Abstract
In cell suspensions subjected to high-shear rotatory motion, human PMN (polymorphonuclear cells) adhered to E-selectin-expressing CHO (Chinese-hamster ovary) cells (CHO-E), and formed homotypic aggregates when challenged by E-selectin–IgG fusion protein, by a mechanism that involved β2 integrins. Both heterotypic and homotypic PMN adhesion was accompanied by tyrosine phosphorylation of a 110 kDa protein (P110). This event was prevented by blocking anti-(β2 integrin) antibodies and by inhibitors of Src-family kinases, suggesting that it was part of an ‘outside-in’ signalling that was initiated by integrin engagement. Interestingly, Src-family kinase inhibitors prevented β2-integrin-mediated (i) homotypic PMN adhesion triggered by E-selectin–IgG, (ii) heterotypic CHO-E/PMN adhesion in mixed-cell suspensions, and (iii) firm adhesion of PMN to CHO-E monolayers under physiological flow. Similarly to PMN treated with Src-family kinase inhibitors, PMN from hck−/−fgr−/− and hck−/−fgr−/−lyn−/− mice showed significant impairment of β2-integrin-mediated adhesion to CHO-E. Moreover, the expression of β2 integrin activation epitopes at the sites of cell–cell contact in CHO-E/PMN conjugates was abolished by Src-family kinase inhibitors. One component of P110 was identified as the FAK (focal adhesion kinase) Pyk2 (proline-rich tyrosine kinase 2), which was phosphorylated in a β2 integrin- and Src-family-kinase-dependent manner. Thus, Src-family kinases, and perhaps Pyk2, mediate a signal necessary for β2 integrin function in PMN tethered by E-selectin.
Keywords: adhesion, β2 integrin, leucocyte recruitment, polymorphonuclear leucocyte, proline-rich tyrosine kinase 2 (Pyk2), Src-family kinase
Abbreviations: AM, acetoxymethyl ester; BCECF, 2′,7′-bis-(2-carboxyethyl)-5(6)-carboxyfluorescein; CHO, Chinese-hamster ovary; CHO-E, CHO cell expressing E-selectin; F-actin, filamentous actin; FAK, focal adhesion kinase; HBSS, Hanks balanced salt solution; HE, hydroethidine; ICAM-1, intercellular cell-adhesion molecule 1; iPr2P-F, di-isopropyl fluorophosphate; LFA-1, lymphocyte function-associated antigen 1; mAb, monoclonal antibody; MAPK, mitogen-activated protein kinase; MFI, mean fluorescence intensity; PFA, paraformaldehyde; PP1, 4-amino-5-(methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine; PP2, 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine; PP3, 4-amino-7-phenylpyrazol[3,4-d]pyrimidine; PSGL-1, P-selectin glycoprotein ligand 1; PMN, polymorphonuclear cells; Pyk2, proline-rich tyrosine kinase 2
INTRODUCTION
E-selectin, a member of the selectin family that is expressed on inflamed endothelium, promotes the initial capture and rolling of PMN (polymorphonuclear cells). As they roll, PMN integrate signals from the activated endothelium that result in full activation, ligand binding by leucocyte β2 integrins, and PMN arrest [1]. Numerous studies have indicated that ‘inside-out’ signalling, following engagement of chemo-attractant receptors, stimulates inactive integrins to adopt an active conformation capable of ligand binding [2,3]. Once bound to their ligands, integrins deliver ‘outside-in’ signals that result in transduction of mechanical force to the cytoskeleton and assembly of signalling complexes at the site of cell adhesion. These post-ligand-binding events may co-operate to induce full integrin function [4–6].
Previous work in our laboratory and others indicates that selectin binding to PMN may, by itself, promote β2 integrin adhesiveness. In a pioneering study, Lo et al. [7] found that either E-selectin expressed on cytokine-treated endothelial cells or immobilized recombinant E-selectin stimulates αMβ2 adhesive function. In studies of PMN adhesion to interleukin-1β-activated endothelial cells in suspension, Kuijpers et al. [8] found that, after pharmacological inhibition or removal of platelet-activating factor from endothelial cell membranes, E-selectin was the major mediator of β2 integrin activation and subsequent adhesion of PMN to endothelial cells. More recently, soluble recombinant E-selectin was shown to stimulate PMN function, including β2 integrin adhesiveness [9,10]. Under conditions of physiological flow, Simon et al. [11] showed that PMN tethering on E-selectin promoted β2-integrin-mediated adhesion to ICAM-1 (intercellular cell-adhesion molecule 1). More recently, the same authors further elucidated the mechanisms by which E-selectin initiates β2 integrin adhesiveness, showing that co-capping of L-selectin and PSGL-1 (P-selectin glycoprotein ligand 1) by E-selectin induces a high-avidity state of β2 integrins in PMN [12].
Studies of another member of the selectin family have revealed that P-selectin promotes the adhesiveness of αMβ2 in suspension [13] and in static conditions [14]. Our previous studies suggested that the activity of Src-family tyrosine kinases downstream of β2-integrin-dependent outside-in signalling was necessary for αMβ2 adhesive function triggered by P-selectin [15]. In the present study, using pharmacological inhibitors in human cells and cells isolated from mice genetically deficient in different members of the Src family, we examined the role of Src-family tyrosine kinases in promoting β2 integrin adhesiveness in PMN tethered by E-selectin.
Our data demonstrate that these signalling molecules mediate post-ligand-binding events, which are necessary to sustain full integrin high affinity and high avidity at the sites of PMN adhesion initiated by E-selectin.
MATERIALS AND METHODS
HE (hydroethidine) was purchased from Molecular Probes Europe (Leiden, The Netherlands); BCECF-AM [2′,7′-bis-(2-carboxy-ethyl)-5(6)-carboxyfluorescein-acetoxymethyl ester], was from Alexis (San Diego, CA, U.S.A.). Hepes and EGTA were from Sigma (St Louis, MO, U.S.A.). PFA (paraformaldehyde) was purchased from Fluka (Milan, Italy). Dextran T500 and Ficoll–Hypaque were from Pharmacia (Uppsala, Sweden) and Aldrich (Milan, Italy) respectively. BCECF-AM and HE were dissolved in DMSO at concentrations of 1 mg/ml and 8 mg/ml respectively, stored at −20 °C and used within 4 weeks. The ECL® (enhanced chemiluminescence)-kit was from Amersham Biosciences (Little Chalfont, Bucks., U.K.). Reagents for electrophoresis and Western-blot analysis were pure grade. The kinase inhibitors PP1 {4-amino-5-(methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine} was purchased from Alexis, PP2 {4-amino-5-(4-chloro-phenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine} and the inactive analogue PP3 {4-amino-7-phenylpyrazol[3,4-d]pyrimidine} were from Calbiochem (La Jolla, CA, U.S.A.). Recombinant human E-selectin–IgG fusion protein was kindly provided by Genetics Institute (Cambridge, MA, U.S.A.). Soluble E-selectin was purified from lysates of E-selectin-expressing CHO (Chinese-hamster ovary) cells by immunoaffinity chromatography using the anti(E-selectin) antibody H18/7.
Antibodies
The following mAbs (monoclonal antibodies) were kindly provided: anti-(β2 integrin) antibody KIM127 [16], by Dr M. K. Robinson (Exploratory Research Cell Tech Therapeutics, Slough, U.K.); and 327C, by D. Staunton (ICOS Corporation, Bothell, WA, U.S.A.) [17]. The anti-(P-selectin) antibody WAPS 12.2 [18], anti-(E-selectin) antibody H18/7 [19], anti-(αL integrin) antibody TS1/22 [20] and anti-(β2 integrin) antibody IB4 [21] were purified from mouse ascites or hybridoma cell supernantant using Protein G–Sepharose affinity chromatography. The anti-(mouse β2 integrin) antibody, Game-46, was purchased from Pharmingen (San Diego, CA, U.S.A.). The PY99 (anti-phosphotyrosine) antibody and the anti-Pyk2 (protein-rich tyrosine kinase 2) antibody (clone C-19) were from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). The anti-Pyk2 antibody recognizes both human and mouse protein. The recombinant horseradish-peroxidase-conjugated anti-phosphotyrosine mAb RC20 was from Transduction Laboratories (Exeter, U.K.). The anti-[phospho-Src-family (Tyr416)] mAb was from Cell Signaling Technology (Beverly, MA, U.S.A.).
Mice
C57BL/6J mice were purchased from Charles River Laboratories (Calco, Lecco, Italy). Generation of double (hck−/−fgr−/−) or triple (hck−/−fgr−/−lyn−/−) mutant mice was as described previously [22]. Animals were housed and cared for by the Animal Care Unit at the Consorzio Mario Negri Sud and used according to procedures approved by the Institutional Animal Care and Use Committee.
Preparation of PMN and culture of CHO cells
All protocols were approved by the Institutional Review Board at Consorzio Mario Negri Sud. Blood was collected from healthy volunteers who had not taken any medications for at least 2 weeks. Nine parts of blood were anti-coagulated with one part of 3.8% (w/v) trisodium citrate. Blood was centrifuged to remove platelet-rich plasma. PMN were isolated by dextran sedimentation followed by Ficoll–Hypaque gradient and hypotonic lysis of erythrocytes, washed and resuspended in ice-cold Hepes/Tyrode buffer [23]. For adhesion assays, PMN (5×107 PMN/ml) were stained with 20 μg of the vital red fluorescent dye HE for 30 min at 4 °C as described previously [23]. Immediately before use, MgCl2 and CaCl2 were added to the suspension of PMN to bring the concentration of both bivalent ions to 1 mM. All of the procedures for PMN isolation were performed at 4 °C using sterile materials.
Wild-type CHO cells or CHO cells stably transfected with the cDNA encoding human E-selectin (CHO-E) were kindly provided by Genetics Institute (Cambridge, MA, U.S.A.) and were cultured as described previously [24]. CHO cells stably transfected with mouse E-selectin were kindly provided by Dr D. Vestweber (Institute of Cell Biology, Center for Molecular Biology of Inflammation, University of Munster, Germany). For mixed-cell suspension assays, CHO and CHO-E cells were grown to confluence and detached with 5 mM of both EGTA and EDTA, washed twice in Hepes/Tyrode buffer, and resuspended in the same buffer at a concentration of 107 cells/ml. For adhesion assay in suspension, CHO and CHO-E cells were loaded with the green fluorescent dye BCECF-AM. For flow assays, wild-type CHO cells and CHO-E cells were cultured on tissue-culture glass slides for 2–3 days and used at confluence.
Mouse bone marrow PMN were isolated from femurs and tibias of five to ten wild-type or double (hck−/−fgr−/−) or triple (hck−/−fgr−/−lyn−/−) mutant mice (18–25 g) for each experiment. Briefly, bone marrow cells were flushed from the bones using Ca2+- and Mg2+-free HBSS (Hanks balanced salt solution) containing 0.1% (w/v) BSA. Cells were aspirated through an 18 gauge needle to disrupt clumps. After removal of contaminating erythrocytes by lysis in 0.15 M NH4Cl, 1 mM KHCO3, and 0.1 mM sodium EDTA, the remaining bone marrow cells were washed twice and resuspended in Ca2+- and Mg2+-free HBSS containing 0.1% (w/v) BSA. PMN were then purified by centrifugation on Ficoll–Hypaque gradient, as described above for human cells. Final cellular suspensions contained 80% PMN as determined by light microscopy.
Flow adhesion assay
PMN adhesion under physiological flow was investigated in a parallel plate flow chamber. CHO or CHO-E cells were grown to confluence on glass slides, the slides were washed and then mounted in the chamber. PMN [(5×106)/ml] suspended in M199 medium (Bio-Whittaker Europe, Verviers, Belgium) containing 0.1% (w/v) BSA were perfused across the CHO cell surface at a flow rate of 4 dyn/cm2 for 2 min. The chamber was then perfused with fresh medium for 2 min at 10 dyn/cm2. The interaction of PMN with CHO cells was observed by phase contrast video microscopy with a ×20 objective (Olympus, Germany). Images were continuously recorded (Pro-Series video camera, High Performance CCD camera, Media Cybernetics, Silver Spring, MD, U.S.A.). The fractions of rolling and firmly adhered PMN were quantified using ad hoc software for image analysis (Image Pro-Plus for Windows, Media Cybernetics), in the last 20 s of flow. In some experiments, E-selectin on CHO-E was blocked by treatment with the mAb H18/7 (20 μg/ml) for 15 min. β2 Integrins were blocked by treating PMN with the mAb IB4 (20 μg/ml) for 15 min in ice. The anti(P-selectin) antibody WAPS 12.2 was used as an isotype-matched control material for both H18/7 and IB4. Where indicated, PMN were treated with Src inhibitors, inactive analogue or DMSO for 5 min at room temperature (23–24 °C).
Mixed-cell suspension assay
PMN alone, or mixed with CHO cells at a PMN/CHO ratio of 5:1, were incubated in a final volume of 500 μl in siliconized glass tubes (internal diameter 6 mm; ChronoLog, Mascia Brunelli, Milan, Italy). The tubes were placed in a PICA (platelet ionized calcium aggregometer; ChronoLog) at 37 °C and stirred at 1000 rev./min using a 4 mm magnetic stirrer for different periods of time (as indicated in the Figure legends). Although the shear rate produced by this stirring speed cannot be quantified precisely, it should be approx. 250 s−1 [25]. For blocking studies, antibodies were incubated at saturating concentration (20 μg/ml) with the desired cell fraction for 15 min at 4 °C. Protein kinase inhibitors or the diluent (DMSO) were incubated with PMN for 2 min at room temperature before mixing them with CHO cells or soluble E-selectin.
The formation of PMN/CHO cell mixed conjugates was quantified by double-colour flow-cytometric analysis as described previously [13,15]. Briefly, BCECF-loaded CHO cells and HE-loaded PMN were incubated together as described above. At different times, an equal volume of 2% (w/v) PFA was added, and the cells fixed for 30 min. After fixation, samples were analysed by flow cytometry as follows. A gate was set on the CHO cells, identified by their green fluorescence (FL1). In each experiment, a sample of CHO cells mixed with PMN in the presence of 10 mM EGTA was used to set the threshold for the red (PMN-specific) fluorescence (FL2). The threshold was set such that 90% of events (CHO) were below the threshold. The percentage of CHO cells in each sample with red fluorescence above the threshold represented the percentage of CHO cells which bound at least one PMN [CHO (+)]. A semi-quantitative estimate of the number of PMN adhered per single CHO (+) (i.e. those CHO cells with red fluorescence above the threshold) was obtained as follows: the FL2 MFI (mean fluorescence intensity) of the CHO (+) population was divided by the FL2 MFI of the PMN population, before multiplying by the percentage of positive CHO cells, to yield an estimate of the number of PMN attached to 100 CHO cells (PMN per 100 CHO).
PMN homotypic aggregation
PMN aggregation can be considered as an index of the adhesive function of integrins αLβ2 and αMβ2 [26,27]. To evaluate the effect of soluble E-selectin on PMN aggregation, PMN [(5×106)/ml] were stirred at 1000 rev./min at 37 °C for 3 min with different amounts of E-selectin–IgG chimaera or purified E-selectin. The reaction was stopped by the addition of an equal volume of 2% (w/v) PFA and the number of non-aggregated PMN (single PMN) was evaluated by light microscopy. These soluble forms of E-selectin did not aggregate PFA-fixed PMN. Similar results were obtained with either the recombinant E-selectin–IgG chimaera or E-selectin that was purified from CHO-E.
Tyrosine phosphorylation experiments
PMN were stirred without or with CHO cells (5:1 PMN/CHO) or E-selectin–IgG chimaera as described in the mixed-cell suspension assay. The reaction was stopped at different times by the addition of an equal volume of 2× reducing Laemmli's buffer, and protein tyrosine phosphorylation was analysed as described previously [15]. Src kinase activity in the PMN lysates subjected to SDS/PAGE (7.5% gel) was detected by immunoblotting with anti-[phospho-Src-family (Tyr416)] antibody.
Pyk2 immunoprecipitation and detection
PMN were pretreated with 5 mM iPr2P-F (di-isopropyl fluorophosphate) in order to assure complete inhibition of intracellular proteases. Samples of iPr2P-F-pretreated PMN (5×106 cells) were incubated with or without 20 μg/ml E-selectin–IgG or mixed with CHO-E (5:1 PMN/CHO-E) and then lysed in reducing Laemmli's buffer containing protease and phosphatase inhibitors (2 mM sodium orthovanadate, 5 mM EGTA, 5 mM EDTA, 10 mM sodium pyrophosphate, 10 mM iodoacetic acid, 1 mM PMSF, 10 mM sodium fluoride, 1 mg/ml trypsin–chymotrypsin inhibitor, 10 μg/ml leupeptin and 10 μg/ml aprotinin), boiled for 10 min, and diluted 1:20 with 1% (v/v) Triton X-100 lysis buffer [1% (v/v) Triton X-100, 150 mM NaCl and 25 mM Tris/HCl, pH 7.4] containing protease and phosphatase inhibitors (as above). After pre-clearing, immunoprecipitation was performed by incubating the samples at 4 °C overnight with 10 μg/ml anti-Pyk2 antibody and Protein G–Sepharose beads (45–165 μm, Pharmacia). Beads were washed three times with 1% (v/v) Triton X-100 lysis buffer, once with PBS, re-suspended with reducing Laemmli's buffer and analysed by SDS/PAGE (7.5% gels). Western blotting was performed with either 0.1 μg/ml anti-Pyk2 antibody or PY99 antibody followed by horseradish-peroxidase-conjugated anti-(goat IgG) and anti-(mouse IgG) respectively. The intensity of the signal was analysed using a digital imaging analysis system (1D Image Analysis Software, Kodak, NY, U.S.A.).
Measurement of ‘activated’ β2 integrins by flow cytometry
The percentage of activated β2 integrin was evaluated by measuring the binding of the activation-dependent mAb KIM127 or 327C in parallel with the binding of the activation-independent β2 integrin mAb IB4 as described by Lu et al. [28]. PMN, alone or stimulated with soluble E-selectin for 2 min without stirring, were incubated with 10 μg/ml KIM127 or IB4 for an additional 5 min at 37 °C in the presence of 1 mM Ca2+ and 1 mM Mg2+ or 2 mM Mn2+. The cells were fixed in 2% PFA, washed and stained with Alexa Fluor®-488-conjugated goat anti-mouse antibody (Molecular Probes) at 4 °C for 30 min. Non-specific fluorescence was determined by incubation of PMN with Alexa Fluor®-488-conjugated goat anti-mouse antibody only and was subtracted from the MFI obtained in the presence of either KIM127 or IB4. The specific MFI for KIM127 was expressed as a percentage of the specific MFI of IB4.
Confocal microscopy
The localization of activated integrins was investigated using the mAbs KIM127 and 327C. After stirring at 1000 rev./min for 2 min, PMN (resting or activated with 20 μg/ml soluble E-selectin–IgG fusion protein or mixed with CHO-E) were incubated for an additional 5 min at 37 °C with 10 μg/ml KIM127 or 327C without stirring and then fixed overnight with 2% (w/v) PFA. Samples were then incubated for 30 min at 4 °C with Alexa Fluor®-488-conjugated goat anti-mouse antibody, permeabilized with PBS containing 0.05% (w/v) saponin (Sigma–Aldrich) and 0.5% (w/v) BSA for 30 min at 4 °C, and F-actin (filamentous actin) was stained with 1 μg/ml rhodamine–phalloidin (Sigma–Aldrich) in PBS containing 0.01% (w/v) saponin and 0.5% (w/v) BSA for 30 min at 4 °C. Samples were re-suspended in Mowiol (Calbiochem), plated on a glass cover-slip, and observed with a Zeiss LSM 510 Laser Scanning Microscope equipped with Axio-3vert 100 M-BP (Carl Zeiss, Jena, Germany), objective ×60. Optical Z-sections from each sample were taken with 0.3 μm Z-step from the top to the bottom of the cell. Immunofluorescence images were acquired at high confocality (pinhole=1 Airy unit) to achieve the thinnest optical slices. Fluorescence intensity corresponding to the binding of KIM127 or 327C was measured using the ‘Display-Profile’ option of LSM510 software (Carl Zeiss). One line was traced along the diameter of the PMN, which crossed the cell from side to side. The intensity of fluorescence was measured along this line both at the adhesion and non-adhesion sites and was expressed in arbitrary units (from 0 to 255).
RESULTS
Tethering by E-selectin triggers β2-integrin-dependent adhesion and a β2-integrin-dependent outside-in signal resulting in tyrosine phosphorylation of a 110 kDa protein, in human PMN
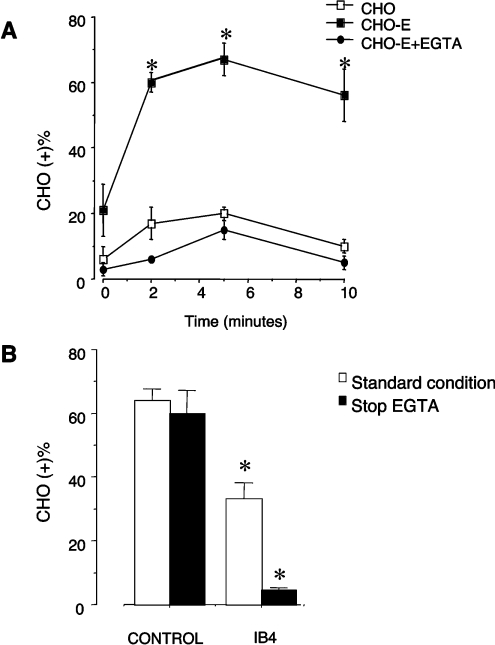
In initial experiments, we evaluated PMN adhesion to wild-type CHO cells or CHO-E in mixed-cell suspensions (5:1 PMN/CHO). When PMN were incubated with CHO-E for 2 min under stirring conditions (see Materials and methods section), 60±3.0% (mean±S.E.M., n=5) of CHO-E bound one or more PMN. By evaluating the relative number of PMN recruited by CHO-E (see Materials and methods section), we found that 60±3.0 CHO-E bound 165±35 PMN (mean 2.8±0.5 PMN per CHO-E). The formation of mixed PMN/CHO-E conjugates occurred rapidly, reaching a maximum at 3 min, and remained stable up to 10 min (Figure 1A). In contrast, only 17±5.0% of wild-type CHO cells interacted with PMN, and each CHO cell bound no more than one PMN. The adhesion of CHO-E stirred with PMN in the presence of 5 mM EGTA was similar to that of CHO cells (Figure 1A). Thus expression of E-selectin is necessary for PMN adhesion to CHO cells.
Figure 1. Adhesion of human PMN to CHO-E in stirred cell suspensions: role of the β2 integrins.
(A) HE-loaded PMN were incubated with BCECF-loaded wild-type CHO or E-selectin-expressing CHO-E cells in the absence or presence of 5 mM EGTA at a PMN/CHO cells ratio of 5:1 (stirring at 37 °C and 1000 rev./min). At different times the interaction was stopped by 1 vol. of 2% (w/v) PFA and mixed-cell conjugates were analysed by flow cytometry. The graph shows the percentage of CHO cells that bound PMN [CHO (+)]. Values are means±S.E.M. for four experiments. Differences between groups were analysed by repeated measurement ANOVA. *P<0.05 compared with CHO or CHO-E+EGTA groups. (B) HE-loaded PMN were pre-incubated for 10 min at 4 °C with or without saturating concentrations of the anti-(β2-integrin) mAb, IB4, and mixed by stirring for 2 min at 37 °C and 1000 rev./min with BCECF-loaded CHO-E. The interaction was stopped by the addition of 1 vol. of 2% (w/v) PFA (standard condition). Alternatively, in order to disrupt the Ca2+-dependent selectin–ligand interaction, 5 mM EGTA was added to mixed cells 30 s before addition of 2% (w/v) PFA (stop EGTA). Samples were then processed for FACS analysis of mixed-cell conjugates. The graph shows the percentage of CHO cells that bound PMN [CHO (+)]. Values are means±S.E.M. for three to five experiments. Differences between groups were analysed by repeated measurement ANOVA. *P<0.05 compared with control group.
Pretreatment of PMN with the β2 integrin-function-blocking mAb IB4 significantly reduced, but did not abolish, PMN binding to CHO-E (Figure 1B), suggesting a role for β2 integrins. In order to elucidate further the functional relevance of integrin–ligand interactions, E-selectin interactions were allowed to occur for 2 min and were then reversed by the addition of 5 mM EGTA for 30 s before fixation to disrupt the Ca2+-dependent, selectin-mediated interactions. The presence of Mg2+ sustains integrin-dependent binding in the absence of Ca2+. Under control conditions, this brief EGTA treatment (stop EGTA, Figure 1B) did not significantly modify the percentage of CHO-E with bound PMN, indicating that once formed, the mixed-cell conjugates are sustained via a selectin-independent mechanism. Importantly, in samples in which PMN were pretreated with IB4, brief exposure to EGTA completely disrupted mixed-cell conjugates (Figure 1B). Thus β2 integrins mediate CHO-E–PMN adhesion. In contrast, IB4 did not modify PMN interaction with wild-type CHO cells, suggesting that the slight capability of these cells to interact with PMN does not involve β2 integrins.
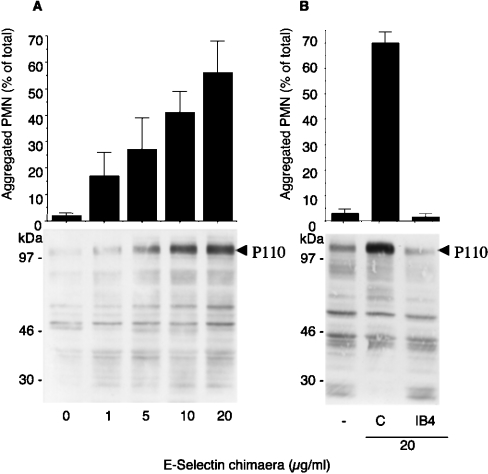
We next investigated the ability of soluble E-selectin to trigger β2 integrin adhesiveness as assessed by the induction of homotypic PMN aggregation. PMN stirred with E-selectin–IgG fusion protein for 3 min formed homotypic aggregates that were dependent on the concentration of E-selectin–IgG (Figure 2A). The formation of homotypic aggregates was accompanied by increased tyrosine phosphorylation of a protein with a molecular mass of approx. 110 kDa (P110). Homotypic adhesion and protein tyrosine phosphorylation were both abolished by the β2 integrin mAb IB4 (Figure 2B), suggesting that β2 integrins mediate homotypic adhesion and trigger tyrosine phosphorylation.
Figure 2. E-selectin–IgG chimaera triggers P110 tyrosine phosphorylation and β2-integrin-dependent PMN homotypic adhesion.
(A) Upper panel: PMN were incubated in standard conditions with increasing concentrations of E-selectin–IgG chimaera for 3 min. The interaction was stopped by 1 vol. of 2% (w/v) PFA and single, non-aggregated PMN were counted by optical microscopy in a Burker chamber. Values (means±S.E.M., n=5) indicate the percentage of aggregated PMN with respect to the total PMN. Lower panel: alternatively, the reaction was stopped at 3 min with reducing Laemmli's sample buffer; samples were subjected to SDS/PAGE (7.5–12.5% gradient gels) and probed with anti-phosphotyrosine antibody RC20. The arrow indicates tyrosine phosphorylation of the 110 kDa proteins. Samples are from a representative of three different experiments. (B) Upper panel: PMN, pre-incubated with or without IB4, were challenged with 20 μg/ml E-selectin–IgG chimaera for 3 min in standard conditions. After addition of 1 vol. of 2% (w/v) PFA, single PMN were counted as in (A). Values (means±S.E.M., n=4) indicate the percentage of aggregated PMN. Lower panel: Alternatively the reaction was stopped at 3 min, with reducing Laemmli's sample buffer, and samples were analysed by Western blot with anti-phosphotyrosine antibody RC20. The Figure shows tyrosine phosphorylation of the 110 kDa protein, indicated by an arrow, in samples from a representative of two different experiments.
Src kinase activity is required for β2-integrin-mediated outside-in signalling and β2-integrin-dependent firm adhesion triggered by E-selectin
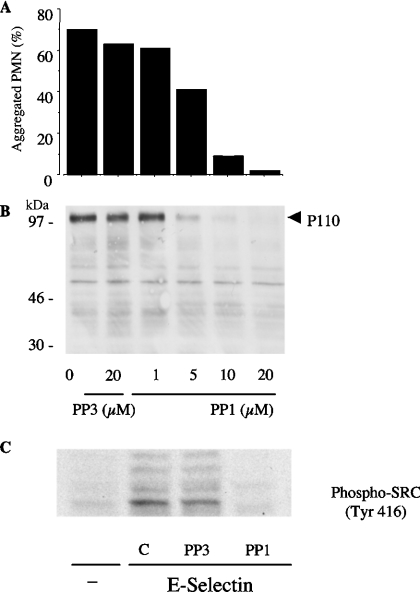
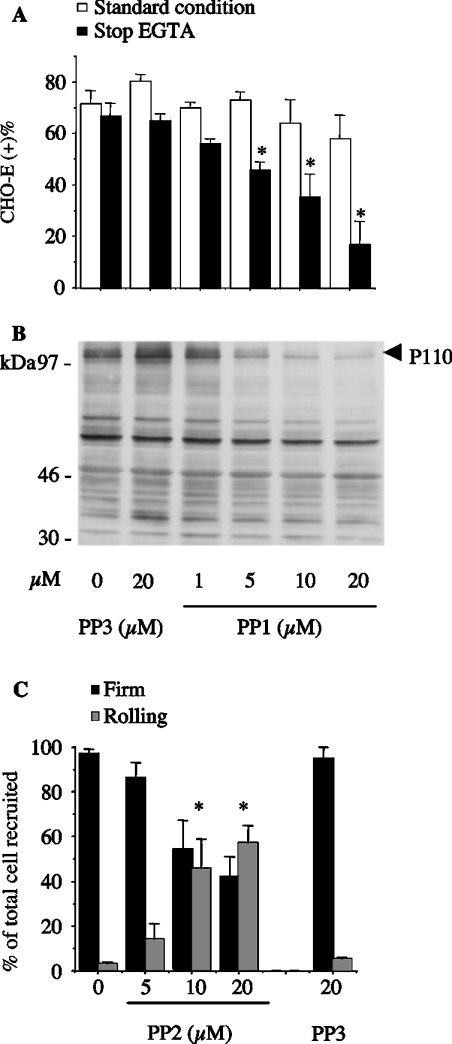
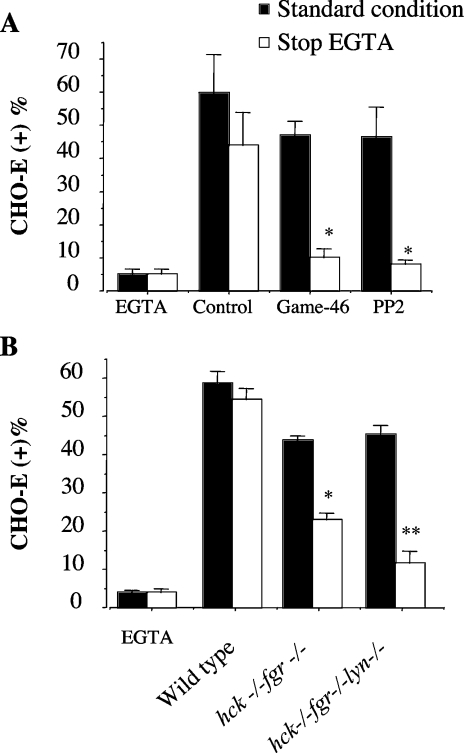
Like β2 integrin blockade (Figure 2B), PP1 (a specific inhibitor of Src-family kinases; [29]), but not PP3 (its inactive analogue), dose-dependently inhibited homotypic PMN adhesion (Figure 3A) as well as P110 tyrosine phosphorylation (Figure 3B) triggered by soluble E-selectin–IgG chimaera. In order to prove that Src-family kinases were activated during the formation of PMN aggregates, lysates were immunoblotted with an antibody recognizing phosphorylated Tyr416 of Src. Autophosphorylation of Src Tyr416 can be used as an index of enzyme activity. As shown in Figure 3(C), phosphorylation of the Tyr416 could be detected in lysates of PMN aggregates induced by soluble E-selectin. This process was completely prevented by PP1, but not by the inactive analogue PP3. Likewise, PMN adhesion to CHO-E, but not to wild-type CHO cells, resulted in tyrosine phosphorylation of P110 (results not shown). Therefore, we tested the effects of Src-family kinase inhibitors on PMN/CHO-E mixed-cell conjugate formation. As was the case with β2 integrin blockade, pretreatment of PMN with PP1 slightly reduced the formation of PMN/CHO-E conjugates. However, when the aggregates were allowed to form and then treated with EGTA to reverse the E-selectin-mediated binding, PP1, but not PP3, dose-dependently reduced the formation of mixed conjugates (Figure 4A). Moreover, PP1 completely prevented P110 phosphorylation in PMN/CHO-E mixed-cell aggregates, whereas the inactive analogue PP3 had no effect (Figure 4B). PP2, another inhibitor of Src-family kinase activity, also prevented the formation of PMN/CHO-E conjugates as well as P110 phosphorylation (results not shown).
Figure 3. Src kinase activity is necessary for β2-integrin-mediated PMN homotypic adhesion triggered by E-selectin–IgG chimaera and for P110 tyrosine phosphorylation in cell suspensions.
PMN were pretreated with DMSO, different concentrations of PP1 or with 20 μM of the inactive analogue PP3 for 1 min at room temperature. (A) PMN were stirred at for 3 min at 37 °C and 1000 rev./min with 20 μg/ml E-selectin–IgG chimaera. The interaction was stopped by 1 vol. of 2% (w/v) PFA, and PMN remaining single (non-aggregated) were counted by optical microscopy in a Burker chamber. Values are means, n=2, and indicate the percentage of aggregated PMN with respect to the total PMN. (B) Alternatively, the reaction was stopped at 3 min with reducing Laemmli's sample buffer; aliquots of 100 μl, corresponding to 1.25×106 PMN total lysate, were analysed by SDS/PAGE (7.5–12.5% gradient gels) and probed with anti-phosphotyrosine antibody RC20. The Figure shows tyrosine phosphorylation of the 110 kDa proteins in samples from a representative of two different experiments. (C) PMN lysates, corresponding to 1.25×106 PMN total lysate per lane, were subjected to SDS/PAGE (7.5% gels) and probed with anti-[phospho-Src-family (Tyr416)] antibody. The Figure shows Western blot of samples from a representative of two experiments.
Figure 4. Src kinase activity is necessary for β2-integrin-mediated PMN adhesion to CHO-E, for P110 tyrosine phosphorylation in mixed-cell suspensions and for PMN firm adhesion to adherent CHO-E in flow.
PMN were pretreated with DMSO, different concentrations of PP1 or PP2, or with 20 μM of the inactive analogue PP3 for 1 min at room temperature. (A) HE-loaded PMN were incubated with BCECF-loaded CHO-E at 37 °C and 1000 rev./min stirring. After 2 min, cells were fixed with 1 vol. of 2% (w/v) PFA (standard condition) or alternatively, 5 mM EGTA was added to mixed cells 30 s before addition of 2% (w/v) PFA (stop EGTA). Samples were then processed for FACS analysis of mixed-cell conjugates. The percentages of CHO cells binding PMN [CHO (+)] are shown. Values are means±S.E.M. for three to five experiments. Differences between groups were analysed by repeated measurement ANOVA. *P<0.05 compared with DMSO-treated group. (B) PMN were incubated with CHO-E at 37 °C and 1000 rev./min stirring. After 2 min, cells were lysed by 1 vol. of reducing Laemmli's sample buffer; samples were subjected to SDS/PAGE (7.5–12.5% gradient gels) and probed with anti-phosphotyrosine antibody RC20. The Figure shows tyrosine phosphorylation of P110 proteins in samples from a representative of three different experiments. (C) PMN [(5×106)/ml], suspended in M199 medium containing 0.1% (w/v) BSA, and pre-incubated with PP2, PP3 or DMSO for 5 min, were perfused at a flow rate of 4 dyn/cm2 for 2 min. The flow was then stepped up to 10 dyn/cm2 and the chamber was perfused with fresh medium for an additional 2 min. The interaction of PMN with CHO cells was observed on a phase contrast video microscope with a ×20 objective. Images were continuously recorded by a video camera. The fractions of rolling and firmly adhered PMN were quantified over the last 20 s of flow. Cells were considered firmly adhered when stationary for 20 s. The values (means±S.E.M., n=4) are the percentages of firmly adhered and rolling PMN relative to the total number of cells recruited. Differences between groups were analysed by repeated measurement ANOVA. *P<0.05 compared with DMSO-treated group.
Although the adhesion model in mixed-cell suspensions is necessary in order to investigate rapid and transient intracellular events such as protein tyrosine phosphorylation, this model does not enable monitoring of the adhesion process in real time. Therefore we used an adhesion assay under flow conditions to investigate further the effect of specific inhibitors of Src family members on β2-integrin-mediated firm adhesion of PMN to adherent CHO-E. We found that the majority of PMN recruited to a CHO-E monolayer at shear stress of 4 dyn/cm2 stopped immediately and became firmly adhered [86±10 PMN/field (mean±S.E.M., n=4)]. Only a few cells arrested after a short rolling movement. Less than 10% of firmly adhered PMN formed homotypic aggregates. PMN did not interact with un-transfected CHO cells. Treatment of the CHO-E monolayer with the anti-(E-selectin) antibody H18/7 abolished PMN recruitment [3±1 PMN/field (n=4)]. The β2 integrin function-blocking mAb IB4 slightly reduced the total number of PMN recruited, to 47±24 PMN/field. However, IB4 substantially reduced the number of arrested PMN, defined as those PMN that moved less than one cell diameter in 20 s, from 84±14 to 18±9 PMN/field (n=4). Like β2 integrin blockade, treatment of PMN with PP2 dose-dependently reduced firm adhesion and increased the fraction of rolling cells; in contrast, the inactive control analogue PP3 did not reduce adhesive interactions (Figure 4C). This finding confirms that β2-integrin-dependent adhesion of PMN tethered to E-selectin requires Src-family kinase activity.
Genetic deficiency of Hck, Fgr and Lyn impairs β2-integrinmediated PMN adhesion to CHO-E
To confirm the specificity of the inhibitory effects of PP1 and PP2, we investigated the ability of PMN from hck−/−fgr−/− and hck−/−fgr−/−lyn−/− mutant mice to form mixed conjugates with CHO-E in mixed-cell suspensions subjected to high-shear rotatory motion. As shown in Figure 5(A), when stirred with wild-type bone-marrow-derived PMN for 2 min, 60±11% (mean±S.E.M., n=6) of CHO-E bound PMN. Mixed-cell conjugates were prevented by the inclusion of 5 mM EGTA; under these conditions only 5±1.6% (mean±S.E.M., n=6) CHO-E bound PMN. As was the case with the human counterparts (Figure 1), treatment of mouse PMN with a function-blocking β2-integrin mAb, Game-46, or with the Src-family kinase inhibitor PP2 (5 μM) only slightly reduced the formation of PMN/CHO-E conjugates; however, when E-selectin-mediated bonds were reversed by the addition of EGTA 30 s before fixation (stop EGTA), both anti-(β2-integrin) antibody and PP2 virtually abolished mixed-cell conjugates. These findings demonstrated that, in mouse PMN tethered by E-selectin, β2-integrin-mediated adhesion is regulated by mechanisms strikingly similar those of human PMN. Therefore we investigated the ability of hck−/−fgr−/− and hck−/−fgr−/−lyn−/− PMN to form conjugates with CHO-E in mixed-cell suspensions. As shown in Figure 5(B), the adhesion of PMN from double hck−/−fgr−/− mutant mice to CHO-E was significantly reduced compared with controls. Interestingly, hck−/−fgr−/−lyn−/− mice displayed an even greater defect, suggesting that Lyn plays an additive role with Hck/Fgr in the regulation of β2 integrin function in our model.
Figure 5. Impaired adhesion to CHO-E of PMN from double hck−/−fgr−/− or triple hck−/−fgr−/−lyn−/− mutant mice in stirred cell suspensions.
(A) HE-loaded PMN from C57BL6 wild-type mice were pre-incubated for 10 min at 4 °C with or without saturating concentrations of the anti-(β2 integrin) mAb, Game-46, or with PP2 (5 μM), and mixed at 37 °C and 1000 rev./min stirring with BCECF-loaded CHO-E, for 2 min. The interaction was stopped by addition of 1 vol. of 2% (w/v) PFA (standard condition). Alternatively, in order to disrupt the Ca2+-dependent selectin–ligand interaction, 5 mM EGTA was added to mixed cells 30 s before the addition of 2% (w/v) PFA (stop EGTA). Samples were then processed for FACS analysis of mixed-cell conjugates. The percentages of CHO-E binding PMN [CHO-E (+)] are shown. Values are means±S.E.M. for three to six experiments. HE-loaded PMN from wild-type or from double hck−/−fgr−/− or triple hck−/−fgr−/−lyn−/− mice were stirred for 2 min at 37 °C and 1000 rev./min with BCECF-loaded CHO-E. The interaction was stopped by the addition of 1 vol. of 2% PFA (standard condition). Alternatively, in order to disrupt the Ca2+-dependent selectin–ligand interaction, 5 mM EGTA were added to mixed cells 30 s before addition of 2% (w/v) PFA (stop EGTA). Samples were then processed for FACS analysis of mixed cell conjugates. Experiments were performed using bone marrow PMN pooled from five mice per experiment. Values are means±S.E.M. for three to six different experiments. Differences between groups were analysed by repeated measurement ANOVA. *P<0.05 compared with control group and **P<0.05 compared with double knock-out.
Src-family kinase activity is required for the formation of sites of firm adhesion based on clustered activated β2 integrins in PMN tethered by E-selectin
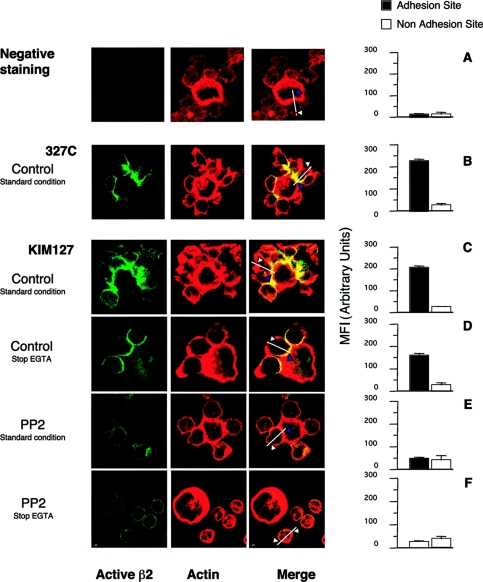
Because Src-family kinases play a central role in phagocyte integrin signalling, the strict dependence of formation of stable homotypic or heterotypic PMN conjugates on both β2 integrins and Src-family kinase activity or expression (Figures 2–5) suggested to us that outside-in signalling ensuing from integrin ligation was required for full integrin function. In order to strengthen this hypothesis, we investigated, by flow cytometry and confocal microscopy, the presence and cellular distribution of activated β2 integrins, as detected by two mAbs that recognize distinct activation-dependent epitopes. The mAbs used were KIM127, which recognizes epitopes in the cysteine-rich repeat region of the β2 chain that are shielded by the α subunit in the closed–inactive conformation and become exposed in the open–active conformation of the integrin [30], and 327C, which recognizes an activation-dependent epitope in the βI-like domain [17].
Using flow cytometry, we sought to determine whether E-selectin–IgG stimulated up-regulation of these epitopes in non-aggregated PMN. To do this, PMN were incubated under static conditions to prevent cell–cell contact and homotypic aggregation. At baseline, 10±1.5% of the total β2 integrins (defined by staining with IB4) were in an activated state (defined by staining with KIM127 or 327C). This value was not significantly modified by the addition of soluble E-selectin (20 μg/ml) to the PMN (8.3±3.6%). In contrast, Mn2+, a direct inducer of integrin conformational changes, up-regulated the KIM127 epitope to 40±14% of total β2 integrins. Similar results were obtained when 327C was used to monitor integrin activation (results not shown). Therefore E-selectin did not stimulate detectable integrin conformational changes.
Interestingly, both KIM127 and 327C epitopes formed clusters specifically located at the contact sites between CHO-E cells and PMN (Figures 6B and 6C). Activated β2 integrins could be detected by KIM127 staining (although at reduced levels) in samples fixed after brief EGTA treatment (Figure 6D), confirming that upon disruption of E-selectin bonds, integrins could sustain PMN/CHO-E adhesion. In contrast, both KIM127 and 327C epitopes were absent at the adhesion sites in mixed-cell conjugates formed by PP2-treated PMN and CHO-E (Figure 6E), indicating that adhesion under these conditions does not involve integrins, but is mainly due to E-selectin-mediated binding. In fact, these conjugates completely disaggregated after brief exposure to EGTA before fixation (Figure 6F).
Figure 6. Activated β2 integrins are specifically clustered at the site of adhesion in heterotypic PMN/CHO-E aggregates: role of Src kinase activity.
PMN, pretreated with DMSO (control) or with 10 μM PP2 for 1 min at 37 °C, were stirred with CHO-E at 37 °C and 1000 rev./min. After 3 min, KIM127 or 327C (both 10 μg/ml) were added and cells were incubated for a further 5 min (under static conditions) and then fixed with 2% (w/v) PFA (standard condition). Alternatively, 5 mM EGTA was added 30 s before KIM127 (stop EGTA). After fixation, Alexa Fluor®-488-conjugated goat anti-mouse antibody revealed KIM127 and 327C localization. (A) Negative staining was performed using samples without KIM127 or 327C. F-actin was stained using rhodamine–phalloidin and samples were processed for confocal laser scanning microscopy. Images represent confocal micrographs from the middle third of cells. Optical Z-sections from each sample were taken with 0.3 μm Z-step from the top to the bottom of the cell. Immunofluorescence images were acquired at high confocality (pinhole=1 Airy unit) to achieve the thinnest optical slices. Fluorescence intensity corresponding to the binding of KIM127 or 327C was measured using the ‘Display-Profile’ option of LSM510 software. We traced one line along the diameter of the PMN, which crossed the cell from side to side. The intensity of fluorescence was measured along this line at both the adhesion and non-adhesion sites and was expressed in arbitrary units (from 0 to 255). Bars are means±S.E.M. for 100–150 cells randomly chosen. (B) and (C) show a single CHO-E surrounded by adherent PMN and stained with 327C and KIM127 respectively. (D) and (E) show a single CHO-E carrying 3 and 5 adherent PMN respectively. (F) A single CHO-E and 4 non-adherent PMN.
Similarly, confocal microscopic examination of E-selectin-induced homotypic aggregates showed that KIM127 or 327C epitopes, virtually absent at the regions of the cell membrane not involved in adhesion, were readily detectable at sites of cell–cell interactions (results not shown).
Pyk2 undergoes tyrosine phosphorylation in a β2-integrin- and Src-family-kinase-dependent manner in PMN aggregated by soluble E-selectin or adherent to CHO-E
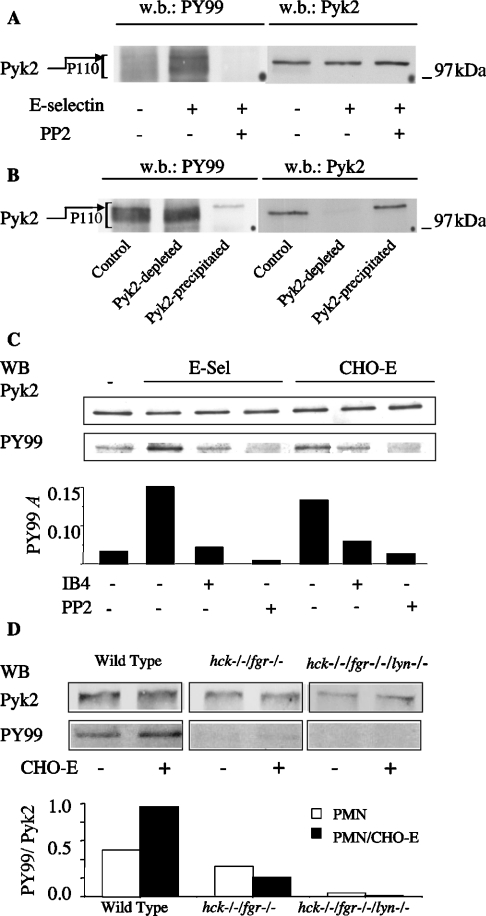
To characterize better the Src-family-kinase-mediated signal(s) involved in the regulation β2 integrin adhesive function, we sought to identify components of the P110 phosphoprotein (Figures 2 and 3). We focused on the proline-rich FAK (focal adhesion kinase) termed Pyk2, which is expressed in cells of myeloid origin and undergoes tyrosine phosphorylation upon β2-integrin-mediated leucocyte adhesion. To detect Pyk2 phosphorylation, lysates from E-selectin-aggregated PMN were immunoblotted for tyrosine phosphorylated proteins and Pky2. The results indicated that Pyk2 could be detected in the upper part of P110 phospho-proteins (Figure 7A). This conclusion was confirmed by immuno-depletion experiments. Lysates from E-selectin-stimulated PMN were subjected to immuno-depletion with an anti-Pyk2 antibody or with goat serum as control. The remaining tyrosine phosphorylated proteins in the supernatants were immunoprecipitated with the anti-phosphotyrosine antibody PY99. The immunoprecipitate was then analysed for tyrosine phosphorylation and Pyk2. Pyk2 was detected in the PY99-immunoprecipitates and migrated as a clear-cut tyrosine-phosphorylated protein in the upper part of P110; this band was no longer detectable in PY99 immunoprecipitates from Pyk2-depleted lysates (Figure 7B). Notably, like P110, Pyk2 tyrosine phosphorylation in PMN stirred with soluble E-selectin or with CHO-E was prevented by β2 integrin blockade, by IB4 and by the Src inhibitor PP2 (Figure 7C).
Figure 7. Pyk2 is a component of P110 and undergoes tyrosine phosphorylation in an Src-kinase- and integrin-dependent manner.
(A) Lysates from unstimulated or E-selectin-activated PMN were analysed by Western blot (w.b.) with anti-phosphotyrosine antibody PY99. After stripping, the same samples were re-probed with anti-Pyk2 antibody. (B) Lysates from unstimulated or E-selectin-activated PMN were subjected to two runs of immunoprecipitation with non-immune goat antiserum (control) or with anti-Pyk2 antibody (Pyk2-depleted) (w.b., Western blot). Tyrosine-phosphorylated proteins in the remaining supernatants were then immunoprecipitated with PY99 anti-phosphotyrosine antibody (Pyk2-precipitated). After stripping, the same samples were re-probed with anti-Pyk2 antibody. In control samples, Pyk2 can be detected in PY99 immunoprecipitates and corresponds to a clear-cut tyrosine-phosphorylated protein in the upper region of P110. This protein is not detectable in Pyk2-depleted PY99 immunoprecipitates. (C) PMN untreated or pretreated with DMSO, 10 μM PP2 or 20 μg/ml IB4 were incubated under stirred conditions for 3 min in the absence or in the presence of soluble E-selectin (E-sel) or CHO-E (5:1 PMN/CHO-E). Pyk2 was immunoprecipitated from cell lysates, and immune complexes were first analysed by Western blot (WB) with PY99 and then re-probed with anti-Pyk2 antibody. Bars indicate absorbances of PY99 Western blots. The Figure shows the results from one representative of three different experiments. (D) PMN from C57BL6 (wild-type), or from double hck−/−fgr−/− or triple hck−/−fgr−/−lyn−/− mice, were incubated in the absence or in the presence of CHO-E for 3 min at 37 °C with stirring at 1000 rev./min. Pyk2 was immunoprecipitated from cell lysates, and immune complexes were first analysed by Western blot (WB) with PY99 and then re-probed with anti-Pyk2 antibody. Bars indicate the ratios between the absorbances of PY99 and Pyk2 Western blots. Values are from one representative of two different experiments.
In order to demonstrate unequivocally that tyrosine phosphorylation of Pyk2 was mediated by Src kinases, we investigated the ability of PMN from hck−/−fgr−/− and hck−/−fgr−/−lyn−/− mutant mice to phosphorylate Pyk2 during adhesion to CHO-E in mixed-cell suspensions subjected to high-shear rotatory motion. As shown in Figure 7(D), Pyk2 tyrosine phosphorylation was readily detectable in PMN stirred in the absence of CHO-E and increased in PMN adherent to CHO-E. In contrast, Pyk2 tyrosine phosphorylation, either in PMN stirred alone or in the presence of CHO-E, was strongly reduced in PMN from hck−/−fgr−/− and virtually abolished in hck−/−fgr−/−lyn−/− mice respectively. We conclude that Src kinases mediate Pyk2 tyrosine phosphorylation in PMN in our model.
DISCUSSION
In the present paper we demonstrate for the first time that a Src-dependent signal is necessary to achieve full β2 integrin activation and to support shear-resistant firm adhesion of PMN tethered by E-selectin. Our results using E-selectin-expressing CHO cells and recombinant, soluble E-selectin confirm previous data indicating that E-selectin-receptor interaction promotes β2 integrin adhesiveness [8–12]. Furthermore, under conditions of rotatory shear motion, we were able to demonstrate the occurrence of protein tyrosine phosphorylation and Src kinase activation in homotypic (PMN/PMN) aggregates triggered by soluble E-selectin or in heterotypic (PMN/CHO-E) conjugates. Both of these events were prevented by β2 integrin blockade. In turn, the use of pharmacological inhibitors or of PMN isolated from hck−/−fgr−/− and hck−/−fgr−/−lyn−/− mice demonstrated that Src kinase activity was required for firm β2-integrin-dependent PMN adhesion in the presence of rotatory shear or laminar flow. Overall, these results indicate that intracellular events initiated by E-selectin–receptor interaction act in concert with a Src-mediated outside-in signal, downstream of β2 integrins, to induce full integrin adhesiveness and shear resistance.
Our conclusions are also supported by detection of activation-dependent epitopes exposed in the open/extended conformation of β2 integrins in PMN. Confocal microscopic analysis revealed that fully active β2 integrins were specifically localized at sites of cell–cell contact in PMN/CHO-E conjugates and in homotypic PMN aggregates triggered by soluble E-selectin. Our results with the two different antibodies indicated that, under our experimental conditions, in adherent PMN, conformational change in integrin molecules involves extension of bent integrin (detected by KIM127) and activation of I-domain (detected by 327C). Exposure of these epitopes at the adhesion sites required Src kinase activity, supporting the conclusion that Src kinases are required for full activation of the integrin. In contrast, flow cytometric analysis of PMN exposed to soluble E-selectin under static conditions, which impedes cell–cell contact and the formation of aggregates, failed to demonstrate an increased expression of these new epitopes, indicating that, under our conditions, conformational changes detected by the antibodies follow integrin–ligand binding.
To determine the nature of the Src-dependent signal, we identified that Pyk2 becomes specifically phosphorylated in a Src- and β2-integrin-dependent manner in E-selectin-induced homotypic PMN/PMN aggregates or in heterotypic PMN/CHO-E conjugates. Pyk2 becomes activated upon stimulation of β2-integrins in leucocytes and may couple integrins to other signalling molecules and the cytoskeleton [31–35]. Moreover, a cross-talk between members of the Src-family kinases and Pyk2 plays an important role in the regulation of migration in macrophages [36–38]. In our model, Pyk2 is activated downstream of β2 integrins in a Src-family-kinase-dependent manner, strongly suggesting that it may be involved in the regulation of integrin function in PMN tethered by selectins. However, the presence of other components of P110 is indicated by PY99 immuno-detection in Pyk2-depleted immunoprecipitates. Experiments attempting to identify the other component(s) of P110 have excluded FAK, phospholipase Cγ or Cbl (results not shown). Clearly, further investigation into the role of Pyk2 and the nature of the other components of the P110 band is needed.
The nature of the signal, as well as the changes of β2 integrins initially generated by E-selectin in our models remain to be determined. However, several other groups have reported that E-selectin may trigger β2 integrin adhesiveness through a p38 MAPK (mitogen-activated protein kinase) pathway. For example, Simon et al. [11] showed that p38 MAPK inhibitors block β2 integrin function initiated by E-selectin under conditions of flow. In agreement with these observations, Kumar et al. [39] found that E-selectin induced chemotaxis and p38 MAPK phosphorylation in human monocytes. Moreover, Green et al. [12] extended these observations to show that inhibition of p38 and p42/44 MAPKs blocked clustering of high-affinity β2 integrin upon co-capping of L-selectin and PSGL-1 driven by E-selectin. Past observations pointed to signals triggered by E-selectin. None of these studies, however, investigated the contribution of β2 integrins in the signalling events. The present results strongly support the conclusion that Src kinase activity in PMN tethered by E-selectin is mainly a consequence of β2 integrin engagement. However, the possibility that Src kinases are also involved in the initial E-selectin–receptor signalling cannot be completely excluded. Structural and functional studies of β2 integrins support the concept that these receptors may exist not only in a closed/bent state or an open/extended high-affinity state, but also in an intermediate conformation that is not able to bind soluble ligands but may sustain lower affinity cell adhesion to immobilized ligands [40,41]. More recently, this concept has been further supported by Shamri et al. [42]. These authors demonstrated that LFA-1 (lymphocyte function-associated antigen 1)-mediated arrest of lymphocytes on ICAM-1 follows a double-step model involving a first instantaneous extension of bent LFA-1, induced by bound chemokines, and a final activation process that is mediated by the ligand. In studies focused on integrin activation by selectins, Ma et al. [14] and Green et al. [12] demonstrated that P-selectin binding to PSGL-1, or E-selectin binding to both L-selectin and PSGL-1, initiates β2 integrin function as a result of an increased avidity due to β2 integrin micro-clustering on the PMN surface. Overall, the present results are in agreement with a model in which E-selectin binding to leucocyte receptor(s) results in an ‘intermediate’ activation state of the integrin, which then translates into a fully active state as a consequence of both ligand binding and activation of Src-signalling pathways.
This process is necessary for shear-resistant β2-integrin-mediated adhesion of PMN tethered by E-selectin under physiological flow. This new role for Src kinases, which are important regulators of leucocyte inflammatory function [43–45], supports the contention that these signalling molecules may prove to be useful targets for anti-inflammatory pharmacological interventions.
Acknowledgments
This work was supported by grants from Ministero della Sanità, Convenzione n. ICS060.2/RF99.74, Italian MIUR-DM L623/96-2002 and AIRC (Associazione Italiana per la Ricerca sul Cancro) codice riferimento 1784 (V.E.) and National Institutes of Health grants HL070304, HL074219, HL78663 and DK064183 (S.S.S.). We thank Drs M. K. Robinson, D. Staunton, C. Beals and D. Vestweber for valuable monoclonal antibodies and transfected CHO cells, Dr Laura Fumagalli for useful suggestions for mouse PMN isolation, and Filomena Cinalli for help in preparing the manuscript.
References
- 1.Ley K. Integration of inflammatory signals by rolling neutrophils. Immunol. Rev. 2002;186:8–18. doi: 10.1034/j.1600-065x.2002.18602.x. [DOI] [PubMed] [Google Scholar]
- 2.Harris E. S., McIntyre T. M., Prescott S. M., Zimmerman G. A. The leukocyte integrins. J. Biol. Chem. 2000;275:23409–23412. doi: 10.1074/jbc.R000004200. [DOI] [PubMed] [Google Scholar]
- 3.Shattil S. J., Kashiwagi H., Pampori N. Integrin signaling: the platelet paradigm. Blood. 1998;91:2645–2657. [PubMed] [Google Scholar]
- 4.Calderwood D. A., Shattil S. J., Ginsberg M. H. Integrins and actin filaments: reciprocal regulation of cell adhesion and signaling. J. Biol. Chem. 2000;275:22607–22610. doi: 10.1074/jbc.R900037199. [DOI] [PubMed] [Google Scholar]
- 5.van Kooyk Y., Figdor C. G. Avidity regulation of integrins: the driving force in leukocyte adhesion. Curr. Opin. Cell. Biol. 2000;12:542–547. doi: 10.1016/s0955-0674(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 6.Zimmerman G. A., McIntyre T. M., Prescott S. M. Adhesion and signaling in vascular cell–cell interactions. J. Clin. Invest. 1996;98:1699–1702. doi: 10.1172/JCI118967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo S. K., Lee S., Ramos R. A., Lobb R., Rosa M., Chi-Rosso G., Wright S. D. Endothelial-leukocyte adhesion molecule 1 stimulates the adhesive activity of leukocyte integrin CR3 (CD11b/CD18, Mac-1, αMβ2) on human neutrophils. J. Exp. Med. 1991;173:1493–1500. doi: 10.1084/jem.173.6.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuijpers T. W., Hakkert B. C., Hoogerwerf M., Leeuwenberg J. F., Roos D. Role of endothelial leukocyte adhesion molecule-1 and platelet-activating factor in neutrophil adherence to IL-1-prestimulated endothelial cells: endothelial leukocyte adhesion molecule-1-mediated CD18 activation. J. Immunol. 1991;147:1369–1376. [PubMed] [Google Scholar]
- 9.Ruchaud-Sparagano M. H., Drost E. M., Donnelly S. C., Bird M. I., Haslett C., Dransfield I. Potential pro-inflammatory effects of soluble E-selectin upon neutrophil function. Eur. J. Immunol. 1998;28:80–89. doi: 10.1002/(SICI)1521-4141(199801)28:01<80::AID-IMMU80>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 10.Ruchaud-Sparagano M. H., Walker T. R., Rossi A. G., Haslett C., Dransfield I. Soluble E-selectin acts in synergy with platelet-activating factor to activate neutrophil β2-integrins: role of tyrosine kinases and Ca2+ mobilization. J. Biol. Chem. 2000;275:15758–15764. doi: 10.1074/jbc.M907390199. [DOI] [PubMed] [Google Scholar]
- 11.Simon S. I., Hu Y., Vestweber D., Smith C. W. Neutrophil tethering on E-selectin activates β2 integrin binding to ICAM-1 through a mitogen-activated protein kinase signal transduction pathway. J. Immunol. 2000;164:4348–4358. doi: 10.4049/jimmunol.164.8.4348. [DOI] [PubMed] [Google Scholar]
- 12.Green C. E., Pearson D. N., Camphausen R. T., Staunton D. E., Simon S. I. Shear-dependent capping of L-selectin and P-selectin glycoprotein ligand 1 by E-selectin signals activation of high-avidity β2-integrin on neutrophils. J. Immunol. 2004;172:7780–7790. doi: 10.4049/jimmunol.172.12.7780. [DOI] [PubMed] [Google Scholar]
- 13.Evangelista V., Manarini S., Sideri R., Rotondo S., Martelli N., Piccoli A., Totani L., Piccardoni P., Vestweber D., de Gaetano G., Cerletti C. Platelet/polymorphonuclear leukocyte interaction. P-selectin triggers protein-tyrosine phosphorylation-dependent CD11b/CD18 adhesion: role of PSGL-1 as a signaling molecule. Blood. 1999;93:876–885. [PubMed] [Google Scholar]
- 14.Ma Y. Q., Plow E. F., Geng J. G. P-selectin binding to P-selectin glycoprotein ligand-1 induces an intermediate state of αMβ2 activation and acts cooperatively with extracellular stimuli to support maximal adhesion of human neutrophils. Blood. 2004;104:2549–2556. doi: 10.1182/blood-2004-03-1108. [DOI] [PubMed] [Google Scholar]
- 15.Piccardoni P., Sideri R., Manarini S., Piccoli A., Martelli N., de Gaetano G., Cerletti C., Evangelista V. Platelet/polymorphonuclear leukocyte adhesion: a new role for SRC kinases in Mac-1 adhesive function triggered by P-selectin. Blood. 2001;98:108–116. doi: 10.1182/blood.v98.1.108. [DOI] [PubMed] [Google Scholar]
- 16.Robinson M. K., Andrew D., Rosen H., Brown D., Ortlepp S., Stephens P., Butcher E. C. Antibody against the Leu-CAM β-chain (CD18) promotes both LFA-1- and CR3-dependent adhesion events. J. Immunol. 1992;148:1080–1085. [PubMed] [Google Scholar]
- 17.Beals C. R., Edwards A. C., Gottschalk R. J., Kuijpers T. W., Staunton D. E. CD18 activation epitopes induced by leukocyte activation. J. Immunol. 2001;167:6113–6122. doi: 10.4049/jimmunol.167.11.6113. [DOI] [PubMed] [Google Scholar]
- 18.Jutila M. A., Bargatze R. F., Kurk S., Warnock R. A., Ehsani N., Watson S. R., Walcheck B. Cell surface P- and E-selectin support shear-dependent rolling of bovine γ/δ T cells. J. Immunol. 1994;153:3917–3928. [PubMed] [Google Scholar]
- 19.Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc. Natl. Acad. Sci. U.S.A. 1987;84:9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez-Madrid F., Krensky A. M., Ware C. F., Robbins E., Strominger J. L., Burakoff S. J., Springer T. A. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc. Natl. Acad. Sci. U.S.A. 1982;79:7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright S. D., Rao P. E., Van Voorhis W. C., Craigmyle L. S., Iida K., Talle M. A., Westberg E. F., Goldstein G., Silverstein S. C. Identification of the C3bi receptor of human monocytes and macrophages by using monoclonal antibodies. Proc. Natl. Acad. Sci. U.S.A. 1983;80:5699–5703. doi: 10.1073/pnas.80.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowell C. A., Soriano P., Varmus H. E. Functional overlap in the src gene family: inactivation of hck and fgr impairs natural immunity. Genes Dev. 1994;8:387–389. doi: 10.1101/gad.8.4.387. [DOI] [PubMed] [Google Scholar]
- 23.Evangelista V., Manarini S., Rotondo S., Martelli N., Polischuk R., McGregor J. L., de Gaetano G., Cerletti C. Platelet/polymorphonuclear leukocyte interaction in dynamic conditions: evidence of adhesion cascade and cross talk between P-selectin and the β2 integrin CD11b/CD18. Blood. 1996;88:4183–4194. [PubMed] [Google Scholar]
- 24.Larsen G. R., Sako D., Ahern T. J., Shaffer M., Erban J., Sajer S. A., Gibson R. M., Wagner D. D., Furie B. C., Furie B. P-selectin and E-selectin: distinct but overlapping leukocyte ligand specificities. J. Biol. Chem. 1992;267:11104–11110. [PubMed] [Google Scholar]
- 25.Remuzzi A., Languino L. R., Costantini V., Guardabasso V., de Gaetano G., Dejana E. Platelet adhesion to subendothelium: effect of shear rate, hematocrit and platelet count on the dynamic equilibrium between platelets adhering to and detaching from the surface. Thromb. Haemostasis. 1985;54:857–861. [PubMed] [Google Scholar]
- 26.Anderson D. C., Miller L. J., Schmalstieg F. C., Rothlein R., Springer T. A. Contributions of the Mac-1 glycoprotein family to adherence-dependent granulocyte functions: structure–function assessments employing subunit-specific monoclonal antibodies. J. Immunol. 1986;137:15–27. [PubMed] [Google Scholar]
- 27.Simon S. I., Rochon Y. P., Lynam E. B., Smith C. W., Anderson D. C., Sklar L. A. β2-integrin and L-selectin are obligatory receptors in neutrophil aggregation. Blood. 1993;82:1097–1106. [PubMed] [Google Scholar]
- 28.Lu C., Ferzly M., Takagi J., Springer T. A. Epitope mapping of antibodies to the C-terminal region of the integrin β2 subunit reveals regions that become exposed upon receptor activation. J. Immunol. 2001;166:5629–5637. doi: 10.4049/jimmunol.166.9.5629. [DOI] [PubMed] [Google Scholar]
- 29.Hanke J. H., Gardner J. P., Dow R. L., Changelian P. S., Brissette W. H., Weringer E. J., Pollok B. A., Connelly P. A. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor: study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 30.Lu C., Shimaoka M., Zang Q., Takagi J., Springer T. A. Locking in alternate conformations of the integrin αLβ2 I domain with disulfide bonds reveals functional relationships among integrin domains. Proc. Natl. Acad. Sci. U.S.A. 2001;98:2393–2398. doi: 10.1073/pnas.041618598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piccardoni P., Manarini S., Federico L., Bagoly Z., Pecce R., Martelli N., Piccoli A., Totani L., Cerletti C., Evangelista V. SRC-dependent outside-in signalling is a key step in the process of autoregulation of β2 integrins in polymorphonuclear cells. Biochem. J. 2004;380:57–65. doi: 10.1042/BJ20040151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gismondi A., Jacobelli J., Mainiero F., Paolini R., Piccoli M., Frati L., Santoni A. Cutting edge: functional role for proline-rich tyrosine kinase 2 in NK cell-mediated natural cytotoxicity. J. Immunol. 2000;164:2272–2276. doi: 10.4049/jimmunol.164.5.2272. [DOI] [PubMed] [Google Scholar]
- 33.Fuortes M., Melchior M., Han H., Lyon G. J., Nathan C. Role of the tyrosine kinase Pyk2 in the integrin-dependent activation of human neutrophils by TNF. J. Clin. Invest. 1999;104:327–335. doi: 10.1172/JCI6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez-Fernandez J. L., Sanchez-Martin L., Rey M., Vicente-Manzanares M., Narumiya S., Teixido J., Sanchez-Madrid F., Cabanas C. Rho and Rho-associated kinase modulate the tyrosine kinase PYK2 in T-cells through regulation of the activity of the integrin LFA-1. J. Biol. Chem. 2001;276:40518–40527. doi: 10.1074/jbc.M102896200. [DOI] [PubMed] [Google Scholar]
- 35.Ren X. R., Du Q. S., Huang Y. Z., Ao S. Z., Mei L., Xiong W. C. Regulation of CDC42 GTPase by proline-rich tyrosine kinase 2 interacting with PSGAP, a novel pleckstrin homology and Src homology 3 domain containing rhoGAP protein. J. Cell Biol. 2001;152:971–984. doi: 10.1083/jcb.152.5.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suen P. W., Ilic D., Caveggion E., Berton G., Damsky C. H., Lowell C. A. Impaired integrin-mediated signal transduction, altered cytoskeletal structure and reduced motility in Hck/Fgr deficient macrophages. J. Cell. Sci. 1999;112:4067–4078. doi: 10.1242/jcs.112.22.4067. [DOI] [PubMed] [Google Scholar]
- 37.Continolo S., Baruzzi A., Majeed M., Caveggion E., Fumagalli L., Lowell C. A., Berton G. The proto-oncogene Fgr regulates cell migration and this requires its plasma membrane localization. Exp. Cell. Res. 2005;302:253–269. doi: 10.1016/j.yexcr.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Okigaki M., Davis C., Falasca M., Harroch S., Felsenfeld D. P., Sheetz M. P., Schlessinger J. Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc. Natl. Acad. Sci. U.S.A. 2003;100:10740–10745. doi: 10.1073/pnas.1834348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar P., Hosaka S., Koch A. E. Soluble E-selectin induces monocyte chemotaxis through Src family tyrosine kinases. J. Biol. Chem. 2001;276:21039–21045. doi: 10.1074/jbc.M009099200. [DOI] [PubMed] [Google Scholar]
- 40.Shimaoka M., Takagi J., Springer T. A. Conformational regulation of integrin structure and function. Annu. Rev. Biophys. Biomol. Struct. 2002;31:485–516. doi: 10.1146/annurev.biophys.31.101101.140922. [DOI] [PubMed] [Google Scholar]
- 41.Takagi J., Springer T. A. Integrin activation and structural rearrangement. Immunol. Rev. 2002;186:141–163. doi: 10.1034/j.1600-065x.2002.18613.x. [DOI] [PubMed] [Google Scholar]
- 42.Shamri R., Grabovsky V., Gauguet J. M., Feigelson S., Manevich E., Kolanus W., Robinson M. K., Staunton D. E., von Andrian U. H., Alon R. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nat. Immunol. 2005;6:497–506. doi: 10.1038/ni1194. [DOI] [PubMed] [Google Scholar]
- 43.Lowell C. A., Fumagalli L., Berton G. Deficiency of Src family kinases p59/61hck and p58c-fgr results in defective adhesion-dependent neutrophil functions. J. Cell Biol. 1996;133:895–910. doi: 10.1083/jcb.133.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mocsai A., Ligeti E., Lowell C. A., Berton G. Adhesion-dependent degranulation of neutrophils requires the Src family kinases Fgr and Hck. J. Immunol. 1999;162:1120–1126. [PubMed] [Google Scholar]
- 45.Lowell C. A., Berton G. Resistance to endotoxic shock and reduced neutrophil migration in mice deficient for the Src-family kinases Hck and Fgr. Proc. Natl. Acad. Sci. U.S.A. 1998;95:7580–7584. doi: 10.1073/pnas.95.13.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]