure 3J), implying that the interaction with cut in the wing margin is specific to certain Lola isoforms. In contrast to lola, loss-of-function psq alleles did not affect the ct53d phenotype (data not shown). If lola interacts with cut during wing-margin development, as our genetic data suggest, lola should be expressed in wing imaginal tissue. Indeed, using a riboprobe that recognizes all variant lola mRNA transcripts, we found that lola is ubiquitously expressed throughout the wing disc (Figure 4C). Together, these results suggest that lola cooperates with cut in wing-margin development.
Overexpression of lolaGS can rescue Cut expression and ectopically induce Wg:
The overexpression data are consistent with lola acting genetically downstream of cut in wing-margin patterning, but do not rule out the possibility that lola suppresses the wing phenotype by restoring Cut expression in the wing discs of cut regulatory mutants. To determine if the lolaGS line rescues Cut expression, we examined the pattern of Cut protein in ct53d wing imaginal discs in either the presence or the absence of driving lolaGS[A916] in the wing margin. In ct53d mutant discs, Cut expression is reduced throughout the wing margin and completely absent at the presumptive wing tip, corresponding to the region of the adult wing most visibly disrupted (Figure 4, D and E). Overexpression of lolaGS[A916] in the wing margin rescues Cut expression in ct53d mutants (Figure 4F), indicating that lola may be involved in regulating Cut expression.
Although C96-Gal4-driven expression at the presumptive margin is broader than the normal Cut expression domain (Figure 4A), ectopic Cut is not observed outside of the margin cells in response to lolaGS[916]. Similarly, when lolaGS[A916] was overexpressed along the anterior/posterior boundary using the patched-Gal4 driver (ptc-Gal4; Figure 4G), ectopic Cut expression was not observed (data not shown). In contrast, ptc-Gal4-directed lolaGS[A916] overexpression resulted in ectopic Wg protein in cells immediately adjacent to the dorsoventral boundary (Figure 4, G–I). Although lolaGS[A916] can be active in wing-blade cells, as shown by ectopic Wg expression, rescued Cut expression remains confined to the margin cells, suggesting that some unknown factor, other than Lola, is involved in restricting Cut expression to margin cells.
These results suggested that lola may be required for wild-type wing-margin morphogenesis. To test this, we generated somatic clones of lola mutant cells using the FLP/FRT method (Xu and Rubin 1993). In homozygous lola mutant clones located adjacent to or bisecting the wing margin, neither Cut expression nor the morphology of wing-margin bristles is disrupted (data not shown). Thus, it appears that lola, although sufficient to rescue decreased levels of Cut expression, is not absolutely required for Cut expression and margin development of otherwise wild-type wing discs, but strongly influences the development of wings with compromised cut activity.
Disruption of Brahma complex activity suppresses the ctK phenotype:
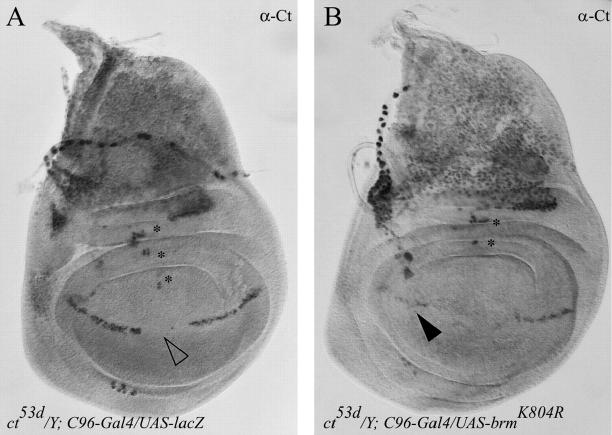
Among the GS lines able to completely rescue the ctK phenotype, we identified a GS vector insertion in the first exon of mor (designated morGS[A897]; Figure 5, A and B, and Table 3). On the basis of its failure to complement the lethality of hypomorphic mor1 mutants, morGS [A897] behaves genetically like a loss-of-function allele (data not shown). In addition, morGS[A897] suppressed ctK independently of the presence of the C96-Gal4 driver. To determine if a reduction of mor function is indeed responsible for suppression, we tested the ability of mor1 to interact with ctK. Adult males of the genotype ctK/Y; mor1/+ display a near-complete restoration of anterior wing-margin structures normally disrupted or missing in ctK mutants, including L1 wing-vein tissue and triple-row sensory bristles (Figure 5C and Table 3). Surprisingly, the deficiencies Df(3R)sbd105 (deficiency suppressor screen) and Df(3R)Exel7327 (Table 3), both covering the mor locus, only weakly suppress the severity of the ctK phenotype. It is not clear why the hypomorphic mor alleles suppress ctK more strongly than a mor deficiency does. Perhaps the cut wing phenotype is particularly sensitive to the level of Mor activity, or the deficiencies have accumulated modifier mutations that are not present in mor hypomorphs, which act to conceal the suppressive effect of reduced Mor function.
Figure 5.—
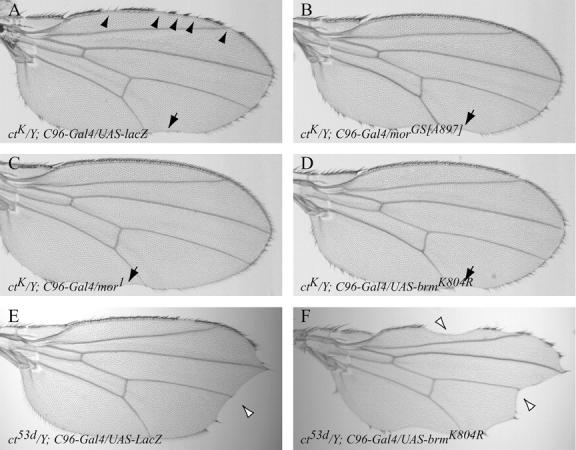
The Brahma complex interacts genetically with cut during wing-margin development. (A–D) The ctK-associated loss of margin bristles is suppressed by disrupting the Brahma complex subunits, Mor and Brm. (A) ctK mutant wings consistently show numerous discontinuities in the stout mechanosensory margin bristles (arrowheads). (B and C) Heterozygous loss-of-function moira mutations, morGS[A897] and mor1, suppress completely the ctK-associated loss of sensory bristles along the anterior wing margin. (D) Margin-specific overexpression of UAS-brmK804R suppresses the ctK wing phenotype in the anterior margin. C96-Gal4-directed overexpression of UAS-brmK804R had no effect on wing development when misexpressed in an otherwise wild-type genetic background. Note that the posterior incisions of wing-blade tissue are not rescued by disrupting Mor or Brm function (arrows in A–D). (E and F) The ct53d wing allele is differentially affected, as compared to ctK, by the disruption of the Brahma complex activity. (E) The ct53d wing phenotype is characterized by the severe loss of wing-blade tissue at the distal wing tip (open arrowhead) and by infrequent gaps in the anterior wing-margin sensory bristles. (F) The overexpression of UAS-brmK804R enhances both the loss of wing-blade tissue (open arrowheads) and sensory bristles. Note that the anterior and posterior wing margin is similarly affected.
TABLE 3.
Summary of the genetic interactions between Brahma complex genes andcut alleles
| Drosophila BRM complex gene |
Yeast homolog | Allele/deficency/transgenic | Penetrance of ctK phenotypea |
n (%) | Penetrance of ct53d phenotypeb |
n (%) |
|---|---|---|---|---|---|---|
| negative control | w | 1380 (99) | 704 (58) | |||
| UAS-lacZ | 1108 (99) | ND | ||||
| brm | SWI2/SNF2 | brm[2] | −/+ | 192 (100) | ++ | 240 (88) |
| Df(3L)brm11 | −/+ | 94 (99) | +++ | 180 (100) | ||
| UAS-brm[K804R] | — | 176 (62) | +++ | 64 (100) | ||
| mor | SWI3 | mor[1] | — | 152 (46) | ++ | 248 (88) |
| mor[GSA897] | — | 120 (18) | ++ | 146 (88) | ||
| Df(3R)Exel7327 | −/+ | 192 (98) | ++ | 168 (87) | ||
| SNF5-related 1(Snr1) | SNF5 | Snr1[01319] | −/+ | 192 (100) | −/+ | 254 (60) |
| BAP111c | High-mobility group (HMG)-like protein |
Df(1)18.1.15 | −/+ | ND | ||
| BAP60c | SWP73/RSC6 | Df(1)N12 | −/+ | −/+ | ||
| BAP55 | Actin-related protein | Df(2R)Exel7147 | −/+ | 168 (100) | −/+ | 144 (65) |
| BAP complex | ||||||
| Osa | SWI1 | Osa[2] | −/+ | 298 (100) | −/+ | 286 (67) |
| UAS-osa[s2] | −/+ | 194 (100) | — | 46 (0) | ||
| UAS-osa[AD] | −/+ | 204 (100) | +++ | 160 (98) | ||
| UAS-osa[RD] | −/+ | 422 (98) | — | 284 (0) | ||
| PBAP complex genes | ||||||
| Polybromo | Rsc1, Rsc2, Rsc4 | Df(3R)slo8 | −/+ | 56 (100) | +++ | 110 (100) |
| BAP170 | Df(2R)ED1552 | −/+ | 146 (100) | +++ | 116 (91) | |
Results from genetic interaction studies are summarized: n, the total number of wings scored; ND, not determined; −/+, no effect; − and +, the degree to which the penetrance of the cut wing phenotype in the anterior wing margin was suppressed and enhanced, respectively.
The wings of the genotype ctK/Y; [specified BRM complex gene]/C96-Gal4 were scored for suppression of anterior margin bristle loss. The penetrance of the ctK wing phenotype is given as a percentage of total wings displaying gaps in the anterior wing margin sensory bristles. Note that the negative control experiments (w and UAS-lacZ) display a completely penetrant ctK wing phenotype (∼99%).
The wings of the genotype ct53d/Y; [specified BRM complex gene]/C96-Gal4 were scored for either the suppression or the enhancement of the ct53d wing phenotype. Note that the negative control experiment (w) displays an incompletely penetrant ct53d wing phenotype (∼58%).
BAP111 and BAP60 were recombined onto both ctK and ct53d X chromoxomes. The phenotype of females heterozygous for the respective deficiencies and homozygous for the cut mutations was compared to females homozygous for the cut mutations only.
mor encodes a core component of the Drosophila SWI/SNF-related ATP-dependent chromatin remodeling complex, the BRM complex (Crosby et al. 1999). The BRM complex is a multimeric complex containing the core catalytic subunit encoded by brm, and it governs an epigenetic mechanism through which the restructuring of nucleosomal DNA establishes and maintains patterns of gene expression (or repression) during development (for review, see Becker and Horz 2002). To determine if loss-of-function mor mutations suppressed ctK via a reduction in BRM complex activity, several brm alleles were tested for the ability to interact with ctK in the wing margin. Contrary to mor mutations, both the amorphic brm2 allele (Kennison and Tamkun 1988) and the brm deficiency, Df(3L)brm11, failed to suppress the ctK phenotype (Table 3). Perhaps the level of Mor protein is limiting with regard to Brm activity and the suppression of the ctK phenotype.
To reduce Brm activity further, a dominant-negative brm transgene, UAS-brmK804R (Elfring et al. 1998), was overexpressed specifically in the presumptive wing margin, using the C96-Gal4 driver. The BrmK804R protein is defective in its ability to hydrolyze ATP, but maintains an association with other BRM complex components. BrmK804R strongly suppresses the ctK-dependent loss of margin sensory bristles (Figure 5D and Table 3), suggesting that reducing energy-dependent BRM complex activity, without disrupting the interactions among components of the complex per se, suppresses the ctK wing-margin phenotype. Thus, manipulating the activity of the BRM complex components Mor and Brm strongly modifies ctK-dependent wing-margin loss.
Disruption of Brahma complex activity enhances the ct53d phenotype:
The gypsy retrotransposon inserted 5′ to the cut coding region in ctK disrupts communication between the distal cut wing enhancer and the proximal core promoter (Jack et al. 1991), possibly by affecting higher-order chromatin structure (Chen and Corces 2001; Byrd and Corces 2003). To determine if the disruption of BRM complex activity suppresses the ctK wing-margin phenotype via a gypsy-dependent or -independent mechanism, we examined the genetic interactions of mor and brm mutants with other gypsy and non-gypsy cut alleles. We find that both mor1 and brm2 heterozygote mutations interact with the non-gypsy ct53d allele. In contrast to the interaction with ctK, however, we observed aggravation rather than suppression of the ct53d phenotype (Table 3). Similarly, wing-margin-specific overexpression of BrmK804R severely enhanced the loss of anterior margin tissue in ct53d (Figure 5, E and F). The loss of wing-margin bristles observed in ctK and ct53d mutants likely reflects a decrease in Cut expression in the wing margin (Figure 6A). Cut expression is substantially restored in ctK/Y;UAS-brmK804R/C96-Gal4 wing imaginal discs (data not shown). Conversely, the aggravated loss of wing-margin bristles of ct53d/Y;UAS-brmK804R/C96-Gal4 correlates with a further decrease in the level of Cut protein throughout the presumptive wing margin in ct53d mutant discs (Figure 6B).
Figure 6.—
The expression of Cut is reduced in response to the disruption of the Brahma complex. (A) In ct53d mutants, Cut expression is reduced at the presumptive wing tip (open arrowhead). (B) Overexpression of a dominant-negative form of Brm further reduces the level of Cut expression in ct53d mutants (solid arrowhead). Note that Cut is still expressed in some sensory precursor cells outside of the C96-Gal4 expression domain (asterisks).
Differences in the nature of the genetic aberrations associated with the cut wing enhancer region may account for the apparent discrepancy in the suppression vs. the enhancement of the wing phenotypes observed in response to disruptions of BRM complex activity. Neither heterozygous mor1 or brm2 mutations nor the overexpression of UAS-BrmK804R modifies the phenotype of the strong cut wing alleles, ct6 (gypsy) or ct2s (non-gypsy) (data not shown). It is conceivable that Cut expression in ct6 and ct2s mutants is reduced to a level beyond which a reduction in BRM complex activity can no longer produce an effect on wing-margin development. In any case, the preceding results demonstrate that the BRM complex contributes to both gypsy-dependent and gypsy-independent regulation of Cut expression in the wing margin (see discussion).
Both BAP and PBAP interact with cut:
In Drosophila, there are two distinct Brm-containing complexes, BAP (Brahma-associated proteins) and PBAP (Polybromo-associated BAP). Both complexes contain the DNA-dependent ATPase Brm and seven core subunits, Mor/BAP155, BAP111, BAP74 (hsp70 cognate hsc4), BAP60, BAP55, actin/BAP47, and Snr1/BAP45 (Mohrmann et al. 2004). Heterozygous loss-of-function mutations in BAP111, BAP60, BAP55, or Snr1/BAP45 did not modify the ctK or ct53d margin bristle phenotype (Table 3), suggesting that decreasing the expression level of these subunits is not limiting for BRM complex activity in vivo.
The BAP and PBAP complexes are distinguished by association with either Osa or Polybromo and BAP170, respectively (Mohrmann et al. 2004). Thus, we examined the ability of osa, polybromo, and BAP170 loss-of-function mutations to enhance the ct53d wing phenotype. Flies heterozygous for the osa2 allele do not display wing defects alone, nor does osa2 cause a strong enhancement of the ct53d phenotype. However, the overexpression of the full-length UAS-osa transgene (Collins et al. 1999) specifically in the wing margin suppressed the loss of anterior margin bristles of the ct53d phenotype (Table 3), implying that an Osa-associated BRM complex interacts with cut during margin development by increasing its activity.
Specific mutations are not available for either polybromo or BAP170. Therefore, we used the deficiencies Df(3R)slo8 and Df(2R)ED1552, covering polybromo and BAP170, respectively, to explore the genetic interaction with ct53d. As with brm or mor mutations, both deficiencies enhanced margin bristle and tissue loss of ct53d (Table 3). Although it cannot be ruled out that one of the other genes disrupted by the deficiencies is responsible for the enhancement of the ct53d phenotype, these data support the idea that cut activity is sensitive to disruptions of PBAP complex components. It should be noted that neither osa mutations nor the polybromo or BAP170 deficiencies were able to modify the phenotype of ctK. Overall, the genetic data suggest that both Brm-containing chromatin-remodeling complexes, BAP and PBAP, may contribute to cut-dependent wing-margin development in a complex manner.
Osa may act as a transcriptional repressor in its interaction with ct53d:
An Osa-containing BRM complex has previously been implicated in the repression of Wg target genes during development of the wing imaginal disc (Collins and Treisman 2000). To study whether Osa acts as a transcriptional activator or repressor with regard to its interaction with cut, we examined the ability of obligatory activator and repressor forms of Osa (Collins et al. 1999) to modify the ct53d phenotype (Table 3). Wing-margin-specific overexpression of the Osa AT-rich interaction domain (ARID)-DNA-binding domain fused to the Engrailed repression domain (UAS-OsaRD[11c]) strongly suppressed the ct53d phenotype, but had no effect in a wild-type background. As previously stated, overexpression of full-length Osa also ameliorates the ct53d phenotype, consistent with the idea that Osa acts as a repressor. Conversely, overexpression of the Osa-ARID domain fused to the VP16 transcriptional activation domain (UAS-OsaAD[20e]) enhanced the wing-margin phenotype of ct53d. Together, these results suggest that the Osa-containing BAP complex must act as a transcriptional repressor to ameliorate cut-dependent wing-margin patterning defects. This is in accordance with the repressive activity of Osa on Wg target genes in the wing disc (Collins and Treisman 2000).
DISCUSSION
The identity of genes that interact with cut during wing-margin patterning: Does Cut regulate cell growth and proliferation?
The secreted morphogen encoded by wg patterns the wing margin by coordinating cell growth and proliferation with cell differentiation (Phillips and Whittle 1993; Johnston and Edgar 1998; Nepveu 2001; Duman-Scheel et al. 2004). Additionally, Wg is required for the survival of margin cells (Johnston and Sanders 2003). Expression of Wg within the presumptive wing margin is maintained by Cut, and in the absence of Cut the wing margin degenerates (Jack et al. 1991). Here, we determined that wing-margin development requires cut activity independently of the maintenance of Wg expression. Inhibiting wing-margin degeneration without rescuing margin bristle development in cut mutants by supplying exogenous Wg expression or the apoptosis inhibitor p35 demonstrates a requirement for Cut in margin sensory organ development, which is separable from its role in maintaining Wg expression. Although Wg is not sufficient for margin bristle formation in the absence of cut, it remains to be determined if transduction of the Wg signal is required cell autonomously within the Cut-positive margin cells for margin sensory organ development. Indeed, expression of the proneural gene achaete in bristle progenitors along the anterior margin depends upon canonical Wg signaling (Phillips and Whittle 1993).
As a means to further elucidate the role of cut in wing-margin patterning, we performed complementary loss- and gain-of-function genetic screens to identify other genes that modify the cut wing phenotype. Several classes of cut modifiers include loci near known genes that regulate processes influencing cell growth and proliferation, including brat, CycE, eIF4A, and eIF4E. The identification of these genes suggests that during wing-margin development Cut activity may be regulated in a manner dependent upon cell cycle phasing and/or may coordinate cell cycle progression with terminal specification of cell identity. This is consistent with the proposed activity of the vertebrate Cut homolog CDP/Cux1, the DNA-binding activity of which is modulated in coordination with cell cycle progression and is postulated to synchronize cell cycle exit with terminal cell differentiation (reviewed in Nepveu 2001)
lola is required in the context of decreased Cut expression for wing-margin development:
lola is known for its role as a regulator of axon growth in Drosophila and is proposed to coordinately control the expression of multiple genes that execute axon guidance decisions (Giniger et al. 1994; Madden et al. 1999; Crowner et al. 2002). We identified a novel role for lola in wing-margin development, revealed by its gain- and loss-of-function genetic interactions with hypomorphic cut alleles. Overexpression directed by lolaGS insertions is sufficient to rescue the reduction in Cut expression of regulatory cut mutants and to suppress the hypoplastic cut wing phenotype. Conversely, loss of lola function aggravates the cut wing-margin defects. It is feasible that Lola modulates Cut expression by interacting directly or indirectly with the cut wing-margin enhancer or with other regulatory regions adjacent to or distant from this enhancer, which may also be involved in promoting Cut expression at the margin. The suppression of the cut wing phenotype by Lola misexpression is consistent with other alternative possibilities, such as that Lola may be involved in the regulation of an unknown Cut target gene, may be a Cut target itself, or both. Consistent with the ability to interact with cut during wing development, lola mRNA is expressed ubiquitously in the imaginal wing disc. However, the requirement for lola in wing development is evident only in cut mutants, since in lola null mutant cell clones Cut expression and wing-margin development is not disrupted. It may be that lola plays a nonessential role in the regulation of processes directing wing-margin development, which only becomes apparent when Cut activity is decreased, akin to the cryptic variations necessary for evolutionary adaptations (Gibson and Dworkin 2004).
Overexpression of Lola in cut mutants suppresses the margin loss phenotype presumed to result from a failure to maintain expression of the secreted factor Wg at the dorsal/ventral boundary of the wing disc. We demonstrate that Lola induces ectopic Wg expression at locations proximal to the dorsal/ventral boundary. Thus, it may be that Lola overexpression rescues wing-blade tissue in cut mutants via the induction of Wg expression. The suppression of sensory bristle loss, however, is likely independent of this effect on Wg expression. It will be interesting to determine if lola contributes to other tissue-specific aspects of cut activity.
The induction and refinement of both Wg and Cut expression at the wing dorsal/ventral boundary requires activation of the Notch signaling pathway (Diaz-Benjumea and Cohen 1995; Micchelli et al. 1997). The induction of Cut and Wg expression in response to Lola overexpression implies that Lola may positively regulate Notch signaling in wing boundary cells. In the eye, however, Lola appears to act in the converse manner, where the loss of Lola function enhances the rough-eye phenotype resulting from the overexpression of a constitutively active form of Notch (Verheyen et al. 1996). Clonal analysis of amorphic lola mutations does not produce the incised wing-margin phenotype indicative of a loss of Notch function, suggesting that Lola activity is not required to regulate Notch signaling. Furthermore, although Cut expression is rescued in the wing-margin in response to broad Lola overexpression, it is not expanded outside of the boundary cells. This is in contrast to the observed expansion of Wg into cells adjacent to the boundary, indicating that the induction of Wg is independent of Cut. Since ectopic expression of both Wg and Cut is induced in the wing disc in response to activated Notch (de Celis et al. 1996), expanded Wg expression due to Lola overexpression may not involve Notch signaling.
The lola locus encodes a family of at least 20 BTB-zinc-finger transcription factors, expressed in partially distinct tissue-specific patterns. The functional significance of the diversity in Lola isoforms and their expression patterns is not entirely clear. In several instances, mutations inactivating a single Lola isoform affect only a subset of axon guidance defects associated with amorphic lola alleles (Goeke et al. 2003). This led to the hypothesis that specific isoforms and interactions with cofactors contribute to the diversity in lola-dependent axon guidance decisions. Lola isoform F has been shown to physically interact in vitro with the chromosomal JIL-1 kinase (Zhang et al. 2003). JIL-1 regulates chromatin structure by influencing the phosphorylation state of histone 3 (Wang et al. 2001). Amorphic lola alleles act as dominant modifiers of a hypomorphic JIL-1 allele, leading to an increase in embryonic viability (Zhang et al. 2003). It is not clear, however, if Lola isoform F is responsible for the in vivo genetic interaction with JIL-1. Similarly, we were unable to determine which Lola isoform(s) is responsible for the interaction with cut in the wing margin. All amorphic lola alleles interact with cut (i.e., enhancement of bristle loss) in a similar manner. Interestingly, the axon guidance decision-selective lolaORE119 allele, thought to disrupt only isoform L, enhanced the cut wing phenotype, whereas the isoform K-specific lolaORC4 allele did not.
Brm-associated chromatin-remodeling complexes regulate multiple aspects of wing development:
In Drosophila, Brm and Brm-associated proteins regulate multiple aspects of wing development. Early in wing development, Brm and Osa modulate the activity of the dorsal wing compartment specific selector gene, Apterous, and the subsequent localization of the Wg-dependent organizer at the dorsal/ventral boundary (Milan et al. 2004). Similarly, Mor is required for the expression of the posterior compartment specific selector gene engrailed (Brizuela and Kennison 1997). The triune of Brm, Osa, and Mor is required to repress the Wg target gene nubbin, a gene required for the growth and patterning of the wing (Collins and Treisman 2000). Finally, Brm activity is required for the cell-type-specific activation and repression of genes involved in wing-vein elaboration (Marenda et al. 2004). Our genetic analysis indicates that cut-dependent wing-margin patterning also relies upon the activity of Brm, as well as upon the activity of several Brm-associated subunits of both the BAP and the PBAP complex. Heterozygous loss-of-function mutations in the core subunits Brm and Mor, although individually having no effect on normal wing-margin development, enhance the loss of wing-margin tissue of the ct53d allele. Deficiencies covering the PBAP subunits, BAP170 and Polybromo, or the overexpression of BAP subunit Osa, exhibit similar interactions with ct53d. The enhancement of the ct53d wing phenotype correlates with a decrease in Cut expression in the presumptive wing margin, thus indicating that the BRM complex activity is required genetically upstream of cut. Together with other studies, our data support the idea that the BRM complex may globally regulate the expression of genes required for wing development.
How might the BRM complex regulate cut expression in the wing? The regulation of the distal cut wing enhancer requires the activity of both enhancer-binding and enhancer-facilitator proteins. Enhancer-facilitator proteins are proposed to structurally facilitate communication between distal enhancer elements and the proximal promoter and are different from enhancer-binding (co)activators in that they do not directly activate the initiation of transcription. A number of genes, including scalloped (sd), mastermind (mam), Chip (Chi), and Nipped-B, are involved in the regulation of cut expression (Morcillo et al. 1996; Rollins et al. 1999). Genetic and biochemical data suggest that sd and mam encode cut wing-margin enhancer-binding transcriptional (co)activators. Consistent with their role as enhancer-binding activator proteins, loss-of-function sd and mam mutations enhance the severity of the cut wing phenotype resulting from deletions in the wing enhancer. In contrast, Chip and Nipped-B encode putative general enhancer-facilitator proteins (Morcillo et al. 1996, 1997; Rollins et al. 1999, 2004) and primarily enhance the cut wing phenotype of cut alleles in which enhancer-promoter communication is partially compromised by the gypsy insulator.
Given the known regulatory mechanisms imposed upon cut expression and the recognized ability of the BRM complex to affect chromatin structure, several mechanisms can be envisioned through which the BRM complex may directly or indirectly regulate cut expression in the wing margin. The BRM complex may indirectly influence cut expression via the expression of cut wing enhancer-binding or enhancer-facilitating proteins. Alternatively, the BRM complex may regulate the local chromatin structure of 5′ cut regulatory regions and affect the access of enhancer-binding proteins and/or the basal transcriptional machinery to DNA. Our data do not distinguish between these possibilities. Similar to sd and mam, both brm and mor display a strong genetic interaction with the ct53d wing-margin enhancer deletion. Furthermore, the overexpression of a dominant negative form of Brm dramatically reduced Cut expression throughout the entire wing margin in the ct53d mutant background, but had a less pronounced effect in a wild-type background. Thus, Cut expression is particularly sensitive to disruptions in BRM complex activity when the wing-margin enhancer is partially inactivated by deletions. Therefore, it is possible that the BRM complex normally acts to positively regulate cut expression through a direct or indirect interaction with enhancer-binding proteins, such as Sd or Mam. However, it is also possible that alterations in chromatin structure may be essential for the remote cut wing enhancer to interact with the proximal promoter. Consistent with this possibility, the BRM complex component Osa physically interacts with the enhancer-facilitator Chip (Heitzler et al. 2003). Similarly, Nipped-B interacts with the Drosophila cohesin complex, a negative regulator of the cut wing enhancer (Rollins et al. 2004). The interaction of the human cohesin complex with chromatin requires hISWI chromatin-remodeling complex activity (Hakimi et al. 2002). Although the nature of the interaction of these factors with the cut enhancer region remains to be determined, our data indicate that enhancer/promoter communication requires BRM complex chromatin-remodeling activity. In addition to mediating cut enhancer activity, BRM complex activity also seems to be required for gypsy-mediated insulation. Thus the BRM complex and other group B candidates may contribute to the regulation of transcription at multiple levels.
Alternatively, the BRM complex may interact with the autoregulation of cut expression. In the embryonic peripheral nervous system, ectopic Cut expression activates the endogenous cut locus. Autoregulatory maintenance of Cut expression appears to be essential for sensory organ development (Blochlinger et al. 1991), and it is possible that the BRM complex activity may assist in this process. Accordingly, subthreshold levels of cut activation in the wing margin, below that required for the maintenance of its own expression, may affect the stochastic, cell-autonomous loss of sensory bristles along the anterior wing margin.
Is the BRM complex required for gypsy insulation activity? Regulatory lesions affecting the activity of the cut wing-margin enhancer are responsive to disruptions of Brm activity. The differential effects of disrupting either Brm or Mor activity on the ctK and ct53d wing phenotypes possibly reflect differences in the nature of these regulatory lesions (i.e., gypsy insertion or partial wing enhancer deletion). In contrast to the ct53d allele interaction, disruptions in BRM complex activity suppressed the discontinuities in the wing bristles of the gypsy ctK allele. The gypsy insulator disrupts communication between the distal cut wing enhancer and the proximal promoter. Su(Hw)] and mod(mdg4) are required for gypsy activity and are postulated to do so by directly interfering with the enhancer-facilitator activity of Chip (Gause et al. 2001). Loss-of-function mutations in either gene suppress the cut wing phenotype resulting from gypsy.
Several lines of evidence suggest that the regulation of higher-order chromatin structure is involved in the control of gypsy activity. First, in diploid cells, Su(Hw) and Mod(mdg4) colocalize with gypsy and other native insulating elements at peri-nuclear locations (Gerasimova and Corces 1998). These sites represent clustering of distant insulator elements. The subnuclear localization of gypsy and its regulatory proteins is suggestive of a higher-order chromatin structure. In Su(Hw) mutants, mod(mdg4) protein and gypsy insulator sequences fail to cluster at peri-nuclear sites and are instead diffusely distributed in the nucleus. The peri-nuclear localization of gyspy, however, does not appear to be required for insulator activity (Xu et al. 2004). Second, mod(mdg4) interacts genetically with several trithorax group (trxG) genes, known regulators of homeotic gene expression (Buchner et al. 2000). trxG genes, including mor and brm, affect the post-translational modification of histone proteins, influencing nucleosome organization and chromatin structure. Therefore, Mod(mdg4) may influence chromatin structure within the confine of gypsy-mediated insulation through an interaction with trxG genes. Finally, mutations in several trxG genes disrupt the peri-nuclear location of Su(Hw)/mod(mdg4)/gypsy complexes, a phenotype resembling Su(Hw) mutations (Gerasimova and Corces 1998). It has been suggested that a general mechanism influencing higher-order chromatin structure may be involved in both regulation of gypsy activity and homeotic gene expression. Consistent with the activity of other trxG genes, we propose that wild-type BRM complex activity is required for the productive interaction between Su(Hw) and the gypsy insulator. The absence or the reduction of BRM complex activity would preclude gypsy insulation and thus restore normal levels of Cut expression.
Acknowledgments
We thank D. Dorsett, E. Giniger, J. Treisman, T. Aigaki, and the Bloomington Stock Center for providing fly stocks. We also thank D. Dorsett and J. Treisman for comments on the manuscript. J.J.K. was supported by a fellowship from the Hearing and Chemical Senses training grant (National Institutes of Health/National Institute on Deafness and Other Communication Disorders 5 T32 DC00011).
References
- Andres, V., M. D. Chiara and V. Mahdavi, 1994. A new bipartite DNA-binding domain: cooperative interaction between the cut repeat and homeo domain of the cut homeo proteins. Genes Dev. 8: 245–257. [DOI] [PubMed] [Google Scholar]
- Becker, P. B., and W. Horz, 2002. ATP-dependent nucleosome remodeling. Annu. Rev. Biochem. 71: 247–273. [DOI] [PubMed] [Google Scholar]
- Blochlinger, K., R. Bodmer, J. Jack, L. Y. Jan and Y. N. Jan, 1988. Primary structure and expression of a product from cut, a locus involved in specifying sensory organ identity in Drosophila. Nature 333: 629–635. [DOI] [PubMed] [Google Scholar]
- Blochlinger, K., R. Bodmer, L. Y. Jan and Y. N. Jan, 1990. Patterns of expression of cut, a protein required for external sensory organ development in wild-type and cut mutant Drosophila embryos. Genes Dev. 4: 1322–1331. [DOI] [PubMed] [Google Scholar]
- Blochlinger, K., L. Y. Jan and Y. N. Jan, 1991. Transformation of sensory organ identity by ectopic expression of Cut in Drosophila. Genes Dev. 5: 1124–1135. [DOI] [PubMed] [Google Scholar]
- Bodmer, R., S. Barbel, S. Sheperd, J. W. Jack, L. Y. Jan et al., 1987. Transformation of sensory organs by mutations of the cut locus of D. melanogaster. Cell 51: 293–307. [DOI] [PubMed] [Google Scholar]
- Brizuela, B. J., and J. A. Kennison, 1997. The Drosophila homeotic gene moira regulates expression of engrailed and HOM genes in imaginal tissues. Mech. Dev. 65: 209–220. [DOI] [PubMed] [Google Scholar]
- Buchner, K., P. Roth, G. Schotta, V. Krauss, H. Saumweber et al., 2000. Genetic and molecular complexity of the position effect variegation modifier mod(mdg4) in Drosophila. Genetics 155: 141–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd, K., and V. G. Corces, 2003. Visualization of chromatin domains created by the gypsy insulator of Drosophila. J. Cell Biol. 162: 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S., and V. G. Corces, 2001. The gypsy insulator of Drosophila affects chromatin structure in a directional manner. Genetics 159: 1649–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, R. T., and J. E. Treisman, 2000. Osa-containing Brahma chromatin remodeling complexes are required for the repression of wingless target genes. Genes Dev. 14: 3140–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, R. T., T. Furukawa, N. Tanese and J. E. Treisman, 1999. Osa associates with the Brahma chromatin remodeling complex and promotes the activation of some target genes. EMBO J. 18: 7029–7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coqueret, O., G. Berube and A. Nepveu, 1998. The mammalian Cut homeodomain protein functions as a cell-cycle-dependent transcriptional repressor which downmodulates p21WAF1/CIP1/SDI1 in S phase. EMBO J. 17: 4680–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby, M. A., C. Miller, T. Alon, K. L. Watson, C. P. Verrijzer et al., 1999. The trithorax group gene moira encodes a brahma-associated putative chromatin-remodeling factor in Drosophila melanogaster. Mol. Cell. Biol. 19: 1159–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowner, D., K. Madden, S. Goeke and E. Giniger, 2002. Lola regulates midline crossing of CNS axons in Drosophila. Development 129: 1317–1325. [DOI] [PubMed] [Google Scholar]
- Dansereau, D. A., M. D. Lunke, A. Finkielsztein, M. A. Russell and W. J. Brook, 2002. Hephaestus encodes a polypyrimidine tract binding protein that regulates Notch signalling during wing development in Drosophila melanogaster. Development 129: 5553–5566. [DOI] [PubMed] [Google Scholar]
- de Celis, J. F., A. Garcia-Bellido and S. J. Bray, 1996. Activation and function of Notch at the dorsal-ventral boundary of the wing imaginal disc. Development 122: 359–369. [DOI] [PubMed] [Google Scholar]
- Diaz-Benjumea, F. J., and S. M. Cohen, 1995. Serrate signals through Notch to establish a Wingless-dependent organizer at the dorsal/ventral compartment boundary of the Drosophila wing. Development 121: 4215–4225. [DOI] [PubMed] [Google Scholar]
- Dorsett, D., 1993. Distance-independent inactivation of an enhancer by the suppressor of Hairy-wing DNA-binding protein of Drosophila. Genetics 134: 1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman-Scheel, M., L. A. Johnston and W. Du, 2004. Repression of dMyc expression by Wingless promotes Rbf-induced G1 arrest in the presumptive Drosophila wing margin. Proc. Natl. Acad. Sci. USA 101: 3857–3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfring, L. K., C. Daniel, O. Papoulas, R. Deuring, M. Sarte et al., 1998. Genetic analysis of brahma: the Drosophila homolog of the yeast chromatin remodeling factor SWI2/SNF2. Genetics 148: 251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, T., L. Gambardella, M. Horcher, S. Tschanz, J. Capol et al., 2001. The transcriptional repressor CDP (Cutl1) is essential for epithelial cell differentiation of the lung and the hair follicle. Genes Dev. 15: 2307–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epps, J. L., and S. Tanda, 1998. The Drosophila semushi mutation blocks nuclear import of bicoid during embryogenesis. Curr. Biol. 8: 1277–1280. [DOI] [PubMed] [Google Scholar]
- Frank, D. J., B. A. Edgar and M. B. Roth, 2002. The Drosophila melanogaster gene brain tumor negatively regulates cell growth and ribosomal RNA synthesis. Development 129: 399–407. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido, A., 1975. Genetic control of wing disc development in Drosophila. Ciba Found. Symp., No. 29: 161–182. [DOI] [PubMed] [Google Scholar]
- Gause, M., P. Morcillo and D. Dorsett, 2001. Insulation of enhancer-promoter communication by a gypsy transposon insert in the Drosophila cut gene: cooperation between suppressor of hairy-wing and modifier of mdg4 proteins. Mol. Cell. Biol. 21: 4807–4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer, F., and M. W. Hentze, 2004. Molecular mechanisms of translational control. Nat. Rev. Mol. Cell Biol. 5: 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimova, T. I., and V. G. Corces, 1998. Polycomb and trithorax group proteins mediate the function of a chromatin insulator. Cell 92: 511–521. [DOI] [PubMed] [Google Scholar]
- Ghosh, D., T. I. Gerasimova and V. G. Corces, 2001. Interactions between the Su(Hw) and Mod(mdg4) proteins required for gypsy insulator function. EMBO J. 20: 2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, G., and I. Dworkin, 2004. Uncovering cryptic genetic variation. Nat. Rev. Genet. 5: 681–690. [DOI] [PubMed] [Google Scholar]
- Giniger, E., K. Tietje, L. Y. Jan and Y. N. Jan, 1994. lola encodes a putative transcription factor required for axon growth and guidance in Drosophila. Development 120: 1385–1398. [DOI] [PubMed] [Google Scholar]
- Glaser, R. L., M. F. Wolfner and J. T. Lis, 1986. Spatial and temporal pattern of hsp26 expression during normal development. EMBO J. 5: 747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeke, S., E. A. Greene, P. K. Grant, M. A. Gates, D. Crowner et al., 2003. Alternative splicing of lola generates 19 transcription factors controlling axon guidance in Drosophila. Nat. Neurosci. 6: 917–924. [DOI] [PubMed] [Google Scholar]
- Gould, A. P., and R. A. White, 1992. Connectin, a target of homeotic gene control in Drosophila. Development 116: 1163–1174. [DOI] [PubMed] [Google Scholar]
- Goulet, B., A. Baruch, N. S. Moon, M. Poirier, L. L. Sansregret et al., 2004. A cathepsin L isoform that is devoid of a signal peptide localizes to the nucleus in S phase and processes the CDP/Cux transcription factor. Mol. Cell 14: 207–219. [DOI] [PubMed] [Google Scholar]
- Grueber, W. B., L. Y. Jan and Y. N. Jan, 2003. Different levels of the homeodomain protein cut regulate distinct dendrite branching patterns of Drosophila multidendritic neurons. Cell 112: 805–818. [DOI] [PubMed] [Google Scholar]
- Gupta, S., M. X. Luong, S. A. Bleuming, A. Miele, M. Luong et al., 2003. Tumor suppressor pRB functions as a co-repressor of the CCAAT displacement protein (CDP/cut) to regulate cell cycle controlled histone H4 transcription. J. Cell. Physiol. 196: 541–556. [DOI] [PubMed] [Google Scholar]
- Gustafson, K., and G. L. Boulianne, 1996. Distinct expression patterns detected within individual tissues by the GAL4 enhancer trap technique. Genome 39: 174–182. [DOI] [PubMed] [Google Scholar]
- Hakimi, M. A., D. A. Bochar, J. A. Schmiesing, Y. Dong, O. G. Barak et al., 2002. A chromatin remodelling complex that loads cohesin onto human chromosomes. Nature 418: 994–998. [DOI] [PubMed] [Google Scholar]
- Hardiman, K. E., R. Brewster, S. M. Khan, M. Deo and R. Bodmer, 2002. The bereft gene, a potential target of the neural selector gene cut, contributes to bristle morphogenesis. Genetics 161: 231–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzler, P., L. Vanolst, I. Biryukova and P. Ramain, 2003. Enhancer-promoter communication mediated by Chip during Pannier-driven proneural patterning is regulated by Osa. Genes Dev. 17: 591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer, J. G., K. Li and T. C. Kaufman, 1995. The Drosophila homeotic target gene centrosomin (cnn) encodes a novel centrosomal protein with leucine zippers and maps to a genomic region required for midgut morphogenesis. Development 121: 3861–3876. [DOI] [PubMed] [Google Scholar]
- Hombria, J. C., and B. Lovegrove, 2003. Beyond homeosis—HOX function in morphogenesis and organogenesis. Differentiation 71: 461–476. [DOI] [PubMed] [Google Scholar]
- Hoover, K. K., T. I. Gerasimova, A. J. Chien and V. G. Corces, 1992. Dominant effects of suppressor of Hairy-wing mutations on gypsy-induced alleles of forked and cut in Drosophila melanogaster. Genetics 132: 691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi, T., E. Giniger and T. Aigaki, 2003. Alternative trans-splicing of constant and variable exons of a Drosophila axon guidance gene, lola. Genes Dev. 17: 2496–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, N., and J. Castelli-Gair, 1999. Study of the posterior spiracles of Drosophila as a model to understand the genetic and cellular mechanisms controlling morphogenesis. Dev. Biol. 214: 197–210. [DOI] [PubMed] [Google Scholar]
- Jack, J. W., 1985. Molecular organization of the cut locus of Drosophila melanogaster. Cell 42: 869–876. [DOI] [PubMed] [Google Scholar]
- Jack, J., and Y. DeLotto, 1995. Structure and regulation of a complex locus: the cut gene of Drosophila. Genetics 139: 1689–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack, J., D. Dorsett, Y. Delotto and S. Liu, 1991. Expression of the cut locus in the Drosophila wing margin is required for cell type specification and is regulated by a distant enhancer. Development 113: 735–747. [DOI] [PubMed] [Google Scholar]
- Jackson, S. M., and C. A. Berg, 1999. Soma-to-germline interactions during Drosophila oogenesis are influenced by dose-sensitive interactions between cut and the genes cappuccino, ovarian tumor and agnostic. Genetics 153: 289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, S. M., and K. Blochlinger, 1997. cut interacts with Notch and protein kinase A to regulate egg chamber formation and to maintain germline cyst integrity during Drosophila oogenesis. Development 124: 3663–3672. [DOI] [PubMed] [Google Scholar]
- Johnston, L. A., and B. A. Edgar, 1998. Wingless and Notch regulate cell-cycle arrest in the developing Drosophila wing. Nature 394: 82–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, L. A., and A. L. Sanders, 2003. Wingless promotes cell survival but constrains growth during Drosophila wing development. Nat. Cell Biol. 5: 827–833. [DOI] [PubMed] [Google Scholar]
- Kennison, J. A., and J. W. Tamkun, 1988. Dosage-dependent modifiers of polycomb and antennapedia mutations in Drosophila. Proc. Natl. Acad. Sci. USA 85: 8136–8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna-Gupta, A., T. Zibello, S. Kolla, E. J. Neufeld and N. Berliner, 1997. CCAAT displacement protein (CDP/cut) recognizes a silencer element within the lactoferrin gene promoter. Blood 90: 2784–2795. [PubMed] [Google Scholar]
- Khanna-Gupta, A., T. Zibello, H. Sun, P. Gaines and N. Berliner, 2003. Chromatin immunoprecipitation (ChIP) studies indicate a role for CCAAT enhancer binding proteins alpha and epsilon (C/EBP alpha and C/EBP epsilon) and CDP/cut in myeloid maturation-induced lactoferrin gene expression. Blood 101: 3460–3468. [DOI] [PubMed] [Google Scholar]
- Kim, S. Y., and G. L. Boulianne, 1998. Characterization of a novel discless mutant in D. melanogaster. Annu. Dros. Res. Conf. 39: 675B. [Google Scholar]
- Kremser, T., K. Hasenpusch-Theil, E. Wagner, D. Buttgereit and R. Renkawitz-Pohl, 1999. Expression of the beta3 tubulin gene (beta Tub60D) in the visceral mesoderm of Drosophila is dependent on a complex enhancer that binds Tinman and UBX. Mol. Gen. Genet. 262: 643–658. [DOI] [PubMed] [Google Scholar]
- Lai, E. C., and V. Orgogozo, 2004. A hidden program in Drosophila peripheral neurogenesis revealed: fundamental principles underlying sensory organ diversity. Dev. Biol. 269: 1–17. [DOI] [PubMed] [Google Scholar]
- Ledford, A. W., J. G. Brantley, G. Kemeny, T. L. Foreman, S. E. Quaggin et al., 2002. Deregulated expression of the homeobox gene Cux-1 in transgenic mice results in downregulation of p27(kip1) expression during nephrogenesis, glomerular abnormalities, and multiorgan hyperplasia. Dev. Biol. 245: 157–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, S. E., S. M. Searle, N. Harris, M. Gibson, V. Lyer et al., 2002 Apollo: a sequence annotation editor. Genome Biol. 3: RESEARCH0082. [DOI] [PMC free article] [PubMed]
- Lievens, P. M., J. J. Donady, C. Tufarelli and E. J. Neufeld, 1995. Repressor activity of CCAAT displacement protein in HL-60 myeloid leukemia cells. J. Biol. Chem. 270: 12745–12750. [DOI] [PubMed] [Google Scholar]
- Liu, S., and J. Jack, 1992. Regulatory interactions and role in cell type specification of the Malpighian tubules by the cut, Kruppel, and caudal genes of Drosophila. Dev. Biol. 150: 133–143. [DOI] [PubMed] [Google Scholar]
- Liu, S., E. McLeod and J. Jack, 1991. Four distinct regulatory regions of the cut locus and their effect on cell type specification in Drosophila. Genetics 127: 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann, I., N. McGinnis, M. Bodmer and W. McGinnis, 2002. The Drosophila Hox gene deformed sculpts head morphology via direct regulation of the apoptosis activator reaper. Cell 110: 457–466. [DOI] [PubMed] [Google Scholar]
- Ludlow, C., R. Choy and K. Blochlinger, 1996. Functional analysis of Drosophila and mammalian cut proteins in files. Dev. Biol. 178: 149–159. [DOI] [PubMed] [Google Scholar]
- Luong, M. X., C. M. van der Meijden, D. Xing, R. Hesselton, E. S. Monuki et al., 2002. Genetic ablation of the CDP/Cux protein C terminus results in hair cycle defects and reduced male fertility. Mol. Cell. Biol. 22: 1424–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden, K., D. Crowner and E. Giniger, 1999. LOLA has the properties of a master regulator of axon-target interaction for SNb motor axons of Drosophila. Dev. Biol. 213: 301–313. [DOI] [PubMed] [Google Scholar]
- Mann, R. S., and S. B. Carroll, 2002. Molecular mechanisms of selector gene function and evolution. Curr. Opin. Genet. Dev. 12: 592–600. [DOI] [PubMed] [Google Scholar]
- Marenda, D. R., C. B. Zraly and A. K. Dingwall, 2004. The Drosophila Brahma (SWI/SNF) chromatin remodeling complex exhibits cell-type specific activation and repression functions. Dev. Biol. 267: 279–293. [DOI] [PubMed] [Google Scholar]
- Merritt, D. J., 1997. Transformation of external sensilla to chordotonal sensilla in the cut mutant of Drosophila assessed by single-cell marking in the embryo and larva. Microsc. Res. Tech. 39: 492–505. [DOI] [PubMed] [Google Scholar]
- Micchelli, C. A., E. J. Rulifson and S. S. Blair, 1997. The function and regulation of cut expression on the wing margin of Drosophila: Notch, Wingless and a dominant negative role for Delta and Serrate. Development 124: 1485–1495. [DOI] [PubMed] [Google Scholar]
- Milan, M., T. T. Pham and S. M. Cohen, 2004. Osa modulates the expression of Apterous target genes in the Drosophila wing. Mech. Dev. 121: 491–497. [DOI] [PubMed] [Google Scholar]
- Miron, M., P. Lasko and N. Sonenberg, 2003. Signaling from Akt to FRAP/TOR targets both 4E-BP and S6K in Drosophila melanogaster. Mol. Cell. Biol. 23: 9117–9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogila, V. A., A. B. Ladvishenko, O. B. Simonova and T. I. Gerasimova, 1992. Intragenic suppression: Stalker, a retrovirus-like transposable element, can compensate for a deficiency at the cut locus of Drosophila melanogaster. Genetica 86: 305–311. [DOI] [PubMed] [Google Scholar]
- Mohrmann, L., K. Langenberg, J. Krijgsveld, A. J. Kal, A. J. Heck et al., 2004. Differential targeting of two distinct SWI/SNF-related Drosophila chromatin-remodeling complexes. Mol. Cell. Biol. 24: 3077–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon, N. S., G. Berube and A. Nepveu, 2000. CCAAT displacement activity involves CUT repeats 1 and 2, not the CUT homeodomain. J. Biol. Chem. 275: 31325–31334. [DOI] [PubMed] [Google Scholar]
- Moon, N. S., P. Premdas, M. Truscott, L. Leduy, G. Berube et al., 2001. S phase-specific proteolytic cleavage is required to activate stable DNA binding by the CDP/Cut homeodomain protein. Mol. Cell. Biol. 21: 6332–6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcillo, P., C. Rosen and D. Dorsett, 1996. Genes regulating the remote wing margin enhancer in the Drosophila cut locus. Genetics 144: 1143–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcillo, P., C. Rosen, M. K. Baylies and D. Dorsett, 1997. Chip, a widely expressed chromosomal protein required for segmentation and activity of a remote wing margin enhancer in Drosophila. Genes Dev. 11: 2729–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepveu, A., 2001. Role of the multifunctional CDP/Cut/Cux homeodomain transcription factor in regulating differentiation, cell growth and development. Gene 270: 1–15. [DOI] [PubMed] [Google Scholar]
- Neufeld, E. J., D. G. Skalnik, P. M. Lievens and S. H. Orkin, 1992. Human CCAAT displacement protein is homologous to the Drosophila homeoprotein, cut. Nat. Genet. 1: 50–55. [DOI] [PubMed] [Google Scholar]
- Phillips, R. G., and J. R. Whittle, 1993. wingless expression mediates determination of peripheral nervous system elements in late stages of Drosophila wing disc development. Development 118: 427–438. [DOI] [PubMed] [Google Scholar]
- Quaggin, S. E., G. B. Heuvel, K. Golden, R. Bodmer and P. Igarashi, 1996. Primary structure, neural-specific expression, and chromosomal localization of Cux-2, a second murine homeobox gene related to Drosophila cut. J. Biol. Chem. 271: 22624–22634. [DOI] [PubMed] [Google Scholar]
- Raisin, S., S. Pantalacci, J. P. Breittmayer and P. Leopold, 2003. A new genetic locus controlling growth and proliferation in Drosophila melanogaster. Genetics 164: 1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, H. E., L. V. O'Keefe, S. I. Reed and R. Saint, 1993. A Drosophila G1-specific cyclin E homolog exhibits different modes of expression during embryogenesis. Development 119: 673–690. [DOI] [PubMed] [Google Scholar]
- Richardson, H., L. V. O'Keefe, T. Marty and R. Saint, 1995. Ectopic cyclin E expression induces premature entry into S phase and disrupts pattern formation in the Drosophila eye imaginal disc. Development 121: 3371–3379. [DOI] [PubMed] [Google Scholar]
- Robertson, H. M., C. R. Preston, R. W. Phillis, D. M. Johnson-Schlitz, W. K. Benz et al., 1988. A stable genomic source of P element transposase in Drosophila melanogaster. Genetics 118: 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins, R. A., P. Morcillo and D. Dorsett, 1999. Nipped-B, a Drosophila homologue of chromosomal adherins, participates in activation by remote enhancers in the cut and Ultrabithorax genes. Genetics 152: 577–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins, R. A., M. Korom, N. Aulner, A. Martens and D. Dorsett, 2004. Drosophila nipped-B protein supports sister chromatid cohesion and opposes the stromalin/Scc3 cohesion factor to facilitate long-range activation of the cut gene. Mol. Cell. Biol. 24: 3100–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santaguida, M., Q. Ding, G. Berube, M. Truscott, P. Whyte et al., 2001. Phosphorylation of the CCAAT displacement protein (CDP)/Cux transcription factor by cyclin A-Cdk1 modulates its DNA binding activity in G(2). J. Biol. Chem. 276: 45780–45790. [DOI] [PubMed] [Google Scholar]
- Schneuwly, S., R. Klemenz and W. J. Gehring, 1987. Redesigning the body plan of Drosophila by ectopic expression of the homoeotic gene Antennapedia. Nature 325: 816–818. [DOI] [PubMed] [Google Scholar]
- Sinclair, A. M., J. A. Lee, A. Goldstein, D. Xing, S. Liu et al., 2001. Lymphoid apoptosis and myeloid hyperplasia in CCAAT displacement protein mutant mice. Blood 98: 3658–3667. [DOI] [PubMed] [Google Scholar]
- Skalnik, D. G., E. C. Strauss and S. H. Orkin, 1991. CCAAT displacement protein as a repressor of the myelomonocytic-specific gp91-phox gene promoter. J. Biol. Chem. 266: 16736–16744. [PubMed] [Google Scholar]
- Sturtevant, M. A., M. Roark and E. Bier, 1993. The Drosophila rhomboid gene mediates the localized formation of wing veins and interacts genetically with components of the EGF-R signaling pathway. Genes Dev. 7: 961–973. [DOI] [PubMed] [Google Scholar]
- Sudarsan, V., S. Anant, P. Guptan, K. Vijayraghavan and H. Skaer, 2001. Myoblast diversification and ectodermal signaling in Drosophila. Dev. Cell 1: 829–839. [DOI] [PubMed] [Google Scholar]
- Toba, G., T. Ohsako, N. Miyata, T. Ohtsuka, K. H. Seong et al., 1999. The gene search system: a method for efficient detection and rapid molecular identification of genes in Drosophila melanogaster. Genetics 151: 725–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truscott, M., L. Raynal, P. Premdas, B. Goulet, L. Leduy et al., 2003. CDP/Cux stimulates transcription from the DNA polymerase alpha gene promoter. Mol. Cell. Biol. 23: 3013–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valarche, I., J. P. Tissier-Seta, M. R. Hirsch, S. Martinez, C. Goridis et al., 1993. The mouse homeodomain protein Phox2 regulates Ncam promoter activity in concert with Cux/CDP and is a putative determinant of neurotransmitter phenotype. Development 119: 881–896. [DOI] [PubMed] [Google Scholar]
- van Wijnen, A. J., M. F. van Gurp, M. C. de Ridder, C. Tufarelli, T. J. Last et al., 1996. CDP/cut is the DNA-binding subunit of histone gene transcription factor HiNF-D: a mechanism for gene regulation at the G1/S phase cell cycle transition point independent of transcription factor E2F. Proc. Natl. Acad. Sci. USA 93: 11516–11521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheyen, E. M., K. J. Purcell, M. E. Fortini and S. Artavanis-Tsakonas, 1996. Analysis of dominant enhancers and suppressors of activated Notch in Drosophila. Genetics 144: 1127–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi-Ito, N., M. P. Belvin, D. A. Bluestein and K. V. Anderson, 2001. fusilli, an essential gene with a maternal role in Drosophila embryonic dorsal-ventral patterning. Dev. Biol. 229: 44–54. [DOI] [PubMed] [Google Scholar]
- Wang, Y., W. Zhang, Y. Jin, J. Johansen and K. M. Johansen, 2001. The JIL-1 tandem kinase mediates histone H3 phosphorylation and is required for maintenance of chromatin structure in Drosophila. Cell 105: 433–443. [DOI] [PubMed] [Google Scholar]
- Wang, Z., A. Goldstein, R. T. Zong, D. Lin, E. J. Neufeld et al., 1999. Cux/CDP homeoprotein is a component of NF-muNR and represses the immunoglobulin heavy chain intronic enhancer by antagonizing the bright transcription activator. Mol. Cell. Biol. 19: 284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Q., M. Li, J. Adams and H. N. Cai, 2004. Nuclear location of a chromatin insulator in Drosophila melanogaster. J. Cell Sci. 117: 1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, T., and G. M. Rubin, 1993. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117: 1223–1237. [DOI] [PubMed] [Google Scholar]
- Zhang, W., Y. Wang, J. Long, J. Girton, J. Johansen et al., 2003. A developmentally regulated splice variant from the complex lola locus encoding multiple different zinc finger domain proteins interacts with the chromosomal kinase JIL-1. J. Biol. Chem. 278: 11696–11704. [DOI] [PubMed] [Google Scholar]
- Zimmer, C., M. C. Tiveron, R. Bodmer and H. Cremer, 2004. Dynamics of Cux2 expression suggests that an early pool of SVZ precursors is fated to become upper cortical layer neurons. Cereb. Cortex 14: 1408–1420. [DOI] [PubMed] [Google Scholar]